rPSGL-Ig for Improvement of Early Liver Allograft Function: A Double-Blind, Placebo-Controlled, Single-Center Phase II Study†
This study was registered on http://www.ClinicalTrials.gov under the identifier NCT00876902.
Abstract
The selectin antagonist known as recombinant P-selectin glycoprotein ligand IgG (rPSGL-Ig) blocks leukocyte adhesion and protects against transplantation ischemia reperfusion injury (IRI) in animal models. This randomized (1:1) single-center double-blind 47-patient phase 2 study with 6-month follow-up assessed rPSGL-Ig's safety and impact on early graft function at 1 mg/kg systemic dose with pretransplant allograft ex vivo treatment in deceased-donor liver transplant recipients. Safety was assessed in all patients, whereas efficacy was assessed in a prospectively defined per-protocol patient set (PP) by peak serum transaminase (TA) and bilirubin values, and normalization thereof. In PP patients, the incidence of poor early graft function (defined as peak TA >2500 U/L or bilirubin >10 mg/dL), average peak liver enzymes and bilirubin, normalization thereof and duration of primary and total hospitalization trended consistently lower in the rPSGL-Ig group compared to placebo. In patients with donor risk index above study-average, normalization of aspartate aminotransferase was significantly improved in the rPSGL-Ig group (p < 0.03). rPSGL-Ig treatment blunted postreperfusion induction versus placebo of IRI biomarker IP-10 (p < 0.1) and augmented cytoprotective IL-10 (p < 0.05). This is the first clinical trial of an adhesion molecule antagonist to demonstrate a beneficial effect on liver transplantation IRI and supported by therapeutic modulation of two hepatic IRI biomarkers.
Abbreviations:
-
- ACR
-
- acute cellular rejection
-
- AE
-
- adverse event
-
- ALT
-
- alanine amino-transferase
-
- AST
-
- aspartate amino-transferase
-
- DCD
-
- donation after cardiac death
-
- DNF
-
- delayed non-function
-
- IRI
-
- ischemia reperfusion injury
-
- HAHA
-
- human antihuman antibody response
-
- HAT
-
- hepatic artery thrombosis
-
- LFT
-
- liver function test
-
- PK
-
- pharmacokinetics
-
- PEGF
-
- poor early graft function
-
- PNF
-
- primary non-function
-
- SAE
-
- serious adverse event
-
- TA
-
- transaminase
-
- TBR
-
- total serum bilirubin
-
- rPSGL-Ig
-
- recombinant P-selectin glycoprotein ligand IgG fusion protein
Introduction
Ischemia/reperfusion injury (IRI) occurs in organ grafts subjected to prolonged periods of warm/cold ischemia followed by reestablishment of blood flow. IRI remains one of the most understudied yet critical clinical problems, as it often leads to poor early graft function (PEGF) or primary nonfunction (PNF) in transplant recipients. Moreover, it can significantly impact transplantation outcome because it is a major risk factor for both early graft failure and late chronic allograft dysfunction (1–5). The etiology of IRI, an innate immunity-dominated local antigen-independent process, is not well understood but known to involve multiple factors resulting in graft inflammation, hepatocyte death and/or necrosis (6).
Deceased donor liver transplantation is currently achieving success rates of greater than 83% 1-year graft survival (7). Although the number of transplants increased 2.4-fold from 1990 to 2004, the waiting list increased 15-fold with a 5-fold increase in wait-list deaths. With demand greatly exceeding supply of donor livers, and the increased mortality of those awaiting suitable organs, extended criteria donor (ECD) livers have been increasingly utilized (8,9). However, such organs are at higher risk for severe IRI resulting in PNF or PEGF (8,10).
Inhibition of IRI could potentially increase the number of usable ECD organs. In animal studies, blockade of the interaction between the adhesion molecule P-selectin and its endogenous ligand, P-selectin glycoprotein ligand 1 (PSGL-1), with a recombinant soluble form of this ligand (sPSGL) decreased neutrophil infiltration, ameliorated hepatocyte injury and improved survival in nonsteatotic rat models of liver IRI (2). A similar recombinant P-selectin antagonist, known as recombinant P-selectin glycoprotein ligand IgG (rPSGL-Ig), was tested in the obese Zucker rat model of severely steatotic syngeneic liver transplantation. Fatty Zucker livers pretreated with rPSGL-Ig ex vivo after retrieval showed significantly decreased portal resistance, increased bile production and diminished hepatic endothelial neutrophil infiltration, as compared with controls. The survival of lean Zucker recipients of steatotic Zucker isografts increased from 40% to 90% after rPSGL-Ig treatment (11). This effect correlated with significantly improved liver function and decreased hepatocyte injury. Hence, our experimental findings from a clinically relevant rat model of prolonged hepatic cold ischemia followed by transplantation suggest the potential clinical utility of rPSGL-Ig in increasing the donor pool through prevention or amelioration of hepatic IRI in high-risk livers.
We present here the results of a single-center double-blind placebo-controlled phase 2 study, which was designed to assess the safety and potential efficacy of rPSGL-Ig in patients undergoing deceased-donor whole liver transplantation.
Patients and Methods
Study design and population
This study was a single-center, randomized, double-blinded, placebo-controlled study in two parts (A and B), approved by the UCLA Office for the Protection of Research Subjects (IRB #06-11-064-03 and #08-01-074-03) and each patient was consented prior to enrolment. Patients were eligible if recipients of an ABO-compatible primary deceased-donor whole liver allograft, ≥18 years old, and no other prior organ transplant. Recipients of a liver from a donation after cardiac death (DCD), or from donors <6 years of age, were excluded. In both parts patients were randomized 1:1 for infusion of the donor liver with 20 mg rPSGL-Ig or placebo after retrieval via the portal vein and an intravenous (IV) dose of 1 mg/kg of rPSGL-Ig or placebo prior to arterial reperfusion. In part A, enrollment was restricted to 11 patients with MELD < 29 and donor livers with <20% macrosteatotic fat. In part B, the above restrictions were lifted, and another 36 patients were enrolled under an almost identical protocol. This study was not powered for high significance. Sample size was based on feasibility and less on statistical considerations.
Study drug
rPSGL-Ig was the active treatment in this double-blind study. Although not an ideal control for an immunological agent, saline was used as placebo for the IV bolus administration, for regulatory and logistical reasons. University of Wisconsin preservation solution (UW) was used as vehicle/control for donor organ flush. As liver transplantation can involve substantial blood loss during hepatectomy, this led to reduced drug-exposure in several part A patients. Therefore, in part B, patients with intraoperative blood loss of >10 units transfused received another 10 mg/kg IV bolus of study drug immediately after abdominal closure.
IV dosing of study drug (rPSGL-Ig or placebo) was initiated as soon as technically feasible after portal and prior to arterial reperfusion. The flush dose (chilled 200 mL UW solution ± a total of 20 mg of rPSGL-Ig) was always administered to the donor liver during back-table preparation prior to implantation after arrival at UCLA rather than at the donor hospital, because of logistic and regulatory considerations. The ‘target’ time interval between the study drug ex vivo flush and implantation was at least 60 min. Thereafter, while the anastomoses were being performed for liver implantation, the high K+ UW solution was flushed out from the liver allograft with approximately 750 mL of physiological solution administered through the portal vein.
Randomization, blinding and study conduct
Randomization was performed by a biostatistician using a fixed block method and provided as numbered stack of sealed envelopes to UCLA's unblinded investigational pharmacist, who performed study drug preparation (rPSGL-Ig or placebo) based on the assignment in the sealed envelope corresponding to the patient number. The patients, investigators, research nurses and sponsor remained blinded to treatment assignment through the end of the study.
Patient and study data were recorded into predesigned triplicate case report forms (CRFs) and entered into a central database maintained at Clinical Trial Consultants Inc. (CTI), Blue Ash, OH, USA. Safety data were reviewed by an independent Data and Safety Monitoring Board (DSMB), consisting of two transplant surgeons and one biostatistician that had previously served on the DSMB of the YSPSL-0001 renal transplant study (17). A senior clinical research associate from CTI monitored on-site all CRFs 100% against the source documentation for each patient within 2 months after transplantation and each per-protocol study visit.
Immunosuppressive therapy
Immunosuppression was limited to tacrolimus, mycophenolate mofetil and steroids. Sirolimus was allowed when clinically indicated because of suspected tacrolimus neurotoxicity. Use of antibody therapy was also permitted per protocol after biopsy-confirmed rejection. However, none of the four patients who experienced rejection during the study received antibody therapy, rather, the immunosuppression in these patients was adjusted and/or patient was treated with a solumedrol taper.
Endpoints
Safety: Stopping rules for this study are summarized under Table 1. Endpoints for safety were the incidence of PNF, defined as nonrecoverable hepatocellular function necessitating emergency retransplantation or death within 72 h posttransplant (12); delayed nonfunction (DNF), defined as initial marginal graft function necessitating retransplantation within 1 month of transplant; PEGF; hepatic artery thrombosis (HAT); clinical bleeding events (necessitating transfusion of at least two units of packed red blood cells) and drug-related adverse events (AEs) or serious AEs (SAEs). The incidence of biopsy-confirmed rejection events (ACR, Banff grade ≥1) at 6 months was also recorded.
| Placebo (N = 24) | rPSGL-Ig (N = 23) | Overall (N = 47) | p-Value | |
|---|---|---|---|---|
| Patient survival | 22 (91.7%) | 22 (95.7%) | 44 (93.6%) | 0.9881 |
| Graft survival2 | 22 (91.7%) | 21 (91.3%) | 43 (91.5%) | 0.5551 |
| SAEs (per patient) | ||||
| Mean | 2.21 | 2.00 | 2.11 | 0.7133 |
| SD | 2.19 | 1.62 | 1.99 | |
- Stopping rules: The stopping rules were as follows: (i) if 2 or greater treatment emergent events of grade 3 NCI toxicity (except for study specific categories as defined in the protocol), then the study would halt for DSMB review and communication to FDA. (ii) In addition to above criterion, two patient deaths prior to 30 days would also halt the study for DSMB review and communication of findings to the FDA. Enrollment would also be halted for DSMB review in case of (iii) more than two cases of PNF; (iv) a treatment emergent HAT event that was not otherwise explained from a technical perspective (i.e., vascular reconstruction, venous compression following closing); (v) two instances of portal vein thrombosis; or (vi) more than four cases of PEGF as defined in the protocol. Any case of PNF counted as well as a PEGF event; hence, one PNF event and three PEGF events would trigger a study halt pending DSMB review. None of these stopping rules were ever triggered, however, the DSMB met per protocol within 1 week after the 6th patient of part A had completed 14 days of follow-up, and again after the 18th patient of part B had completed 14 days of follow up. The DSMB allowed the study to proceed after both meetings. The minutes were submitted to FDA. One patient in part B who died in the operating room from severe reperfusion syndrome was emergency unblinded per protocol and determined to have received placebo.
- 1Log-rank (Mantel–Cox) test.
- 2Within study duration (180 ± 21 days).
- 3Two-tailed unpaired t-test rPSGL-Ig vs. placebo.
Efficacy: Because of the exploratory nature of this study, it did not have a single primary endpoint. The impact of rPSGL-Ig on IRI was rather evaluated by the following five exploratory endpoints:
- 1
Categorical classification of transplant IRI based on maximum aspartate aminotransferase (AST) in the first 72 h posttransplant: group 1 <600 U/L; group 2, 600–2500 U/L; group 3, >2500–5000 U/L and group 4 >5000 U/L (13,14).
- 2
PEGF incidence, with PEGF defined as a peak AST or alanine aminotransferase (ALT) level >2500 U/L during the first three postoperative days (POD) (15) and/or by total serum bilirubin (TBR) >10 mg/dL between POD2 and 7 (16).
- 3
Early liver function as assessed by average peak serum levels of AST and ALT (POD1–3), and TBR (POD2–7) in treatment versus placebo patients.
- 4
Treatment groups were also compared for normalization of liver function as assessed by the area under the curve of liver enzyme and TBR from POD1 through 14 (AUC14).
- 5
Liver biopsy postreperfusion histology as assessed by an UCLA pathologist blinded to study drug treatment. Histologic IRI was defined by a four-point grading scale (13). Presence of IRI was defined as grading scale >0.
Pharmacokinetic (PK) and immunogenicity analysis
Study drug and anti-rPSGL-Ig antibodies in serum were determined as previously described (17) on serum samples drawn at the same timepoints.
Statistical analysis
Because of the exploratory nature of this study, no statistical analysis plan was put in place, rather, safety and efficacy endpoints were scored as stipulated in the study protocol. All statistical tests were two-sided and at the 5% significance level if not specified otherwise. All summaries and analyses were performed using data pooled from parts A and B. Missing data were not estimated or carried forward in any of the analyses. All safety analyses were performed on the Intent-to-Treat (ITT, n = 47) analysis set defined as all randomized patients who received study drug.
Efficacy analysis was performed on a prospectively defined ‘per protocol’ (PP) patient set. Inclusion into the PP set required: (i) the graft was NOT from a living or DCD donor. (ii) Patient AND donor organ were dosed. (iii) Study drug was administered IV to patient PRIOR to arterial reperfusion. (iv). The time elapsed between portal and arterial reperfusion did not significantly exceed 1 h. In part A, all 11 enrolled patients met these conditions. In part B, six patients out of 36 (three rPSGL-Ig-patients and three placebo-patients) did not meet one of these conditions and were therefore excluded from the PP set, which hence included 41 patients.
All statistical analysis was performed using Prizm (GraphPad, San Diego, CA, USA) or SAS (SAS Institute, Cary, NC, USA) software.
The PP patient set, for efficacy analysis, was further stratified into patients that received high-risk versus low-risk livers. Risk was estimated using the donor risk index (DRI) proposed by Feng et al. (18). Patients with DRI above study average 1.56 (10 placebo and 8 rPSGL-Ig-treated patients) were defined as high risk, whereas those with DRI<1.56 (11 placebo and 12 rPSGL-Ig-treated patients) were defined as low risk (Figure 1).
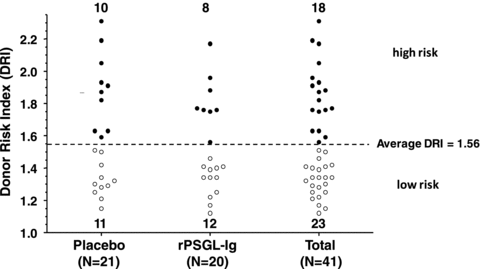
Distribution of per protocol patient (PP) set into patients with high and low donor risk index (18) grouped by treatment assignment.
Biomarker analysis
Liver biopsies: Two biopsies were taken from all patients except from one placebo patient who died intraoperatively, and one rPSGL-Ig-treated patient. The prereperfusion biopsy was taken from the allograft while on ice on the back-table, prior to the organ flush. The postreperfusion biopsy was taken immediately prior to abdominal closure at the end of surgery on average ∼2 h after hepatic arterial anastomosis. Biopsy specimens were placed in RNAlater® solution (Ambion, Austin, TX, USA), refrigerated and shipped within 3 days for analysis at Johns Hopkins University.
RNA isolation and quantitative PCR analysis: Thirteen biomarker transcripts (Figure 6) from liver biopsy RNA were scored essentially as described (19,24). RNA was successfully isolated and transcribed from a total of 45 matched pairs from 22 rPSGL-Ig-treated and 23 placebo patients.
Results
Demographics
Recipient and donor demographics, as well as cold and warm ischemia time and intraoperative blood loss for rPSGL-Ig, placebo, and all patients in the ITT set (N = 47) are presented in Tables 2–4.
| Treatment N | Placebo 24 | Active 23 | Overall 47 |
|---|---|---|---|
| Sex | |||
| Male | 11 (45.8%) | 18 (78.3%) | 29 (60.4%) |
| Female | 13 (54.2%) | 5 (21.7%) | 18 (37.5%) |
| p-value (Fisher's exact test) | 0.035 | ||
| Age (years) | |||
| Mean | 55.8 | 57.3 | 56.5 |
| SD | 10.2 | 9.0 | 9.6 |
| Min–max | 26.7–71.2 | 37.2–73.9 | 26.7–73.9 |
| p-value (unpaired t-test) | Not significant | ||
| Race/ethnicity | |||
| Caucasian | 13 (54.2%) | 14 (60.9%) | 27 (56.3%) |
| African American | 1 (4.2%) | 1 (4.3%) | 2 (4.2%) |
| Hispanic/Latino | 6 (25.0%) | 8 (34.8%) | 14 (29.2%) |
| Asian | 4 (16.7%) | 0 (0.0%) | 4 (8.3%) |
| Pacific Islander | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Primary cause of liver failure | |||
| Hepatitis C | 9 (37.5%) | 11 (47.8%) | 20 (41.7%) |
| Malignant neoplasm | 9 (37.5%) | 11 (47.8%) | 20 (41.7%) |
| Laennec's cirrhosis | 3 (12.5%) | 7 (30.4%) | 10 (20.8%) |
| Nonalcoholic steatohepatitis (NASH) | 3 (12.5%) | 2 (8.7%) | 5 (10.4%) |
| Autoimmune hepatitis | 1 (4.2%) | 0 (0.0%) | 1 (4.2%) |
| Metabolic disease | 2 (8.3%) | 1 (4.3%) | 3 (6.3%) |
| Other | 7 (29.2%) | 2 (8.7%) | 9 (18.8%) |
| MELD | |||
| Mean | 27.0 | 25.8 | 26.4 |
| SD | 6.3 | 5.4 | 5.9 |
| Min–max | 7–40 | 13–39 | 7–40 |
| p-value (unpaired t-test) | Not significant | ||
- There were no significant differences in recipient demographics between treatment and control groups, except for recipient sex, that was significantly (p < 0.05) skewed toward male in the rPSGL-Ig-treated versus the placebo patients.
| Treatment N | Placebo 24 | Active 23 | Overall 47 |
|---|---|---|---|
| Sex | |||
| Male | 12 (50.0%) | 15 (65.2%) | 27 (56.3%) |
| Female | 12 (50.0%) | 8 (34.8%) | 20 (41.7%) |
| p-value (Fisher's exact test) | Not significant | ||
| Age (years) | |||
| Mean | 41.4 | 38.2 | 39.9 |
| SD | 13.5 | 14.7 | 14.1 |
| Min–max | 17–63 | 17–63 | 17–63 |
| p-value (unpaired t-test) | Not significant | ||
| Race/ethnicity | |||
| Caucasian | 15 (62.5%) | 12 (52.2%) | 27 (56.3%) |
| Black or African American | 0 (0.0%) | 3 (13.0%) | 3 (6.3%) |
| Hispanic/Latino | 7 (29.2%) | 8 (34.8%) | 15 (31.3%) |
| Asian | 1 (4.2%) | 0 (0.0%) | 1 (2.1%) |
| Pacific Islander | 1 (4.2%) | 0 (0.0%) | 1 (2.1%) |
| Donor cause of death | |||
| CVA | 10 (41.7%) | 5 (21.7%) | 15 (31.3%) |
| Trauma | 7 (29.2%) | 6 (26.1%) | 13 (27.1%) |
| Anoxic injury | 4 (16.7%) | 11 (47.8%) | 15 (31.3%) |
| Other | 1 (4.2%) | 1 (4.3%) | 2 (4.2%) |
| Living donor1 | 1 (4.2%) | 0 (0.0%) | 1 (2.1%) |
| Donor risk index (18) | |||
| Mean | 1.64 | 1.52 | 1.58 |
| SD | 0.40 | 0.29 | 0.35 |
| Min–max | 1.15–2.69 | 1.08–2.17 | 1.08–2.69 |
| p-value (unpaired t-test) | Not significant | ||
| Donor terminal peak LFTs | |||
| AST | |||
| Mean (U/L) | 184.0 | 401.3 | 290.4 |
| SD (U/L) | 320.4 | 559.3 | 461.5 |
| Min–max | 21–1267 | 27–1827 | 21–1827 |
| p-value (unpaired t-test) | Not significant | ||
| ALT | |||
| Mean (U/L) | 188.5 | 211.9 | 199.9 |
| SD (U/L) | 319.9 | 302.7 | 308.5 |
| Min–max | 15–1258 | 18–1189 | 15–1258 |
| p-value (unpaired t-test) | Not significant | ||
| Bilirubin | |||
| Mean (mg/dL) | 1.80 | 2.92 | 2.35 |
| SD (mg/dL) | 1.48 | 5.34 | 3.88 |
| Min–max | 0.4–6.0 | 0.2–26.0 | 0.2–26.0 |
| p-value (unpaired t-test) | Not significant | ||
- 1One of the patients enrolled in this study received a liver from a living donor in the context of a domino liver transplantation. This patient was excluded per protocol from efficacy analysis.
| Treatment N | Placebo 24 | Active 23 | Overall 47 |
|---|---|---|---|
| Cold ischemia time (hours) | |||
| Mean | 7.42 | 7.10 | 7.26 |
| SD | 2.47 | 2.89 | 2.66 |
| Min–max | 4.22–14.40 | 2.58–11.92 | 2.58–14.40 |
| p-value (unpaired t-test) | Not significant | ||
| Warm ischemia time (minutes)1 | |||
| Mean | 73.1 | 78.3 | 73.1 |
| SD | 14.8 | 28.9 | 14.8 |
| Min–max | 52–104 | 55–1982 | 52–1982 |
| p-value (unpaired t-test) | Not significant | ||
| Intraoperative blood loss (units) | |||
| Mean | 14.8 | 14.3 | 14.6 |
| SD | 10.6 | 16.4 | 13.6 |
| Min–max | 2–43 | 2–752 | 2–752 |
| p-value (unpaired t-test) | Not significant | ||
| Patients that received a second IV dose of rPSGL-Ig at the end of surgery3 | |||
| N | 5 | 6 | 11 |
| % | 20.8% | 26.1% | 23.4% |
| p-value (Fisher's exact test) | Not significant | ||
- 1Defined as the time elapsed from the moment liver was taken out of ice until arterial anastomosis.
- 2The 198 min of warm ischemia time (WIT) in one patient were due to major complications during the arterial anastomosis and this patient was excluded per protocol from the efficacy analysis. The second and third longest WIT in this study was 101 min in a placebo patient and 99 min in a rPSGL-Ig patient. In the patient with 198 min WIT, extraordinary blood loss (75 units) also occurred because of the operative complications. The second largest intraoperative blood loss was 35 units in a placebo patient.
- 3Five placebo patients and three rPSGL-Ig-treated patients did not receive a second dose of IV rPSGL-Ig although their intraoperative blood loss was >10 units. One rPSGL-Ig patient received a second dose of rPSGL-Ig, although patient's intraoperative blood loss was less than 10 units, in violation of the study protocol.
Safety
Deaths: No death occurred in part A but three deaths occurred in part B, two in the placebo arm and one in the rPSGL-Ig-treated arm. One of the deaths in the placebo group was due to PNF while the cause of death of the other placebo patient could not be determined. The one death in the rPSGL-Ig-treated patient group was due to HAT and sepsis. All deaths were determined as unrelated to study drug by the PI and the DSMB.
Graft loss: In addition to three grafts lost due to death, one other rPSGL-Ig-treated patient experienced graft loss within the duration of the study (6 months ± 21days follow-up posttransplant) because of chronic rejection necessitating retransplantation on POD186.
HAT: Two cases of HAT were noted in two rPSGL-Ig-treated patients. One of these eventually died on POD14. This patient was excluded from the PP set, because 2.5 h had elapsed between portal and arterial anastomosis. The other case of HAT was diagnosed over 3 months posttransplant and eventually necessitated retransplantation well outside the study period on POD250. Both cases of HAT were deemed by the PI unrelated to study drug and are fully characterized in Table 5.
| Treatment | Date of transplant | Complication | Date discovered | Cause |
|---|---|---|---|---|
| rPSGL-Ig | 3/28/07 | Bile leak | 7/14/07 | T-tube removal |
| rPSGL-Ig | 8/10/08 | Bile leak | 8/26/08 | T-tube exit site leak |
| rPSGL-Ig | 8/8/08 | HAT | 8/17/08 | Complex reconstruction of donor replaced right hepatic artery, median arcuate ligament syndrome release, splenic artery ligation, anastomosis redone with infra-renal iliac graft. Did well. POD #9: ARDS, hypotensive DX. HAT. Died 8/23/2008 |
| rPSGL-Ig | 9/4/08 | HAT | 1/14/09 | 10/2/08—HA stenosis on CT 5/12/09—re-OLT. Celiac and splenic arteries found thrombosed at hepatectomy |
| Placebo | 10/22/08 | Bile duct anastomotic stricture | 11/22/08 | Technical; balloon dilated 11/25/08 |
| Placebo | 12/17/08 | Hyperkalemia after T-tube removal | 12/17/08 | Non-related |
Clinically significant bleeding events: Seven incidences of clinically significant bleeding were noted in four placebo and three rPSGL-Ig-treated patients.
Serious adverse events (SAEs): There were a total of 99 SAEs, with 28 SAEs reported in Part A and 71 SAEs in Part B. All SAEs were expected events, and were defined as ‘unrelated’ or ‘unlikely related’ to study drug. SAE numbers, frequencies and incidences were similar between the treatment and placebo arms (Table 1). There were also no significant differences between rPSGL-Ig and placebo-treated patients in terms of SAEs grouped by System Order Class (MedDRA10, Table 6).
| System organ class (MedDRA10) | NCI-grade | Placebo | YSPSL | Total | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Blood and lymphatic system disorders | All | 4 | 16.7 | 1 | 4.3 | 5 | 10.6 |
| Moderate | 4 | 16.7 | 1 | 4.3 | 5 | 10.6 | |
| Cardiac disorders | All | 2 | 8.3 | 3 | 13.0 | 5 | 10.6 |
| Mild | 1 | 4.2 | 0 | 0.0 | 1 | 2.1 | |
| Moderate | 0 | 0.0 | 1 | 4.3 | 1 | 2.1 | |
| Life-threatening | 1 | 4.2 | 2 | 8.7 | 3 | 6.4 | |
| Gastrointestinal disorders | All | 3 | 12.5 | 3 | 13.0 | 6 | 12.8 |
| Moderate | 3 | 12.5 | 1 | 4.3 | 4 | 8.5 | |
| Severe | 0 | 0.0 | 2 | 8.7 | 2 | 4.3 | |
| Immune system disorders (excluding rejection) | All | 1 | 4.2 | 0 | 0.0 | 1 | 2.1 |
| Severe | 1 | 4.2 | 0 | 0.0 | 1 | 2.1 | |
| Rejection | All | 2 | 8.3 | 2 | 8.7 | 4 | 8.5 |
| Moderate | 2 | 8.3 | 1 | 4.3 | 3 | 6.4 | |
| Life-threatening | 0 | 0.0 | 1 | 4.3 | 1 | 2.1 | |
| Infections and infestations | All | 6 | 25.0 | 5 | 21.7 | 11 | 23.4 |
| Moderate | 4 | 16.7 | 0 | 0.0 | 4 | 8.5 | |
| Severe | 2 | 8.3 | 3 | 13.0 | 5 | 10.6 | |
| Life-threatening | 0 | 0.0 | 1 | 4.3 | 1 | 2.1 | |
| Death | 0 | 0.0 | 1 | 4.3 | 1 | 2.1 | |
| Injury, poisoning and procedural complications | All | 8 | 33.3 | 5 | 21.7 | 13 | 27.7 |
| Moderate | 2 | 8.3 | 2 | 8.7 | 4 | 8.5 | |
| Severe | 2 | 8.3 | 2 | 8.7 | 4 | 8.5 | |
| Life-threatening | 3 | 12.5 | 1 | 4.3 | 4 | 8.5 | |
| Death | 1 | 4.2 | 0 | 0.0 | 1 | 2.1 | |
| Investigations | All | 4 | 16.7 | 4 | 17.4 | 8 | 17.0 |
| Mild | 1 | 4.2 | 1 | 4.3 | 2 | 4.3 | |
| Moderate | 3 | 12.5 | 2 | 8.7 | 5 | 10.6 | |
| Severe | 0 | 0.0 | 1 | 4.3 | 1 | 2.1 | |
| Metabolism and nutrition disorders | All | 3 | 12.5 | 2 | 8.7 | 5 | 10.6 |
| Mild | 2 | 8.3 | 1 | 4.3 | 3 | 6.4 | |
| Severe | 1 | 4.2 | 1 | 4.3 | 2 | 4.3 | |
| Psychiatric disorder | All | 0 | 0.0 | 1 | 4.3 | 1 | 2.1 |
| Severe | 0 | 0.0 | 1 | 4.3 | 1 | 2.1 | |
| Nervous system disorders | All | 2 | 8.3 | 0 | 0.0 | 2 | 4.3 |
| Severe | 2 | 8.3 | 0 | 0.0 | 2 | 4.3 | |
| Renal and urinary disorders | All | 4 | 16.7 | 4 | 17.4 | 8 | 17.0 |
| Moderate | 3 | 12.5 | 2 | 8.7 | 5 | 10.6 | |
| Severe | 1 | 4.2 | 1 | 4.3 | 2 | 4.3 | |
| Life-threatening | 0 | 0.0 | 1 | 4.3 | 1 | 2.1 | |
| Respiratory, thoracic and mediastinal disorders | All | 6 | 25.0 | 4 | 17.4 | 10 | 21.3 |
| Moderate | 2 | 8.3 | 2 | 8.7 | 4 | 8.5 | |
| Severe | 4 | 16.7 | 2 | 8.7 | 6 | 12.8 | |
| Skin and subcutaneous tissue disorders | All | 0 | 0.0 | 1 | 4.3 | 1 | 2.1 |
| Severe | 0 | 0.0 | 1 | 4.3 | 1 | 2.1 | |
| Unknown | All | 1 | 4.3 | 0 | 0.0 | 1 | 2.1 |
| Death | 1 | 4.3 | 0 | 0.0 | 1 | 2.1 | |
- Hepatobiliary and vascular complications are listed separately in Table 5.
Acute rejection: Four incidences of biopsy-confirmed ACR were noted in two rPSGL-Ig patients and in two placebo patients (one incidence per patient). One of these in a rPSGL-Ig patient progressed to chronic rejection and necessitated retransplantation on POD186.
Efficacy
Binary endpoints: Presence of IRI was scored by the blinded pathologist in 39 out of the total of 41 postreperfusion biopsies (82.3%) in the PP patient set. In fact, mild to severe IRI was also scored in three of the prereperfusion biopsies in the PP set. Only two placebo patients scored into peak AST category 3, whereas none scored into category 4. PEGF was experienced by five patients, four of which had received placebo. After stratification by low and high DRI (cf. ‘Patients and Methods’), four out of 10 high-risk patients treated with placebo experienced DGF versus zero out of 8 high-risk patients treated with rPSGL-Ig (p = 0.0915, cf. Table 7).
| PP patient set | DRI >1.56 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Presence of IRI | Peak AST category | PEGF | ||||||
| 1 | 2 | 3 | 4 | N | PEGF | ||||
| rPSGL-Ig | 20 | 16 (80.0%) | 6 (30.0%) | 14 (70.0%) | 0 (0.0%) | 0 (0.0%) | 1 (5.0%) | 8 | 0 (0.0%) |
| Placebo | 21 | 18 (85.7%) | 9 (42.9%) | 10 (47.6%) | 2 (9.5%) | 0 (0.0%) | 5 (23.8%) | 10 | 4 (40.0%) |
| p-Value | ns1 | ns2 | ns1 | 0.09151 | |||||
- DRI, donor risk index (18).
- 1Fisher's exact test (90% confidence interval).
- 2Mantel-Haenzel chi-square test.
Early liver allograft function: Serum liver enzyme and total bilirubin (TBR) levels were determined in all patients at least once a day during hospitalization at UCLA. For all evaluable patients, and for the high DRI stratum, the peak values and their normalization (area under the curve days 0–14, AUC14) were determined and averages tabulated in Table 8. For the minority of patients who were discharged from hospital prior to POD13, the postdischarge contribution to AUC14 was computed by linear interpolation of LFTs between the day of discharge and the POD14 study visit.
| Group | Patient set | N | ALT | AST | TBR | ||||
|---|---|---|---|---|---|---|---|---|---|
| Peak U/L | AUC14 (U/L) × days | Peak U/L | AUC14 (U/L) × days | Peak mg/dL | AUC14 (mg/dL) × days | ||||
| Placebo | PP | 21 | AVG | 516.2 | 2166.5 | 1005.9 | 1897.4 | 4.09 | 44.9 |
| SEM | 105.5 | 391.0 | 190.4 | 383.0 | 1.08 | 11.9 | |||
| High DRI | 10 | AVG | 676.1 | 2820.6 | 1286.0 | 2650.4 | 5.68 | 63.3 | |
| SEM | 203.2 | 702.6 | 358.1 | 703.7 | 2.14 | 23.4 | |||
| YSPSL | PP | 20 | AVG | 455.6 | 1917.7 | 885.9 | 1494.4 | 3.72 | 34.7 |
| SEM | 81.7 | 283.0 | 125.3 | 192.6 | 0.93 | 6.3 | |||
| High DRI | 8 | AVG | 515.6 | 2319.4 | 950.5 | 1529.2 | 2.51 | 24.9 | |
| SEM | 125.6 | 527.7 | 156.7 | 248.1 | 0.51 | 4.6 | |||
| Overall | PP | 41 | AVG | 486.6 | 2045.2 | 947.3 | 1700.8 | 3.91 | 39.9 |
| SEM | 66.5 | 241.0 | 114.1 | 217.2 | 0.71 | 6.8 | |||
| High DRI | 18 | AVG | 604.8 | 2597.8 | 1136.9 | 2152.1 | 4.27 | 46.2 | |
| SEM | 124.1 | 447.0 | 209.4 | 418.5 | 1.24 | 13.7 | |||
- None of the above differences in endpoints was statistically significant (p < 0.1, unpaired two-tailed t-test) for rPSGL-Ig versus placebo patients.
Average postoperative peak AST, peak ALT and peak TBR were consistently lower in the treatment arm versus placebo. AST, ALT and TBR also appeared to normalize more rapidly in the treated patients versus the placebo patients. These differences were more pronounced when computed for the high-risk stratum only (Figure 2). The more rapid AST normalization in the high-risk strata of the rPSGL-Ig-treated versus placebo patients was statistically significant (p = 0.024). Because the LFTs of patients discharged early (prior to POD13) were already low when compared to patients with longer primary hospitalizations, the LFT interpolation between discharge and POD14 study visit did not impact considerably on the treatment group averages.
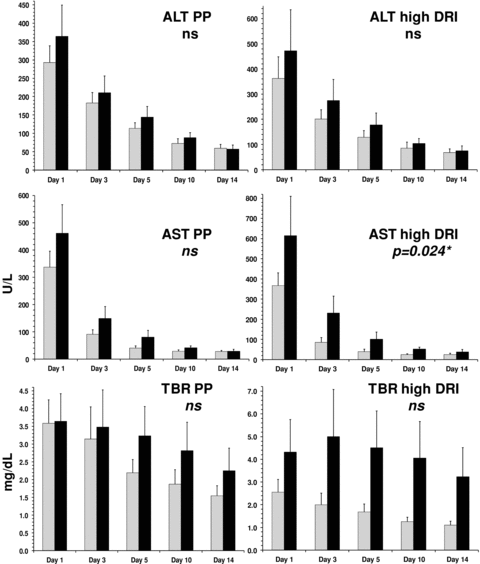
Liver function normalization in per protocol patients compared to high DRI patients grouped by treatment assignment. ░, rPSGL-Ig; █, placebo; ns, not significant; *, random-effect generalized least squares (GLS) regression analysis. Error bars represent standard error of the mean (SEM).
Biomarkers of hepatic ischemia reperfusion injury
The mRNA levels encoding a number of previously suggested hepatic IRI biomarkers were measured by Q-PCR in 45 matched pre- and postreperfusion biopsy pairs and compared to transcripts encoding three housekeeping genes. Relative transcript levels in the postreperfusion biopsies were then compared to the prereperfusion levels. In part A, biomarker transcript levels were also compared to those in a commercial normal liver RNA control. Average transcript levels and postreperfusion induction are tabulated in Table 9. All biomarkers except HMGB-1, TLR4 and, unexpectedly HO-1, were induced postreperfusion, significantly so for Bcl-1, Bcl-2, E-selectin, IL-10, IFN-γ, IP-10, MCP-1 and TNFα. As shown in Figure 3, in placebo patients, IP-10, a marker previously implicated in hepatic IRI in animal models, was induced 20-fold on average upon allograft reperfusion, whereas in rPSGL-Ig-treated patients this induction was blunted (3.3-fold induction only, p < 0.1). Vice versa, postreperfusion induction of the ‘protective’ biomarker IL-10 was significantly augmented (p = 0.043) in the rPSGL-Ig-treated (3.8-fold) versus placebo patients (2.2-fold). Although the average differences were borderline statistically significant, we noted large variations in biomarker responses between individual patients, which warrant caution in interpretation of linear statistics. IL-6 induction also tended to be blunted in rPSGL-Ig-treated patients (data not shown). Postreperfusion levels of transcripts encoding HO-1 and antiapoptotic Bcl-1 and Bcl-2 were not impacted by rPSGL-Ig in our patients.
| Liver reference1 | Bcl2 0.39 | HMOX12 31.26 | HMGB1 3.59 | IFNβ 0.00 | IFNγ 0.00 | IL6 0.02 | IL10 0.79 | IP 10 0.05 | TLR42 2.42 | TNFα2 0.04 | BC113 | MCP13 | SELE3 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | % hkpg | AVG | 0.88 | 15.03 | 1.76 | 1.80 | 0.06 | 2.62 | 1.41 | 0.68 | 3.00 | 0.12 | 28.72 | 14.36 | 0.67 |
| SEM | 0.20 | 3.07 | 0.29 | 0.87 | 0.02 | 2.33 | 0.33 | 0.24 | 0.35 | 0.04 | 5.64 | 3.88 | 0.25 | ||
| Post | % hkpg | AVG | 6.79 | 19.59 | 2.07 | 4.68 | 0.48 | 10.20 | 4.89 | 2.41 | 2.59 | 0.33 | 47.59 | 150.36 | 5.71 |
| SEM | 2.37 | 3.99 | 0.61 | 2.07 | 0.19 | 6.32 | 1.53 | 0.72 | 0.44 | 0.07 | 11.71 | 47.77 | 1.67 | ||
| Ind. | FC | AVG | 16.99 | 1.34 | 1.80 | 65.52 | 44.31 | 72.81 | 5.33 | 22.42 | 0.87 | 4.61 | 2.08 | 31.01 | 132.95 |
| SEM | 5.99 | 0.20 | 0.38 | 53.37 | 21.86 | 32.95 | 1.10 | 12.24 | 0.10 | 1.01 | 0.38 | 16.06 | 69.23 | ||
| p-value4 | 0.014 | 0.201 | 0.513 | 0.178 | 0.023 | 0.069 | 0.021 | 0.024 | 0.189 | 0.032 | 0.033 | 0.005 | 0.003 | ||
- Pre = preperfusion biopsy; post = postperfusion biopsy; % hkpg =% of average of three house-keeping genes in same sample; FC = fold change; AVG = arithmetic mean; SEM = standard error of the mean; SELE = E-selectin.
- Biomarker transcript levels were normalized to transcripts encoding glyceraldehyde 3-phosphate dehydrogenase, β-actin and phosphoglycerate kinase-1 in the same sample.
- 1Normal liver mRNA from Ambion.
- 2Part A only (11 paired samples).
- 3Part B only (34 paired samples).
- 4Two-tailed paired t-test.
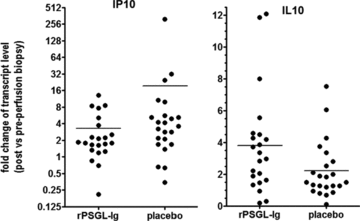
Postreperfusion induction of biomarkers IP-10 and IL-10 in rPSGL-Ig-treated versus placebo patients.
Hospital resource utilization
We measured the length of time spent postoperatively in the intensive care unit (ICU), as well as primary and total hospitalization in all evaluable patients and compared these between treatment arms (Figure 4). Although not reaching statistical significance due to the low number of patients enrolled, the rPSGL-Ig-treated patients experienced less primary and total hospitalization, with the latter reduced by 14 days on average.
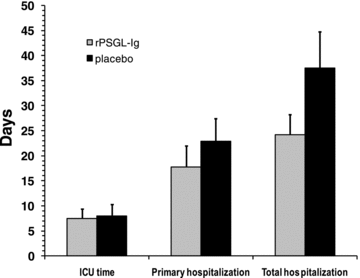
ICU time (defined as the time elapsed between end of transplantation surgery and transfer from the ICU onto the floor) and average primary and total hospitalization days in the PP patient set (within 6-month follow up) grouped by treatment assignment. Error bars represent standard error of the mean (SEM).
rPSGL-Ig pharmacokinetics (PK)
rPSGL-Ig concentrations appeared to peak at 12 h. Thereafter, drug levels declined steadily through POD30. By POD90 rPSGL-Ig-levels had declined approximately 10-fold and on POD180 only very low rPSGL-Ig-levels if any were detected. In part A, drug exposure was noted >2-fold reduced in patients that had experienced significant intraoperative blood loss. Therefore, in part B, a second IV bolus of study drug was administered to patients after an intraoperative blood loss of >10 units. Average cmax, AUC and serum half-life in the rPSGL-Ig-treated patients are tabulated in Table 8.
rPSGL-Ig-immunogenicity
All anti-rPSGL-Ig antibodies samples were determined to be nonreactive. Thus, at least within the duration of the study, rPSGL-Ig did not elicit human antihuman antibody responses.
Discussion
rPSGL-Ig is a recombinant inhibitor of the initial tethering of leukocytes to activated platelets and endothelium, which is mediated by selectins binding to PSGL-1. In small animal models of solid organ transplantation, perioperative treatment with rPSGL-Ig was safe, and ameliorated IRI (11,20,21).
Here, we report the results of a phase 2a study to establish the safety of rPSGL-Ig in de novo deceased-donor orthotopic whole liver transplant recipients. Efficacy assessments explored trends of potential impact of rPSGL-Ig on early liver allograft function. Overall, the administration of rPSGL-Ig in all patients was uneventful and no specific safety concerns were raised from using this drug in the perioperative period, in agreement with previous clinical data (17,22,23). The distribution of the SAEs was similar between the rPSGL-Ig-treated and placebo arm, no SAE deemed related to study drug and all of them were expected in this patient population.
Although this study was not designed and powered for efficacy, our results suggest trends toward improvement of early liver function posttransplantation effected by rPSGL-Ig as compared with placebo, in terms of peak liver enzyme and bilirubin levels and normalization thereof. Although these effects were not statistically significant on their own, the average AST, ALT and bilirubin peak values and normalizations (AUC day 0–14) were all lower in the treatment arm versus the placebo arm. When evaluable patients were stratified into receiving a high or low donor risk graft using a published index, rPSGL-Ig's potential benefit appeared to be restricted to the high DRI patients, bordering at statistical significance for AST normalization. Although we analyzed both AST and ALT it is not surprising that there is a more rapid normalization of AST due to its shorter half-live. Furthermore, although ALT may be more ‘liver specific’, AST has been shown to be a very sensitive maker of early graft dysfunction (14). In the high-risk stratum, rPSGL-Ig also further reduced the PEGF incidence, which was significant at the 90% confidence interval. Other stratifications, by MELD score, by histological IRI, or by macro and/or microsteatotic fat content did not augment the observed treatment effect and also did not reveal significant correlations to short-term outcome.
rPSGL-Ig did not appear to impact hepatic IRI evidenced by histopathology. However, this method of determining IRI is limited by sampling bias and the fact that a subset of patient had evidence for IRI already in the preperfusion biopsy. In fact, experimental animal data on IR liver damage, assessed by histological Suzuki's criteria, do not always correlate with liver function as reflected by serum ALT/AST levels (Kupiec-Weglinski, unpublished data).
Biomarkers of IRI are sought for as more objective surrogate endpoints and predictors of outcome. In our recent renal biomarker study (24) within our double-blinded randomized placebo-controlled renal transplant clinical trial (17), targeting the selectin cascade with rPSGL-Ig attenuated the postreperfusion renal increase of MCP-1 and TGFβ (p < 0.05) transcripts. In hepatic IRI animal models, IP-10, IL-10 and HO-1, along with antiapoptotic genes, were suggested to correlate or inversely correlate with local inflammation and hepatocyte death (20,25,26). Here, we compared biomarker transcript levels in pre- and postreperfusion liver biopsies. We found that proinflammatory and antiapoptotic transcripts were induced upon reperfusion. rPSGL-Ig treatment appeared to reduce induction of IP-10 and IL-6, and augment induction of the cytoprotective biomarker IL-10. Although due to the large variations in individual patient's biomarker responses these data need to be interpreted with caution and substantiated in larger sample sizes, our present findings in humans support the concept that IP-10 expression can serve as marker for hepatic IRI, which is most likely mediated through the TLR4 signaling pathway (26,27). The augmented IL-10 expression in the rPSGL-Ig treatment group is also consistent with our previous animal model data (26,28). However, unlike in most animal studies, we failed to validate HO-1 as a biomarker of hepatic IRI. HO-1 promoter studies based on (GT)m microsatellite polymorphisms suggest that stronger HO-1 expression protects from organ IRI, and conversely, polymorphisms correlating with lower HO-1 response worsen the pathology (29). Hence, more analyses are needed to precisely attribute a cytoprotective action to HO-1 in transplant patients. Immunohistochemical determination of active P-selectin on the biopsies was also considered, but not implemented because of technical obstacles.
Although the statistical significance of our data is limited by the sample size, we believe this is the first clinical trial to demonstrate a beneficial effect of an adhesion molecule antagonist on IRI in liver transplantation. Other clinical strategies reported to ameliorate IRI in liver transplantation include donor treatment with steroids (30), caspase inhibition (31), ATG induction therapy (32), donor organ flush with calcineurin inhibitor (33) and nitric oxide (NO) inhalation (34). All these studies report somewhat improved posttransplant liver function resulting from experimental treatments, with ATG allowing for more compromised liver grafts to be transplanted with less clinical evidence of IRI and improved function. However, except for the NO trial, these studies involved immunosuppressive agents or post hoc analysis. Importantly, rPSGL-Ig is the first systemically administered non-immunosuppressive agent shown in a prospective randomized double-blinded clinical trial to reduce liver IRI, with improved early liver function correlating to beneficial modulation of hepatic IRI biomarkers. Our data provide an adequate safety profile and indicate a beneficial impact of rPSGL-Ig on hepatic IRI and early liver allograft function in recipients of deceased donor liver transplants. Further studies with appropriate dose ranging are required to establish rPSGL-Ig as a safe and effective treatment modality for transplantation IRI in deceased-donor transplantation. Such a modality, in addition of providing short and potentially long-term benefit to patients, might also increase the pool of usable donor livers, because currently about 20% of available donor livers are not being utilized because they are deemed high risk for hepatic IRI and subsequent poor graft function and survival.
Acknowledgments
This study was funded by Y's Therapeutics, Inc. and by the Dumont-UCLA Research Foundation. GSL was supported in part by a grant from the National Institutes of Health (5K08HD057555-03). The authors thank Charles Lassman for expert review of liver histological specimens.
Disclosure
The authors of this manuscript have conflicts of interest to disclose as described by the American Journal of Transplantation. SH is a salaried employee of Y's Therapeutics, Inc. ECS was also a salaried employee of Y's Therapeutics, Inc. (until April 2007). No other conflicts of interest.




