The Molecular Phenotype of 6-Week Protocol Biopsies from Human Renal Allografts: Reflections of Prior Injury but Not Future Course
Abstract
We assessed the molecular phenotype of 107 6-week protocol biopsies from human renal allografts, using Affymetrix microarrays. Transcript changes were summarized as nonoverlapping pathogenesis-based transcript sets (PBTs) reflecting inflammation (T cells, macrophages, IFNG effects) and the injury–repair response of the parenchyma, stroma and microcirculation-increased (‘injury-up’) and decreased (‘injury-down’) transcripts. The molecular changes were highly correlated with each other, even when all rejection and borderline cases were excluded. Inflammation and injury-down PBTs correlated with histologic inflammation and tubulitis, and the inflammation transcripts were greater in kidneys diagnosed as T cell-mediated or borderline rejection. Injury-up PBTs did not correlate with histopathology but did correlate with kidney function: thus functional disturbances are represented in transcript changes but not in histopathology. PBT changes correlated with prior delayed graft function. However, there was little difference between live donor kidneys and deceased donor kidneys that had not shown delayed graft function. Molecular changes did not predict future biopsies for clinical indications, rejection episodes, functional deterioration or allograft loss. Thus while detecting T cell-mediated inflammation, the molecular phenotype of early protocol biopsies mostly reflects the injury–repair response to implantation stresses, and has little relationship to future events and outcomes.
Abbreviations:
-
- PBTs
-
- pathogenesis-based transcript sets
-
- DGF
-
- delayed graft function
-
- LD
-
- live donor
-
- DD
-
- deceased donor
-
- TCMR
-
- T cell-mediated rejection
-
- ABMR
-
- antibody-mediated rejection
-
- IFTA
-
- interstitial fibrosis and tubular atrophy
-
- eGFR
-
- estimated glomerular filtration rate
-
- BFC
-
- biopsies for clinical indication
-
- QCAT
-
- T cell transcripts, reflecting the T cell burden
-
- QCMAT
-
- macrophage transcripts, reflecting the macrophage burden
-
- AMATs
-
- alternative macrophage activation transcripts
-
- GRIT1
-
- transcripts reflecting IFNG effects
-
- ENDAT
-
- endothelial transcripts
-
- KT1
-
- kidney transcripts
-
- KT2
-
- kidney solute carrier transcripts
Introduction
Protocol biopsies of stable kidney transplants are performed at specified times and exclude kidneys that have phenotypes that would otherwise trigger a biopsy for clinical indications. Protocol biopsies in the first months posttransplantation often show silent inflammation, sometimes meeting the arbitrary criteria for the diagnosis of T cell-mediated rejection (TCMR) or ‘borderline changes’, previously defined for biopsies for clinical indications (BFC) (1,2). Indeed, some infiltrates may represent an early stage of TCMR that will soon progress to a phenotype. Other lesions may never have functional significance, instead representing a forme fruste of TCMR that never progresses to functional changes. In addition, some infiltrates may represent the injury–repair response, since interstitial infiltration and tubulitis can occur after renal injury. Silent antibody-mediated rejection (ABMR) can produce inflammation but has microcirculation changes, and is rare in protocol biopsies from nonsensitized patients (3). Thus silent mononuclear cell infiltration and tubulitis in protocol biopsies probably represents one of three processes: early TCMR, forme fruste TCMR, or the injury–repair response.
In recent years, the frequency of silent inflammation in early protocol biopsies (4–8) has declined, presumably related to more effective immunosuppression, and no benefit of protocol biopsies on outcomes has been demonstrable (9). Furthermore, the argument for detecting asymptomatic TCMR lesions is questionable: in the absence of ABMR, even overt TCMR has little impact on function and outcome when appropriately treated (10,11).
In addition to detecting silent inflammation, protocol biopsies have been claimed to predict future allograft loss, functional deterioration, interstitial fibrosis and tubular atrophy (IFTA), and clinical rejection episodes (12–15). This is based on a belief that early injury triggers mysterious, relentlessly progressive deterioration with IFTA, independent of known diseases. However, recent studies indicate that most renal allograft loss is due to specific diseases like recurrent or de novo glomerular disease and ABMR, frequently as the consequence of nonadherence. Neither of these are present or predictable in early protocol biopsies (16–19), contradicting the belief that early injury/inflammation triggers relentless deterioration. In protocol biopsy series, by 6 months posttransplant up to 60–80% of renal allografts show some IFTA, frequently associated with inflammation but remain stable indefinitely (20). Thus early inflammation with later mild IFTA is often the natural history of the injury–repair response to implantation stresses, and stabilizes after the first 6 months or so, like wound healing (4,6,19,21).
We hypothesized that the molecular phenotype could provide novel insights beyond those provided by histopathology, either by detecting silent rejection or by predicting outcomes. We had previously characterized the histopathology findings in a protocol biopsy population (20). In this study we selected a subset of these biopsies representing a standard risk population with stable allograft function at the time of the 6-week protocol biopsy. We compared the molecular phenotype of 6-week protocol biopsies from renal allografts to histologic lesions and function at the time of biopsy, to previous events, and to future rejection episodes, function and outcome. We summarized the microarray results as nonoverlapping pathogenesis-based transcript sets previously defined in experimental systems (http://transplants.med.ualberta.ca/Nephlab/data/gene_lists.html). The PBT system annotates groups of related transcripts representing discrete biological events relevant in organ transplants, like inflammation (infiltration by T cells or macrophages; γ-interferon effects) and the injury–repair response in parenchyma, stroma, or microcirculation cells: increased transcripts (‘injury-up’) and decreased transcripts (‘injury-down’) (22–25).
Materials and Methods
Study population
In 2000, the Division of Nephrology at the Hannover Medical School, Hannover, Germany established as standard of care a protocol biopsy program after kidney transplantation (26). All kidney allograft recipients were approached for consent to participate in protocol biopsies taken at 6 weeks, 3 months and at 6 months posttransplantation. All biopsies were processed for paraffin histopathology (PAS, HE, trichrome, C4d staining) and assessed following the up-dated 2009 Banff criteria (27). Following local standard of care all cases with a Banff diagnosis of TCMR were treated with steroid pulse therapy; patients with borderline changes were not treated. In addition to diagnostic biopsy cores one 18- or 16-gauge needle core was immediately stabilized (either snap frozen or in RNA-Later®) and stored at –80°C for future RNA extraction. Between January 1, 2001 and December 31, 2006 a total of 618 6-week protocol biopsies were performed. For this study 107 6-week protocol biopsies were retrospectively selected according to the following criteria: stable allograft function (<20% increase above baseline serum creatinine), no clinical rejection episode within 1 week before the 6-week protocol biopsy, no recurrent or de novo disease, no BK nephropathy, no focal or diffuse C4d staining in the 6-week protocol biopsy, negative cross-match (CDC-AHG) at the time of transplantation, at least 2 years of follow-up and sufficient material for microarray analysis. They were not selected on the basis of outcomes. Delayed graft function (DGF) was defined as the need for dialysis within the first week posttransplantation. Local institutional ethic review boards approved both, the protocol biopsy program in Hannover and the microarray studies in Edmonton.
Microarray experiments
RNA extraction, labelling and hybridization to HG_U133_Plus_2.0 GeneChips (Affymetrix, Santa Clara, CA, USA) were carried out according to protocols published at http://www.affymetrix.com and as previously described (23). Microarray data was preprocessed by RMA. Fold change for each gene was calculated as the ratio of expression values in each protocol biopsy versus the average value of eight normal controls: histologically normal kidney tissue samples from tumor nephrectomies. The controls were all Caucasian, six males/two females; 60–85 (mean 69.1) years of age, and showed low variance in their PBT score.
Pathogenesis based transcript sets (PBTs)
Microarray gene expression results for the 107 6-week protocol biopsies were summarized as PBT scores: the geometric mean of fold changes across all probesets in that PBT. By the PBT approach, large scale and cumbersome microarray gene expression results are collapsed into a small number of PBT scores representing measurements of the respective biological processes in the tissue (23,28–32). The PBTs were derived from experimental models (mouse kidney allografts and human cell lines) and thus not derived from the present data. We recently showed that the PBT significantly associated with histological rejection lesions in human renal and heart allograft biopsies taken for clinical indication (23,33).We applied the following PBTs to the present set of protocol biopsies for assessing the respective biological processes in the tissue: infiltration of cytotoxic T cells (QCATs, n = 25 probesets) (32), γ-interferon effects on the tissue (GRIT1, n = 68) (30), infiltration of macrophages (QCMATs, n = 71) (34), alternative macrophage activation (AMAT1, n = 27) (10,35), or injury and repair induced transcripts (IRITD3, n = 802, and IRITD5, n = 569) (31), endothelial transcripts (ENDATs, n = 119) (25), and kidney parenchymal transcripts (e.g. solute carriers) highly expressed in ‘normal’ kidneys but showing decreased expression during injury and rejection (KT1, n = 1333, and KT2, n = 64) (29). The probe sets in each PBT as well as the algorithms for PBT generation are available on our homepage (http://transplants.med.ualberta.ca/Nephlab/data/gene_lists.html).
Statistical analysis
Data analyses were performed using SPSS 15.0 statistical software package (SPSS Inc., Chicago, IL, USA), Bioconductor version 2.4, and R version 2.9.1. Comparisons between continuous variables (PBT scores, allograft function) and ordinal variables (Banff scores) were assessed as Spearman correlations. To indentify patterns/clusters of correlations in between the PBTs, a distance matrix was calculated using Euclidean distance between the PBT rows. The R function ‘hclust’ for standard hierarchical cluster analysis was used on the distance matrix. Unsupervised principal component analysis was used to discover classes related to donor type and DGF using the PBTs scores. Principal components analysis (PCA) is an exploratory data analysis/clustering technique that reduces the dimensionality of multivariate data in order to map the variation into a visualizable space of either two or three dimensions. By projecting the multivariate relationships into a smaller number of dimensions representative of the original (multivariate) distances, the relationship between the dominant components of that variation can be extracted and visualized. Each dimension (i.e. Principal Component) is defined to capture the maximum amount of variation in the data not already captured by higher PCs. For example, PC2 represents the maximum direction of variation present after taking into account the PC1 variation. Group comparison for mean PBT scores was done by the Mann–Whitney U-test. Allograft function was analyzed using the four variable MDRD formula for estimated glomerular filtration rate (eGFR) (36). Changes in function were calculated as the delta eGFR between two time points. Results are given as correlation coefficients, means and p-values with the level of significance was set at p < 0.05.
Results
Biopsy and patient demographics
Table 1 summarizes donor and recipient demographics, immunosuppression data, histopathology diagnosis and allograft function at various times posttransplant. Nine protocol biopsies (8%) met the criteria for TCMR, and 20 (19%) for borderline. Most of the 107 patients biopsied at 6 weeks also had protocol biopsies at 3 and 6 months posttransplant.
| All biopsies | |
|---|---|
| Mean recipient age | 48 (19–71 years) |
| Recipient gender (% male) | 61 (57%) |
| Ethnicity, Caucasian | 107 (100%) |
| Previous transplant | 13 (12%) |
| Mean donor age | 47.6 (7 – 82 years) |
| Donor gender (% male) | 64 (60%) |
| Donor type (% deceased donor transplants) | 93 (87%) |
| ABO-incompatible or cross-match positive transplants | 0 |
| Maintenance immunosuppressive regimens at biopsy | |
| MMF, Tacrolimus, steroid | 13 (12%) |
| MMF, Tacrolimus | 1 (1%) |
| MMF, Cyclosporine, steroid | 50 (47%) |
| MMF, Steroids | 6 (6%) |
| Azathioprine, Cyclosporine, Steroids | 1 (1%) |
| mTOR inhibitor | 0 |
| Others | 36 (34%) |
| Mean% PRA at transplantation | 2.3 (0–85%) |
| Grafts with DGF | 28 (26.2%) [27/28 = deceased donors] |
| Mean number of dialysis posttransplantation in grafts with DGF | 4 (1–10) |
| Diagnosis at 6-week PB: no rejection – borderline – TCMR (n = 107) | 78 (72.9%) – 20 (18.7%) – 9 (8.4%) |
| Diagnosis at 12-week PB: no rejection – borderline – TCMR (n = 104) | 75 (70.1%) – 16 (15.0%) – 13 (12.1%) |
| Diagnosis at 6-month PB: no rejection – borderline – TCMR (n = 105) | 80 (74.8%) – 17.0 (15.9%) – 8 (7.5%) |
| Number of patients with BFC before 6-week PB | 23 (1–2 biopsies) |
| Number of patients with BFC after 6-week PB | 31 (1–8 biopsies) |
| Mean eGFR (MDRD) at 6-week PB | 49 (13–96 mL/min) |
| Mean ΔeGFR 6-weeks to 2-years posttransplantation | 1.2 (−49.1–37.0 mL/min) |
| Mean ΔIFTA 6-weeks to 6-months | 4.2 (−5.1–28.7%) |
- BFC = biopsy for cause; PB = protocol biopsy.
Mean lesions scores for the protocol biopsies are summarized in Table 2. As expected, atrophy and fibrosis increased between 6 weeks and 6 months, while interstitial inflammation and tubulitis (the mean Banff i- and t-scores) remained stable during the same period. The protocol biopsies with inflammation at 6 weeks tended to have inflammation at 3 and 6 months (correlation between Banff total i-score at 6 weeks and 3 months: r = 0.3, p = 0.003; between 6 weeks and 6 months: r = 0.4, p < 0.001). Mild glomerulitis and peritubular capllaritis of unknown significance was occasionally seen but did not meet the criteria for ABMR.
| Mean Banff lesion scores* | 6-week PB n = 107 | 12-week PB n = 100 | 6-month PB n = 103 | p-Value** 6-week vs. 12-week | p-Value** 6-week vs. 6-months |
|---|---|---|---|---|---|
| Mean number of glomeruli | 12.1 ± 6.8 (0–32) | 10.2 ± 5.9 (1–38) | 12.3 ± 7.5 (2–40) | ns | ns |
| Mean number of arteries | 2.3 ± 1.7 (0–8) | 2.4 ± 1.6 (0–9) | 2.5 ± 1.8 (0–8) | ns | ns |
| i-score | 0.5 ± 0.7 | 0.5 ± 0.8 | 0.5 ± 0.7 | ns | ns |
| total i-score | 0.5 ± 0.7 | 0.5 ± 0.7 | 0.5 ± 0.8 | ns | ns |
| t-score | 0.4 ± 0.6 | 0.5 ± 0.8 | 0.4 ± 0.7 | ns | ns |
| ci-score | 0.09 ± 0.3 | 0.2 ± 0.4 | 0.4 ± 0.5 | 0.005 | <0.0001 |
| ct-score | 0.3 ± 0.5 | 0.5 ± 0.5 | 0.8 ± 0.5 | 0.046 | <0.0001 |
| v-score | 0.03 ± 0.2 | 0.03 ± 0.2 | 0 | ns | ns |
| cv-score | 0.01 ± 0.1 | 0.05 ± 0.3 | 0.2 ± 0.1 | ns | ns |
| g-score | 0 | 0.04 ± 0.2 | 0.06 ± 0.3 | ns | 0.03 |
| cg-score | 0 | 0.01 ± 0.1 | 0.04 ± 0.2 | ns | ns |
| mm-score | 0.1 ± 0.4 | 0.1 ± 0.4 | 0.2 ± 0.6 | ns | ns |
| ptc-score | 0.1 ± 0.4 | 0.2 ± 0.6 | 0.1 ± 0.5 | ns | ns |
| ah-score | 0.1 ± 0.4 | 0.1 ± 0.3 | 0.2 ± 0.4 | ns | ns |
| C4d | 0.2 ± 0.5 | 0.1 ± 0.5 | 0.2 ± 0.6 | ns | ns |
- PB = protocol biopsy. Banff scores: i = interstitial inflammation; t = tubulitis; ci = interstitial fibrosis; ct = tubular atrophy; v = endothelialithis; cv = intimal fibrosis; g = glomerulitis; cg = transplant glomerulopathy; mm = mesangial matrix increase; ptc = peritubular capillaritis; ah = arteriolar hyalinosis; C4d = immunofluorescence stain in peritubular capillaries.
- *Arithmetic mean.
- **Uncorrected p-value.
The molecular phenotype in 6-week protocol biopsies
The molecular changes in protocol biopsies compared to normal kidneys were expressed as PBTs, summarizing the inflammation and the injury–repair response—increased expression (injury-up) and decreased expression (injury-down). Note that the PBTs were derived in experimental systems and were not trained on the present data.
The molecular changes were highly stereotyped: i.e. the PBT scores correlate strongly with each other, as previously described in renal allograft biopsies for clinical indications (BFC) (23,37) and in heart allograft biopsies (33) (Figure 1A). There was increased expression of the inflammation PBTs, reflecting the T cell burden (QCAT), macrophage burden (QCMAT) and IFNG effects (GRIT1), particularly in biopsies diagnosed as TCMR or borderline (see ribbon at the top of Figure 1A). The inflammation PBTs correlated moderately with the injury-up PBTs (IRITD3, IRITD5), endothelial transcripts (ENDAT) and alternative macrophage activation associated transcripts (AMAT) (Figure 1B). Note that the ENDATs behaved primarily as an injury-up transcript set, reflecting changes in the microcirculation in the injury–repair response. The injury-down changes were highly correlated with each other, reflecting coordinate loss of the transcripts highly expressed in normal kidney parenchyma (KT1, KT2) and inversely correlated with inflammation and injury-up PBTs.
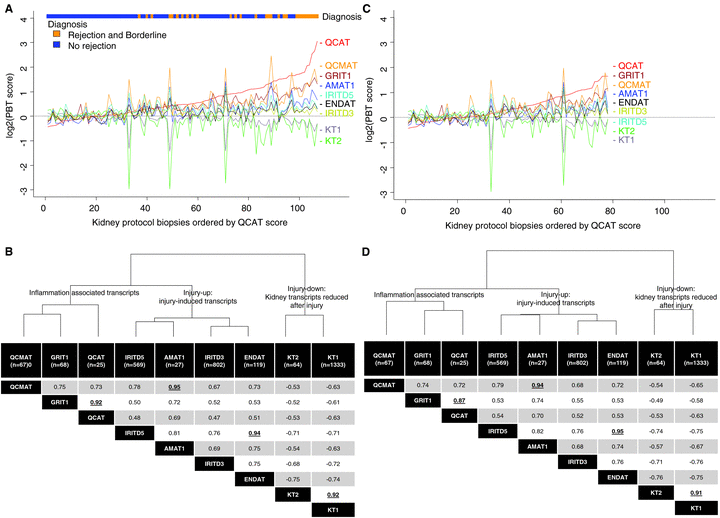
The molecular phenotype in 6-week protocol biopsies. The PBT scores in all biopsies (A) and those in nonrejecting (C) are lined up in order of increasing scores for cytotoxic T cell associated transcripts (QCATs), revealing the strong correlation among PBTs. Spearman correlations of PBTs in all protocol biopsies (n = 107) are shown in (B), with the highest correlation coefficients in bold and underlined. The exclusion of all biopsies showing histopathology TCMR or borderline changes did not change the fundamental relationships or the correlation coefficients in the remaining biopsies (n = 78) (C and D). The highest correlations were between the QCATs and GRITs; CMATs and AMATs; IRITD5 and ENDATs and KT1s and KT2s. All r-values are significant at p < 0.01. The dendrograms at the tops of (B and D) are based on Euclidian distance derived from the entire data set (n = 107), and illustrate how the inflammation (QCAT, QCMAT, GRIT1), injury-up (IRITD3, IRITD5, AMAT1), and injury-down PBTs (KT1, KT2) cluster/group together, indicating the coordinated molecular phenotype in the tissue.
The relationships among PBTs were not primarily driven by the allogeneic response: the pattern was similar when all cases with histologic TCMR and borderline lesions were excluded (Figure 1C and D).
Although the PBTs are nonoverlapping, high correlations (spearman correlation coefficients >0.9) were observed between certain PBT groups: T cell burden and IFNG effects; macrophage burden and alternative macrophage activation; IRITD5 and ENDATs; and both sets of kidney transcripts (Figure 1B). Again, these relationships were similar after excluding biopsies with TCMR and borderline diagnoses, restricting the analysis to biopsies with minimal abnormalities by histopathology (Figure 1D).
The dendrograms in Figure 1 indicate that the PBTs due to their coordinated expression pattern grouped/clustered into inflammation, injury-up and injury-down groups. Of interest, the alternative macrophage activation transcripts (AMATs) displayed ambiguous loyalty, correlating strong with macrophage transcripts (correlation coefficients >0.9) but grouping with injury-up transcripts in the dendrogram.
Associations of the molecular phenotype with other variables at the time of biopsy
As in BFC (23,37), inflammation PBTs, alternative macrophage activation PBTs and injury-down PBTs correlated with histologic inflammation: Banff i- and t-scores, and thus with the diagnosis of TCMR or borderline (Table 3). There were no histologic inflammation lesions that correlated with the injury-up PBTs. IRITD3 (injury-up) weakly correlated with atrophy and scarring (ci-score, ct score) and kidney function at 6 weeks (eGFR), in agreement with our previous findings in BFC (22). Endothelial transcripts (ENDAT) weakly correlated with the ah-score.
| Banff scores | Inflammation associated PBTs with increased expression | Injury and repair associated PBTs with increased expression | Kidney parenchymal PBTs with decreased expression | ||||||
|---|---|---|---|---|---|---|---|---|---|
| QCMAT | GRIT1 | QCAT | IRITD5 | AMAT1 | IRITD3 | ENDAT | KT2 | KT1 | |
| i-score | 0.30** | 0.53*** | 0.55*** | 0.18 | 0.31** | 0.18 | 0.23** | −0.39*** | −0.36*** |
| total i-score | 0.26** | 0.48*** | 0.53*** | 0.13 | 0.26** | 0.10 | 0.18 | −0.27** | −0.26** |
| t-score | 0.32*** | 0.50*** | 0.47*** | 0.13 | 0.33*** | 0.09 | 0.16 | −0.23** | −0.26** |
| Diagnosis (0 = no rejection, 1 = borderline, 2 = TCMR) | 0.34*** | 0.51*** | 0.49*** | 0.14 | 0.32*** | 0.14 | 0.19 | −0.28** | −0.29** |
| v-score | 0.18 | 0.17 | 0.17 | 0.08 | 0.13 | 0.05 | 0.07 | −0.09 | −0.06 |
| ci-score | 0.08 | 0.13 | 0.17 | 0.01 | 0.06 | 0.20* | 0.05 | −0.12 | −0.11 |
| ct-score | −0.10 | −0.17 | −0.17 | −0.10 | −0.11 | −0.02 | −0.05 | 0.00 | 0.08 |
| cv-score | 0.05 | 0.07 | 0.10 | 0.09 | 0.04 | 0.14 | 0.08 | −0.15 | −0.13 |
| ah-score | −0.11 | −0.09 | −0.10 | −0.17 | −0.14 | −0.12 | −0.20* | 0.14 | 0.17 |
| ptc-score | −0.03 | 0.14 | 0.08 | 0.02 | 0.00 | 0.06 | 0.02 | −0.11 | −0.03 |
| eGFR at 6 weeks | −0.16 | −0.07 | −0.09 | −0.08 | −0.13 | −0.26** | −0.06 | 0.08 | 0.08 |
- None of the 6-week protocol biopsies showed Banff g- and cg-score >0 or diffuse C4d positivity.
- *Uncorrected p-value < 0.05.
- **Uncorrected p-value < 0.01.
- ***Uncorrected p-value < 0.001.
The molecular phenotype correlates with previous delayed graft function
Although all kidneys were functioning at the time of biopsy, 27/93 deceased donor (DD) kidneys and one of 14 live donor (LD) kidneys had previously manifested DGF. PBT disturbances were consistently greater in kidneys with a history of DGF and correlated with the number of dialyses needed after transplantation (Table 4). No other donor, recipient and transplant variables before 6 weeks posttransplantation correlated with the PBT expression in the biopsy.
| Inflammation associated PBTs with increased expression | Injury and repair associated PBTs with increased expression | Kidney parenchymal transcripts with decreased expression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| QCMAT | GRIT1 | QCAT | IRITD5 | AMAT1 | IRITD3 | ENDAT | KT2 | KT1 | |
| Donor age in years | −0.02 | 0.05 | 0.08 | 0.04 | −0.01 | 0.08 | 0.05 | −0.07 | 0.02 |
| Recipient age at transplantation in years | 0.06 | −0.05 | −0.07 | −0.02 | 0.04 | 0.15 | −0.06 | 0.11 | 0.09 |
| Cold ischemia time in hours | 0.01 | −0.01 | −0.03 | 0.12 | 0.01 | 0.19 | 0.18 | −0.10 | −0.08 |
| Delayed graft function (0 = no/1 = yes) | 0.24* | 0.20* | 0.24* | 0.32*** | 0.25** | 0.40*** | 0.37*** | −0.41*** | −0.42*** |
| Number of dialysis needed posttransplantation | 0.27** | 0.21* | 0.24* | 0.34*** | 0.26** | 0.41*** | 0.38*** | −0.43*** | −0.41*** |
| Donor type (1 = deceased (n = 93), 2 = living (n = 14)) | −0.05 | −0.06 | −0.01 | 0.00 | −0.01 | −0.13 | −0.05 | 0.13 | 0.17 |
- *Uncorrected p-value < 0.05.
- **Uncorrected p-value < 0.01.
- ***Uncorrected p-value < 0.001.
Since DGF was primarily observed after deceased donation, we repeated the analysis only including 6-week protocol biopsies from grafts procured from DD (n = 93). This revealed essentially identical correlations between PBTs and variables before, at, and after the 6-week protocol biopsy (Tables S1–S3).
We compared the mean PBT scores between the two donor types without DGF and those with DGF. We observed no significant differences between LD and DD without DGF, while those 6-week protocol biopsies from grafts with DGF showed significant greater expression of all PBTs (Figure 2).
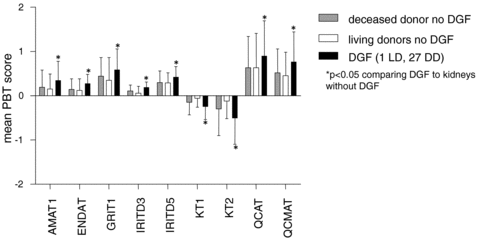
Pathogenesis based transcript sets (PBTs) scores and delayed graft function (DGF). Mean PBT scores are compared for 6-week protocol biopsies from kidneys from deceased donors or live donors that never manifested DGF; and those that had manifested DGF but were now functioning (27 from deceased donors, 1 from a live donor). No significant differences between deceased and living donors without DGF were observed.
These findings were confirmed by PCA of the PBT scores (Figure 3). There is no separation between LD and DD, while those allografts with DGF separated with higher scores in principal component 1 (x-axis) from those without DGF. This finding was further confirmed by a significant correlation between the principal component 1 score and the number of dialysis posttransplantation (r = 0.35, p < 0.001). In addition principal component 1 score were not different between DD and LD who did not develop DGF, while they were significantly higher for those 6-week protocol biopsies which previously experienced DGF compared to those without (p = 0.005).
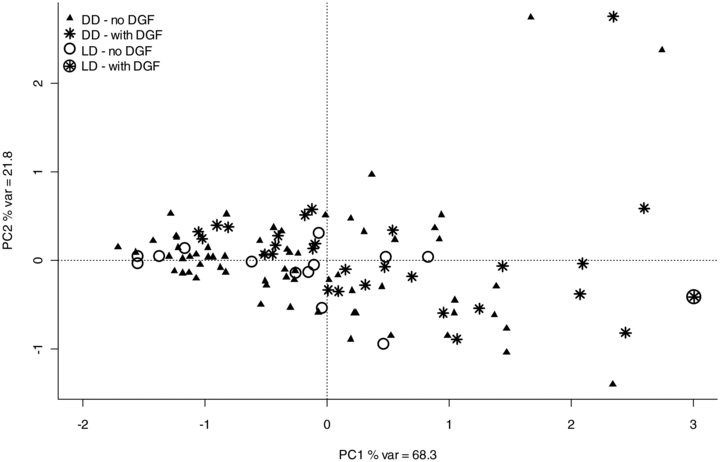
Principal component analysis using the nine PBTs included in this study for exploring the relationship between gene expression and prior DGF. Cases with a star displayed required dialysis posttransplantation. PCA reduces the dimensionality of multivariate data in order to map the variation. Principal component 1 (PC1) shown on the x-axis summarises 68% of the variance from all data. There is no difference in principal component 1 scores between deceased donors (DD) and live donors (LD), while those 6-week protocol biopsies from allografts with DGF show higher principal component 1 scores, i.e. they trend to separate towards the right of the diagram (see also results section for statistical comparison of PC1 scores).
Poor correlation of the molecular phenotype of PBs with future rejection and function
The disturbances in PBTs in 6-week protocol biopsies showed no strong correlations with the future findings in the 3- and 6-month protocol biopsies (Table 5). Because histologic inflammation at 6 weeks predicts inflammation in subsequent biopsies, we anticipated some correlation between the PBT scores at 6 weeks and the subsequent findings in those biopsies. Inflammation associated transcripts, in particular the interferon-γ–inducible (GRIT1), macrophage associated (QCMAT, AMAT1) and injury-up PBTs (IRITD5) correlated weakly with interstitial inflammation in sequential protocol biopsies at 3 and 6 months (Table 5). No correlations were found between the 6-week PBT scores and the future Banff diagnosis of borderline and subclinical TCMR. The PBTs in 6 week PBs did not correlate with the number of future BFCs, a surrogate for overt rejection episodes. Increased macrophage burden (QCMAT) and injury-up PBTs (IRITD3, IRITD5, AMAT1) and one kidney parenchymal PBT (KT1) correlated weakly with increased IFTA from the 6-week to the 6-month protocol biopsy.
| Inflammation associated PBTs | Injury–repair PBTs with increased expression | Kidney parenchymal PBTs with decreased expression | |||||||
|---|---|---|---|---|---|---|---|---|---|
| QCMAT | GRIT1 | QCAT | IRITD5 | AMAT1 | IRITD3 | ENDAT | KT2 | KT1 | |
| Banff total i-score in 12-week sequential protocol biopsy | 0.27** | 0.35*** | 0.39*** | 0.20* | 0.28** | 0.15 | 0.19 | −0.23* | −0.26** |
| Banff total i-score in 6-month sequential protocol biopsy | 0.22* | 0.27** | 0.25* | 0.26** | 0.25* | 0.25* | 0.28** | −0.34*** | −0.30*** |
| Banff diagnosis at 12-week sequential protocol biopsy (0 = no rejection, 1 = borderline, 2 = TCMR) | 0.19* | 0.17 | 0.19 | 0.07 | 0.17 | −0.03 | 0.04 | −0.05 | −0.08 |
| Banff diagnosis at 6-month sequential protocol biopsy (0 = no rejection, 1 = borderline, 2 = TCMR) | 0.14 | 0.11 | 0.10 | 0.16 | 0.16 | 0.08 | 0.20* | −0.16 | −0.16 |
| ΔeGFR 6-weeks to 2-years | −0.02 | −0.04 | −0.08 | −0.13 | −0.09 | −0.06 | −0.06 | −0.00 | 0.04 |
| ΔIFTA 6-weeks to 6-months | 0.27** | 0.19 | 0.14 | 0.23* | 0.30** | 0.24* | 0.17 | −0.14 | −0.20* |
| Number of biopsies for cause after 6-week protocol biopsy | 0.12 | 0.05 | 0.07 | 0.13 | 0.14 | 0.14 | 0.15 | −0.06 | −0.13 |
- *Uncorrected p-value < 0.05.
- **Uncorrected p-value < 0.01.
- ***Uncorrected p-value < 0.001.
The PBTs failed to correlate (>0.2) with a change in allograft function within the first 2 years posttransplantation. The PBTs also failed to correlate with outcomes, although there were too few events to support robust conclusions. In 55.3 ± 22.2 months follow-up (15–100.7, median 54) for 107 transplants, we observed four deaths with a functioning graft and four death-censored graft failures. Three of the four graft failures were due to nonadherence at 17, 28 and 47 months posttransplant, presenting with multiple rejection episodes, both T cell and finally mixed with signs of antibody-mediated injury. The fourth graft failed at 56.4 months and the loss remains unexplained.
No evidence for benefit from treating subclinical TCMR
As standard of care cases fulfilling current Banff criteria of TCMR (i ≥ 2 + t ≥ 2 and/or v > 0) were treated with steroid boli. To assess whether this practice affected the future course of the allografts we compared the nine TCMR cases to the 20 borderline cases, which were not treated. Both TCMR and borderline cases showed significantly higher inflammation PBT scores (QCAT, QCMAT and GRIT1) compared to nonrejecting cases (Figure 4A). AMAT1 and ENDAT were increased and kidney parenchyma PBTs (KT1, KT2) were decreased in borderline cases compared to nonrejecting cases. Thus, in this relative small series no difference between subclinical borderline and TCMR in terms of their molecular phenotype could be observed in 6-week protocol biopsies. Despite treatment of TCMR cases no difference in change in eGFR from 6-weeks to 2-years posttransplantation, progression of IFTA from 6 weeks to 6 months, or total i-score in the sequential 6-month protocol biopsies could be observed (Figure 4B).
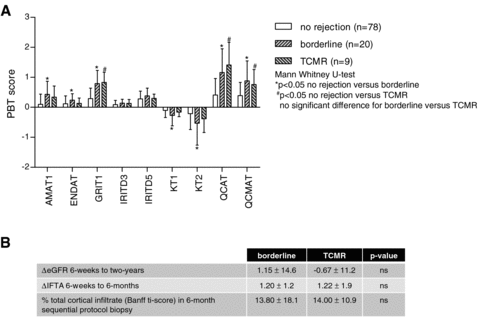
Potential influence of treatment: No differences in terms of the molecular phenotype of 6-week protocol biopsies with TCMR (n = 9) or borderline (n = 20) findings was observed (A). Despite the fact that TCMR cases were treated and borderline cases not, no difference in terms of future allograft function or biopsy findings could be found (B).
Discussion
This study used Affymetrix microarrays to assess the molecular phenotype of 6-week protocol biopsies from kidney transplants and the relationship of the molecular changes to other variables at, prior to, and after the protocol biopsy. The transcript changes expressed as PBTs presented in three groups: inflammation PBTs; injury-up PBTs; and injury-down PBTs, e.g. solute carriers highly expressed in normal nephrons. The close correlations among the PBTs were not driven primarily by rejection: the correlation coefficients were similar when all TCMR and borderline cases were excluded. The inflammatory and injury-down PBTs correlated with histologic inflammation and tubulitis in the biopsy, and the disturbances were greater in kidneys with a history of DGF. But these subclinical molecular and pathological findings did not translate into future TCMR episodes, loss of allograft function, or graft loss, despite treatment of subclinical TCMR and some persisting inflammation and progression of IFTA in sequential protocol biopsies at 3 and 6 months posttransplant.
The iconic relationship among inflammation, injury up and injury-down transcripts is a robust feature of transplant biopsies that is primarily a reflection of the stereotyped injury–repair response, a preprogrammed response of the kidney to injury and to diseases such as TCMR. Organs such as kidney and heart respond to injury in stereotyped ways, with disease-specific features superimposed on the basic pattern of the injury–repair response. The inverse correlation of injury-up and injury-down changes were first described in mouse kidney isografts and shown to be augmented in allografts, and later in human kidney allograft biopsies for cause and heart transplant biopsies (23,33). Other groups have confirmed this pattern in protocol biopsies (38–42). This disturbance correlates with the history of DGF and with the extent of inflammation by histopathology and thus with the lesions of TCMR. The fact that molecular changes at 6 weeks correlate with prior delayed graft function indicates that the driver of PBT changes is primarily the injury of transplantation, with a silent T cell-mediated inflammation—either TCMR or borderline—superimposed to a variable degree.
On balance this study and recent trials suggest that silent/subclinical mononuclear cell infiltration and its typical molecular changes—T cell and macrophage transcripts and IFNG effects—usually represent a self-limited forme fruste TCMR or the injury–repair response to implantation injury rather than true TCMR that will later evolve to functional damage. As recently reviewed (43), clinical TCMR is a local interstitial inflammatory process in which cognate effector T cells organize an inflammatory compartment with de-differentiation of the local epithelium and loss of function. But it is entirely possible that this occurs in a forme fruste variant in some immunosuppressed patients that does not cause enough damage to require treatment. Effector T cells entering kidney interstitium in small numbers may produce silent inflammation then undergo antigen-induced programmed cell death, as part of T cell adaptation to persistent antigen. However, some inflammation may be a manifestation of the injury–repair responses. In this limited series, subclinical TCMR and borderline showed an identical molecular and nearly identical histological phenotype. And despite treatment of subclinical TCMR no change in outcomes could be observed. This argues that the TCMR and borderline changes are more likely to represent a continuous spectrum of injury–repair inflammation or forme fruste TCMR than full-blown TCMR requiring treatment. However, this needs further confirmation in larger retrospective series or more preferably in well designed randomized prospective studies. Nevertheless, our findings reminds us that our current criteria for diagnosing TCMR merely represent arbitrary consensus might have to be adjusted for different clinical variables, e.g. time posttransplantation, allograft function, presence of HLA-antibodies, etc.
The observation that at 6 weeks deceased (DD) and live donor (LD) kidneys that never manifested DGF presented with a similar molecular phenotype indicates that brain death injury has become background activity by 6 weeks posttransplantation in DD kidneys without DGF, and that no qualitative signature for brain death survives beyond the early transplant period. As previously described 1 h biopsies at the time of transplant show three categories of kidneys: LD, good DD and bad DD, which often manifest DGF (44). At 6 weeks, kidneys that had manifested DGF (almost all DD kidneys) showed greater disturbances in PBT scores, but DD kidneys that never manifested DGF were not distinguishable from LD kidneys. However, both DD and LD kidneys displayed a spectrum of inflammation and injury at the molecular level, reflecting the universal stresses of implantation that all transplants endure.
The correlation of the molecular and histologic phenotype of inflammation at 6 weeks with both, prior injury (i.e. DGF) and future atrophy and scarring by 6 months suggests that this is the natural history of the injury–repair response, as seen in wound healing. The association between early inflammation with later scarring (40–42) did not impact long-term outcome and thus does not mean that all inflammation is maladaptive and causes relentless scarring.
The molecular phenotype of these protocol biopsies had little predictive value for future function and outcomes. Because of the excellent outcomes now obtained in kidney transplantation, this series and similar studies find little relationship of the molecular phenotype to future events. But interpretation of such studies is limited by the paucity of late events in kidney transplants that are functioning at 6 weeks with no indication for biopsy. (This may be different in a high-risk population, e.g. cross-match positive patients.) Nevertheless, even allowing for underpowering, this study shows that the subclinical inflammation and injury changes do not trigger relentless progression.
The molecular phenotype of protocol biopsies does not provide a rationale for routine protocol biopsies, either for detecting silent rejection or for predicting the future course. This adds to other recent challenges to the rationale for protocol biopsies, including the declining incidence of silent inflammation, the lack of improvement after protocol biopsies (9), and the emergence of a new understanding of the role of specific diseases and late nonadherence in late allograft failures. However, protocol biopsies may be justified in allografts at high risk for early onset of specific disease processes like ABMR, i.e. in cross-match positive patients (45).
Acknowledgments
This work was presented in part at the 2009 American Transplant Congress (Concurrent Session 55), the 2009 annual meeting of the American Society of Nephrology (TH-PO961) and at the 2009 European Society of Transplantation Congress (O-339).
Funding sources: This research has been supported by Alberta Health Services, Genome Canada, Genome Alberta, the University of Alberta Hospital Foundation, Roche Molecular Systems, and Alberta Advanced Education and Technology. Michael Mengel and Banu Sis are holding research grants from the Roche Organ Transplantation Research Foundation.
Disclosures
P. Halloran has a conflict of interest to declare as described by the American Journal of Transplantation. P. Halloran has Shares in TSI, a company with an interest in molecular diagnostics. None of the other authors have conflicts of interest to declare as described by the American Journal of Transplantation.




