Organ Donor Screening Practices for Trypanosoma cruzi Infection among US Organ Procurement Organizations
Abstract
Donor-derived Trypanosoma cruzi infection in solid organ transplant recipients is associated with significant morbidity and mortality. Little is known about T. cruzi screening practices among U.S. organ procurement organizations (OPOs). We distributed a questionnaire to all U.S. OPO directors, requesting data on T. cruzi screening strategies, laboratory methods, number of donors screened, disposition of organs from positive donors and attitudes toward screening. Fifty-eight (100%) U.S. OPOs responded to the survey. Donor screening began in 2002 and is presently performed by 11 (19%) OPOs. Among screening OPOs, four screen all donors and seven use a risk-based strategy. Three different T. cruzi serology tests are used for donor screening. During 2008, 9/993 (0.9%) donors screened positive by a T. cruzi screening test, 6/9 (66%) had confirmatory tests performed and 4/6 (66%) had positive confirmatory tests. These results led to the nonuse of five donors and 17 organs. Five organs from three seropositive donors were transplanted in 2008 without recognized disease transmission. Variability of T. cruzi donor screening strategies, laboratory methods and disposition of organs from positive donors currently exists. Further research is needed to identify the risk of donor-derived T. cruzi infections to help inform the best screening strategy.
Abbreviations:
-
- AOPO
-
- Association for Organ Procurement Organizations
-
- CDC
-
- Centers for Disease Control and Prevention
-
- FDA
-
- Food and Drug Administration
-
- OPO
-
- Organ procurement organization
-
- RIPA
-
- Radioimmunoprecipitation assay
Introduction
Chagas disease is caused by infection with the protozoa Trypanosoma cruzi. Infected triatomine insects transmit T. cruzi to humans in rural areas of Mexico, Central America and South America but T. cruzi can also be spread by congenital transmission, blood transfusion and organ transplantation (1,2). Following T. cruzi infection, only 20–30% of persons will develop the cardiomyopathy or gastrointestinal manifestations of Chagas disease. An asymptomatic period of infection with potentially intermittent parasitemia lasts many decades prior to onset of clinical disease; therefore, only serological screening is sensitive for identifying infected patients (3).
Eight to eleven million persons in Latin America (4) and over 300 000 persons living in the United States are thought to be infected with T. cruzi (5). The U.S. Food and Drug Administration (FDA) licensed serologic testing for blood screening in 2007 and since then over 1000 confirmed cases of T. cruzi infection have been identified (6). Over the last decade, solid organ donor-derived infections with T. cruzi have been identified in the United States and were associated with significant morbidity and mortality despite the use of antiparasitic agents, typically instituted after onset of clinically overt disease (2,7,8). Given this large number of potentially infected organ donors in the United States and the potential consequences of donor-derived infection, some organ procurement organizations (OPOs) have begun screening donors for T. cruzi infection.
Screening for T. cruzi infection in solid organ donors is routine in regions of the world where infection is highly endemic, such as Brazil and Argentina (9). However, in the United States, where the disease prevalence is lower, no consensus exists on which donors should be screened for T. cruzi infection. Given the lack of data and guidance on screening for T. cruzi infection in organ donors, there is likely wide variability in practices among OPOs. In an effort to better understand the present day donor screening practices and use of organs from donors who screen positive for T. cruzi we conducted a prospective survey of all U.S. OPOs.
Material and Methods
A 26-item questionnaire (available as online supplemental material) was developed by the study team using a web-based electronic survey website (http://www.surveymonkey.com). The survey focused on five data types: (i) T. cruzi donor screening strategies; (ii) laboratory methods for diagnosis of T. cruzi infection; (iii) total number of donors screened for T. cruzi; (iv) disposition of organs from donors screened positive for T. cruzi and (v) attitudes toward future screening in nonscreening OPOs. The questionnaire was distributed by e-mail in May of 2009 via the Executive Director's office of the Association of Organ Procurement Organizations (AOPO). The questionnaire was sent to the executive director of each of the 58 OPOs in the United States. The executive director was asked to complete the survey or pass it on to someone in the organization who has knowledge in this area and could complete it. A follow-up reminder was sent to the executive directors via e-mail until all 58 OPOs responded; the final survey was completed in August 2009. Data were compiled and deidentified by AOPO Executive Director's office and provided to the rest of the study team for analysis.
Results
Screening strategies
All 58 (100%) OPOs in the United States responded to the survey. Screening for T. cruzi infection is performed at 11 (19%) of OPOs. Among the screening OPOs, four (36%) are screening all donors while seven (64%) are using a risk-based strategy. Risk factors used to determine need for T. cruzi screening were reported as birth or prior residency in Mexico, Central America or South America by all seven OPOs using risk-based strategies. Five of seven OPOs who used risk-based screening also included travel to an endemic country as a reason for T. cruzi screening. The earliest reported T. cruzi screening began in 2002 and has increased over the following 7 years (Figure 1).
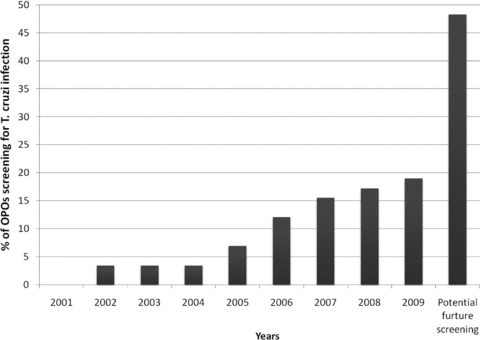
Percentage of OPOs screening for T. cruzi infection among donors over the last 9 years and expected future screening rates. The category ‘potential future screening’ was calculated by adding all OPOs screening in 2008 plus all OPOs who answered the survey results as ‘considering future screening’.
Laboratory-based screening methods
Three different T. cruzi serology tests are used by OPOs for donor screening. These testing methods include the ORTHO T. cruzi ELISA Test System (Ortho-Clinical Diagnostics, Inc.; Raritan, New Jersey), Chagatest (Wiener Laboratorios; Rosario, Argentina) and Hemagen Chagas Kit (Hemagent Diagnostics; Columbia, Maryland). In the United States, only the ORTHO T. cruzi ELISA Test System and Abbott PRISM Chagas are approved for organ or tissue donor screening. The Hemagen Chagas Kit assay was used most frequently (Figure 2A). Seven (64%) OPOs used a confirmatory test when they had a positive screening test and utilized ORTHO T. cruzi ELISA Test System, Hemagen Chagas Kit and T. cruzi radioimmunoprecipitation assay (RIPA), while others reported using another test (Figure 2B). Three (21%) OPOs reported that they would send a positive sample to the Centers for Disease Control and Prevention (CDC) for confirmation all the time, two (18%) would send it sometimes and six (55%) would not send to the CDC.
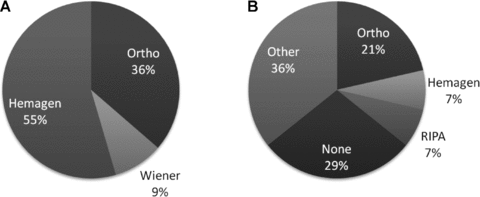
(A) T. cruzi screening tests used by OPOs by %; (B) confirmatory T. cruzi testing used by OPOs by %. Ortho = ORTHO T. cruzi ELISA Test Syst (Ortho-Clin Diag, Inc), Wiener = Chagatest (Wiener Labs), Hemagen = Hemagen Chagas Kit (Hemagen Diag.). RIPA = radioimmunoprecipitation assay. Other: one OPO does confirmatory testing at local blood lab, three send to CDC.
Disposition of organs from seropositive donors
During 2008 a total of 993 donors were screened by OPOs for T. cruzi infection with a median of 90.3 donors screened per screening OPO (range 1–460). Nine (0.9%) donors screened positive and 6/9 (66%) had a confirmatory tests performed. Four of six (66%) confirmatory tests were positive. These nine positive screening results resulted in the nonuse of five potential donors and 17 organs. Five organs (one heart, two kidneys and two livers) from three donors who tested positive for T. cruzi were transplanted by three OPOs in 2008. Since the start of screening for T. cruzi infection six organs from four positive donors have been transplanted.
Attitude toward future screening
Among the OPOs not presently screening for T. cruzi, 17/47 (36%) are considering future screening. Among those considering screening, 6/28 (21%) would screen all donors, 13 (46%) would use a risk-based strategy and 9 (32%) were not sure. Among the 17 considering screening four (23%) OPOs plan to use ORTHO T. cruzi ELISA Test System, while the remainder is unsure of which testing method they would use.
Discussion
The majority of U.S. OPOs are not screening donors for T. cruzi infection. Among OPOs utilizing T. cruzi screening, most screen only those at risk since most cases of donor-derived T. cruzi infection have occurred in donors from endemic regions. However, risk-based screening methods are not 100% sensitive for identifying infected blood donors and this method may be even less effective for screening solid organ donors (10). Given this limitation, there may be some rationale for universal screening in selected areas with large populations of migrants from Mexico, Central or South America.
There is also significant variability in the screening methods used. Of the screening OPOs, most reported using the Hemagen Chagas kit which is cleared by the United States FDA for clinical testing but not for donor screening. As such, there are limited data on the sensitivity and specificity of the assay when applied as a screening intervention. Of concern, one retrospective study using the Hemagen Chagas kit to look for T. cruzi infected solid organ donors demonstrated low specificity of the assay (11). This decreased specificity is concerning because 36% of OPOs did not use a confirmatory test when the screening test was positive, and 55% never involved the CDC for additional testing. Obtaining additional testing through the CDC in all such cases is critical as they can facilitate confirmation in addition to providing additional testing, advice and treatment, if needed, for recipients of organs from infected donors.
OPOs differed in their approach to the disposition of organs from T. cruzi positive donors. In 2008, most organs were discarded but five organs from seropositive donors were transplanted. These inconsistencies in T. cruzi screening and organ utilization from seropositive donors likely reflects multiple factors including geographic variability of at-risk populations (6), paucity of data on performance of T. cruzi screening tests in solid organ donors, and lack of information on specific donor and recipient risk factors which contribute to donor-derived infection. Since this survey focused on T. cruzi screening practice and did not follow-up on the outcomes of recipients of T. cruzi-infected donor organs, this study cannot provide information on the risk of transmission from such donors. Although there are few studies demonstrating safety of pre-emptive monitoring and treatment following receipt of organ from seropositive donors, there is a growing experience of successful transplantation of T. cruzi infected organs to uninfected recipients in the United States which has not been published to date. A group of experts have recently prepared proposed guidelines for the screening of donors for T. cruzi and for the utilization of organs from patients with initially positive results.
We believe that the results of this survey illustrate the need for further research on disease transmission from organ donors. All groups screening donors should confirm the initial positives with a second alternative test, preferably in collaboration with experts at the CDC, with findings collected centrally. A national registry would be useful in assessing risk factors for disease transmission, testing strategies and outcomes in regions of both high and low endemicity. Studies are needed to inform effective strategies for early recognition and treatment of donor derived T. cruzi infections. When using organs from seropositive donors, expert advice, from local, state or federal health authorities, such as the CDC, should be utilized.
Acknowledgments
We would like to thank Elling Eidbo for his review of the manuscript, Bruce Wilson for his assistance with organizing the survey and the Chagas in Transplant Working Group (a collaborative working group of AOPO, CDC and an independent group of experts) for their advice.
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.




