Activation of A3 Adenosine Receptor Provides Lung Protection Against Ischemia-Reperfusion Injury Associated with Reduction in Apoptosis
Abstract
Apoptosis has been described in various models of ischemia-reperfusion (IR) injury, including lung transplantation. A3 adenosine receptor (AR) has been linked to a variety of apoptotic processes. The effect of A3AR activation on lung injury and apoptosis, following IR, has not been reported to date. In a spontaneously breathing cat model, in which the left lower lobe of the lung was isolated and subjected to 2 h of ischemia and 3 h of reperfusion, we tested the effect of IB-MECA, a selective A3AR agonist, on lung apoptosis and injury. Significant increase in the extent of apoptosis was observed following lung reperfusion. IB-MECA, administered before IR, and before or with reperfusion, markedly (p < 0.01) attenuated indices of injury and apoptosis including the percentage of injured alveoli, wet/dry weight ratio, myeloperoxidase activity, in situ terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate nick end-labeling (TUNEL) positive cells, and caspase 3 activity and expression. The protective effects of IB-MECA were completely blocked by pretreatment with the selective A3AR antagonist MRS-1191. In summary, even when given after the onset of ischemia, the A3AR agonist IB-MECA conferred a powerful protection against reperfusion lung injury, which was associated with decreased apoptosis. This suggests a potentially important role for A3AR in lung IR injury.
Introduction
Ischemia-reperfusion (IR) lung injury occurs in the context of pulmonary thromboembolectomy, thrombolysis, cardiopulmonary bypass or following lung transplantation when blood supply is reintroduced to the ischemic graft at the completion of the implantation procedure. In the lung, kidney and heart, activation of A1 or A2a adenosine receptors (AR) modulates IR injury (1–6). Also, in the kidney and heart, A3AR activation worsens or protects against IR injury, respectively (7–11). Although A3AR subtype is expressed in the lung (12,13), its role in IR-induced lung injury has not yet been reported.
Apoptosis is important in the cellular response to IR injury. In the heart, liver, kidney and intestine, IR initiates apoptosis (14–18). Similarly in the transplanted lung, recent studies reported significant increases in the numbers of cells undergoing apoptosis after graft reperfusion (19,20). The authors suggested that this may contribute to the severe organ dysfunction reported in 20% of patients after lung transplantation. Previous studies in mouse and rat heart documented significantly improved contractile recovery after attenuation of IR-induced apoptosis (17) or following administration of caspase 3 inhibitor during reperfusion (21,22), suggesting that inhibition of the apoptotic response to reperfusion may be critical. The effect of the A3 receptor on apoptosis appears to be dual and opposite. Both agonists and antagonists have been shown to induce apoptosis when administered in high concentration, whereas nanomolar concentrations of selective agonists tend to inhibit apoptosis (23–25).
Based on these data, we wished to study the effects of a selective A3AR agonist in an intact chest, spontaneously breathing animal model of IR lung injury (6,26). If the proposed studies show evidence of benefit from the administration of A3 agonists before or during ischemia, it may suggest that targeting the A3AR could be a novel and useful approach to protecting the lung during ischemia and reperfusion.
Materials and Methods
Adult cats, weighing 2.5–3.5 kg, were used in this investigation. All experiments were performed in accordance with the guidelines of the Animal Care and Use Committee of the Hebrew University, Hadassah School of Medicine and with the approval of the Institutional Animal Care and Use Committee.
In vivo animal model
A standard reperfusion lung model of injury in intact chest, spontaneously breathing cat was employed, as described previously in detail (6,26). Briefly, in barbital-anesthetized cats, with the aid of fluoroscopy, a specially designed 6F triple-lumen catheter was advanced from the left external jugular vein into the lobar artery of the left lower lobe (LLL). Also, with the use of fluoroscopy, a 4F bronchial blocker was inserted into the LLL bronchus. After heparinization, the LLL was perfused at 35 mL/min with blood withdrawn from the aorta through a catheter in the femoral artery, using a Harvard peristaltic pump. The LLL was isolated by distending a balloon with contrast dye on the LLL arterial catheter. After 1-h period of stabilization, ischemia of the LLL was induced by discontinuing the Harvard peristaltic pump for 2 h (ischemia period), and the perfusion circuit was then attached to a femoral vein catheter. The balloon on the tip of the bronchial blocker was distended with contrast dye to block ventilation to the lobe. After 2 h of ischemia, the balloon on the bronchial blocker was deflated, the perfusion circuit was reattached to the arterial catheter in the LLL, and the LLL was reperfused (reperfusion period) for 3 h at a rate of 35 mL/min, using a Harvard peristaltic pump, as described above. In all groups, hemodynamic measurements and arterial blood gases were obtained before ischemia, after 1 h and 2 h of ischemia and after 1 h and 3 h of reperfusion.
Experimental protocol
Cats were randomly assigned to six treatment groups (n = 6). (I) Nonischemic group: The LLL was perfused for 4 h (no ischemia). (II) IR group: Animals were subjected to ischemia and reperfusion of the LLL. (III) A3AR agonist group: To test the hypothesis that A3AR activation affects apoptosis and injury following IR, 0.3 mg/kg of the highly selective A3AR agonist, N6-(3-iodobenzyl)-N-methyl-5′-carbamoyladenosine, (IB-MECA, Research Biochemical International, Natick, MA, USA) was administered as an i.v. bolus 15 min before ischemia. (IV) To test the hypothesis that A3AR activation affects apoptosis and injury when administered after induction of ischemia, animals received the highly selective A3AR agonist IB-MECA, as in group III, however, 1 h after beginning of the ischemic period. (V) To test the hypothesis that A3AR activation affects apoptosis and injury when administered at the beginning of reperfusion, animals received the highly selective A3AR agonist IB-MECA, as in group III, however, 5 min after reperfusion. (VI) A3AR antagonist + agonist group: To ascertain that IB-MECA-induced modulation of lung apoptosis is mediated by A3AR. In further studies, the ability of an A3AR antagonist to block the effect of IB-MECA was evaluated. IB-MECA (0.3 mg/kg, i.v.) was given 15 min before ischemia (as in group III), with pretreatment 15 min earlier with the highly selective A3AR antagonist 3-ethyl-5-benzyl-2-methyl-4-phenylethynykyk-6-phenyl-1,4-(±)-dihydropyridine-3,5 dicraboxylate (MRS-1191, Research Biochemical International, Natick, MA, USA) (1mg/kg, i.v.).
The doses of the A3AR agonist (27,28) and antagonist (1,7) and their pretreatment times (1,7,9) were selected based on previous in-vivo studies in mice, rats and rabbits.
Injury and apoptosis assessment
After 3 h of reperfusion, animals received an overdose of pentobarbital sodium (30 mg/kg). For light microscopy, samples of lung tissue were embedded in paraffin, cut into 4 μm slices, and stained with hematoxylin and eosin. The slides were coded and examined in a blinded manner by a single examiner. Fifty microscopic fields at ×40 magnification were examined in each section and the total number of alveoli in the 50 microscopic fields was scored. The severity of alveolar injury was assessed according to the percentage of injured alveoli as defined before (6,26,29). Briefly, an alveolus was defined as injured if it contained exudate, more than two leukocytes (macrophages or neutrophils) or more than two erythrocytes. The severity of alveolar injury was assessed according to the percentage of injured alveoli (number of injured alveoli divided by the total number of alveoli in the 50 microscopic fields). Excised samples of lung tissue were also snap frozen in liquid nitrogen and stored at −70°C for determination of lung myeloperoxidase (MPO) (30–32). The remainder of the left and right lower lobe was utilized for determination of lung wet: dry weights ratio, after sequential weighs demonstrated maximal dehydration at 80°C.
Apoptosis was assessed through TdT-mediated TUNEL assay. This was performed using the Deadend Fluorometric TUNEL System (Promega, Madison, WI) according to the manufacturer's instructions on formaldehyde-fixed lung sections. TUNEL-stained tissue sections were examined with a fluorescent confocal microscope. First, the PI staining (red) was examined through a 520 nm filter at a magnification of ×100. PI stains all nucleated cells in the same manner. The magnification is then increased to ×400 and a color photomicrograph was taken. The same area was then similarly examined for apoptotic staining (bright green), using 590 nm filter at a magnification ×400. Six randomly chosen fields were used for cell counts. PI-stained cells (representing the total number of cells: alive + necrotic + apoptotic cells) were counted first, followed by TUNEL-positive cells (represent the number of apoptotic cells). Only apoptotic cells that could clearly be identified as individual cells in the pulmonary parenchyma were counted. Alveolar macrophages, phagotized cells or cells floating in the alveolar space, were not included in the count.
Lungs were also tested after the reperfusion period for caspase 3 activity and expression, as outlined previously in detail (17,33). Caspase is derived from a proenzyme at the onset of apoptosis and plays an important role in the final common pathway of programmed cell death. During IR injury, increased caspase 3 activity is indicative of increased programmed cell death signal. To detect caspase 3 activity, 150 μg of lysate from each lung was combined with fluorogenic caspase 3 substrate, diluted to 300 mg/L in caspase assay buffer (250 mmol/L PIPES, 50 mmol/L EDTA, 2.5% CHAPS, and 125 mmol/L DTT), and measured immediately on a fluorometer at an excitation wavelength of 400 nm and an emission wavelength of 505 nm. Measurements were repeated every 10 min for 1 h, and the slope of fluorescent units per hour was calculated. Values were compared with known standards to determine enzymatic activity. To study apoptosis-related caspase expression levels by Western blotting, the homogenate was sonicated and centrifuged at 35 000 g for 15 min. A total of 100 μg of protein was loaded onto 10% sodium dodecyl sulfate polyacrylamide gel for electrophoresis and then transferred onto a nitrocellulose membrane. The membrane was blocked with non-fat dry milk, and probed with the polyclonal antibody to active caspase-3 (1/1000, Santa Cruz Biochemical, Santa Cruz, CA) for 8 h at 4°C, and then incubated with a 1/1000 dilution of horseradish peroxidae-conjugated secondary antibody (Sigma). Hyperfilm ECL was exposed to blots treated with ECL solution, developed in a film processor and scanned using a Molecular Dynamics 300A laser densitometer (Sunnyvale, CA). Membrane were subsequently stripped (62.5 mM Tris-HCl pH 6.8, 20% SDS, 100 mM β-mercaptoethanol) and n-probed for actin. To allow comparison between groups, data were shown as percent density of bands versus nonischemic (group I) lungs.
Statistical analysis
Data were analyzed with Student's t-test when comparing means of two groups or with one way analysis of variance (ANOVA) with Bonferroni correction for multiple comparisons between groups. Differences were considered significant at p < 0.05. Results are presented as means ± SEM. Data were analyzed using SigmaStat (Jandel, San Rafael, CA).
Results
The surgical interventions (insertion of the catheter into the lobar artery and inflation of the balloon), and perfusion of the LLL with a peristaltic pump, had no effect on lung-injury indices. Both microscopic findings (% injured alveoli 2.7 ± 1.2%) in the LLL of group I (nonischemic control group) as well as wet/dry weight ratio (4.9 ± 0.5), MPO activity (1.3 ± 0.2 U/g lung tissue), and percentage of TUNEL positive cells (1 ± 0.8%) were not significantly different from those observed in the corresponding right lower lobe (3 ± 1%, 5.2 ± 0.5, 1.5 ± 0.3 U/g lung tissue, 1 ± 0.5%, respectively), in which no manipulations were performed. Also, caspase 3 activity and expression in the LLL were not increased when compared to the right lower lobe.
Effect of IR on lung injury and apoptosis
The inflammatory response caused by IR injury was evaluated by measuring tissue MPO activity and histological study (Table 1). LLL-MPO activity in the IR group was 3.6-fold higher (p < 0.01) than in the nonischemic group which exhibited minimal activity. In addition, the percentage of injured alveoli was significantly higher compared to the nonischemic group (p < 0.001). IR also caused marked lung edema as assessed by wet to dry weight ratios of the LLL.
| Group | Injured alveoli (%) | MPO activitya | Wet/dry weight ratio |
|---|---|---|---|
| I | 2.7 ± 1.2b | 1.3 ± 0.2b | 4.9 ± 0.5b |
| II | 49.2 ± 3.1 | 4.7 ± 0.5 | 8.1 ± 0.6 |
| III | 17.1 ± 2.5b | 2.1 ± 0.3b | 4.4 ± 0.4b |
| IV | 18.7 ± 3.0b | 1.9 ± 0.2b | 4.6 ± 0.4b |
| V | 23.1 ± 3.3b | 2.3 ± 0.3b | 4.6 ± 0.3b |
| VI | 44.8 ± 3.3 | 4.9 ± 0.6 | 8.0 ± 0.5 |
- Values shown are means ± SEM; n = 6 cats/group. The groups were as follows: I, nonischemic group; II, ischemia-reperfusion; III, IB-MECA was administered before ischemia; IV, IB-MECA was administered 1 h after beginning of ischemia; V, IB-MECA was administered 5 min after beginning of reperfusion; VI, MRS-1191 pretreatment before IB-MECA, as in group III, and beginning of ischemia.
- aTissue myeloperoxidase (MPO) activity is expressed in units of myeloperoxidase/g of lung weight, each of which was defined as the activity degrading 1 μmol of peroxide per min at 25°C.
- bp < 0.05 compared with group II and VI.
To clarify the occurrence of apoptosis caused by IR injury, TUNEL and caspase 3 activity and expression studies were performed, the results of which are summarized in Figures 1 and 2. As shown in Figure 2, we detected TUNEL-positive cells in the nonischemic lung (1 ± 0.8%), as well as in lungs subjected to ischemia and reperfusion (35 ± 6%, p < 0.001) . Quantitative analysis revealed a significant increase in the number of TUNEL-positive cells in the ischemic-reperfused lungs compared with nonischemic lungs (Figures 1A and 2). A change in expression of apoptosis-related caspase 3 protein during the course of IR was analyzed by Western blotting. Activated caspase 3 protein significantly increased after IR injury (361 ± 37% increase over group I). Caspase 3 activity was also significantly increased in samples from the IR group (267 ± 23% increase over group I), compared with the nonischemic group (Figure 1B, C).

Terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL)-positive cells (A), changes in caspase 3 activity (B) and in activated caspase 3 expression as analyzed by Western blotting (C), in the LLL of the different groups after ischemia and reperfusion and with IB-MECA and MRS-1191 pretreatment. Values shown are means ± SEM. (I), nonischemic group; (II), ischemia-reperfusion (IR); (III), IB-MECA was administered before ischemia; (IV), IB-MECA was administered 1 h after beginning of ischemia; (V), IB-MECA was administered 5 min after beginning of reperfusion and (VI), MRS-1191 pretreatment before IB-MECA, as in group (III) and beginning of ischemia. *p < 0.05 compared to groups (II) and (V).
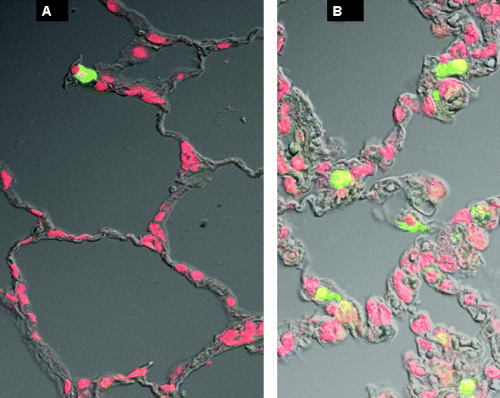
Representative results of TUNEL staining. Alveolar parenchyma from the left lower lobe of a cat from the nonischemic group (group I) (A), compared with a cat with ischemia-reperfusion injury (group III) (B). Note more bright green cells in B versus A, indicating more apoptosis.
Protective effects of A3AR agonist
The effects of the A3AR agonist and antagonist on indicators of lung injury and apoptosis are summarized in Table 1 and Figure 1. When administered before ischemia, activation of A3AR with IB-MECA (group III) caused significant attenuation of IR-induced lung injury and apoptosis; the average percentage of injured alveoli was 65% lower and MPO activity and wet to dry weight ratio were nearly halved compared to group II (Table 1). Markers of apoptosis decreased approximately to one fifth (expression levels of activated caspase 3 protein: 65 ± 19% increase over group I) or one third (% of TUNEL-positive cells 12 ± 2% and caspase 3 activity 84 ± 10% increase over group I) of the corresponding IR values (361 ± 37% increase over group I, 35 ± 6%, 267 ± 23% increase over group I, respectively, p < 0.01), however, they did not reach nonischemic values (group I). Likewise, administration of IB-MECA during the ischemic period (group IV) or during reperfusion (group V) showed significant attenuation of lung injury (Table 1) and apoptosis (% of TUNEL-positive cells and caspase 3 activity and expression in group IV were 11 ± 3%, 75 ± 8 % increase over group I and 59 ± 16% increase over group I; group V, 15 ± 3%, 93 ± 10 % increase over group I and 73 ± 19% increase over group I, respectively).
The highly selective A3AR antagonist MRS-1191 given before IB-MECA (group VI), completely abolished the protection provided by pretreatment with IB-MECA; in this group, lung injury and apoptosis parameters were indistinguishable from those measured in the IR group. The lung-protective effects of IB-MECA cannot be ascribed to the vehicle DMSO because the same dose of DMSO had no effect on IR lung damage (data not shown).
Effects on hemodynamics and arterial blood gases
At baseline, heart rate and mean arterial blood pressure were similar in all groups (Table 2). The administration of IB-MECA (group III) or MRS-1191 (group VI) produced no systemic hemodynamic effects: mean heart rate before administration of the drugs: 118 ± 6 and 127 ± 9 bpm, respectively, and 15 min later: 122 ± 8 and 132 ± 7 bpm, respectively; mean blood pressure before administration of the drugs: 127 ± 9 and 123 ± 7 mmHg, respectively, and 15 min later: 121 ± 7 and 124 ± 11 mmHg, respectively.
| Groups | Mean arterial blood pressure (mmHg) | Heart rate (beats/min) | Mean lobar arterial pressure (mmHg) |
|---|---|---|---|
| I | 125 ± 7 | 124 ± 8 | 3.9 ± 0.3 |
| II | 121 ± 6 | 132 ± 9 | 4.7 ± 0.6 |
| III | 125 ± 5 | 127 ± 8 | 4.1 ± 0.4 |
| IV | 132 ± 7 | 131 ± 7 | 4.7 ± 0.6 |
| V | 125 ± 5 | 126 ± 8 | 4.0 ± 0.5 |
| VI | 129 ± 8 | 121 ± 9 | 4.5 ± 0.7 |
- Values shown are means ± SEM; n = 6 cats/group. The groups were as follows: I, nonischemic group; II, ischemia-reperfusion; III, IB-MECA was administered before ischemia; IV, IB-MECA was administered 1 h after beginning of the ischemia; V, IB-MECA was administered 5 min after beginning of reperfusion and VI, MRS-1191 pretreatment before IB-MECA, as in group III, and beginning of ischemia.
In all the groups, no acidosis, no significant increase in partial pressure of carbon dioxide or decrease in partial pressure of oxygen was observed during ischemia or reperfusion (Table 3).
| Group | I | II | III | IV | V | VI |
|---|---|---|---|---|---|---|
| Po2, mmHg | ||||||
| Baseline | 93 ± 5 | 103 ± 5 | 104 ± 5 | 101 ± 6 | 96 ± 5 | 101 ± 4 |
| Ischemia (2 h) | 94 ± 4 | 95 ± 3 | 102 ± 4 | 100 ± 6 | 97 ± 4 | |
| Reperfusion (3 h) | 95 ± 4a | 104 ± 6 | 102 ± 4 | 101 ± 3 | 98 ± 4 | 97 ± 5 |
| Pco2, mmHg | ||||||
| Baseline | 42 ± 2 | 38 ± 1 | 35 ± 1 | 36 ± 3 | 38 ± 3 | 35 ± 3 |
| Ischemia (2 h) | 35 ± 2 | 39 ± 2 | 40 ± 2 | 39 ± 2 | 37 ± 2 | |
| Reperfusion (3 h) | 37 ± 3a | 34 ± 2 | 39 ± 1 | 36 ± 1 | 35 ± 2 | 34 ± 2 |
| pH | ||||||
| Baseline | 7.37 ± 0.01 | 7.35 ± 0.01 | 7.35 ± 0.02 | 7.40 ± 0.02 | 7.38 ± 0.02 | 7.39 ± 0.01 |
| Ischemia (2 h) | 7.36 ± 0.02 | 7.36 ± 0.01 | 7.38 ± 0.03 | 7.40 ± 0.02 | 7.37 ± 0.01 | 7.40 ± 0.02 |
| Reperfusion (3 h) | 7.36 ± 0.02a | 7.39 ± 0.02 | 7.40 ± 0.01 | 7.37 ± 0.01 | 7.40 ± 0.02 | 7.39 ± 0.02 |
- Values shown are means ± SEM; n = 5–6 cats/group. The groups were as follows: I, nonischemic group; II, ischemia-reperfusion; III, IB-MECA was administered before ischemia; IV, IB-MECA was administered 1 h after beginning of the ischemia; V, IB-MECA was administered 5 min after beginning of the reperfusion and VI, MRS-1191 pretreatment before IB-MECA, as in group III, and beginning of ischemia.
- aThe value obtained in this group after 4 h of perfusion in nonischemic control.
Discussion
This is the first report that evaluates the role of adenosine A3 receptors in IR-induced apoptosis and lung injury. Using an in-vivo IR injury model we demonstrated that: (i) pharmacological activation of A3 receptors before ischemia confers significant lung protection; (ii) A3AR activation before or with reperfusion also attenuates IR-induced lung injury; (iii) there was no evidence of drug-induced changes in vital signs and (iv) although the precise mechanism for the inhibition of IR injury remains unclear, it appears from the present study that the anti-apoptotic action of the A3AR agonist plays a role in ameliorating the damage.
Apoptosis is a fundamental process in cell survival and cell death that occurs via activation of distinct signaling pathways involving mitochondria, regulatory proteins and caspase activation. Ultimately, cells undergo nuclear chromatin fragmentation, and formation of apoptotic bodies (34). Apoptosis associated with IR injury has been reported in several organs including the heart, kidney, liver and intestine (14–18). In the lung, apoptotic cell death appears to play an important role in IR injury (19,20). Fischer et al. (19) evaluated lung tissue from 20 patients undergoing lung transplantation and found that after graft reperfusion, but not with ischemia, there were significant increases in the number of cells undergoing apoptosis and that the increase in apoptosis was time dependent. Using a rat single lung transplant model, this group of investigators (20) showed that the predominant mode of cell death after 6 h and 12 h of preservation and reperfusion is apoptotic, compared with necrotic mode, which was observed after longer periods of preservation and reperfusion. In the current study, ischemia and reperfusion resulted in 34 ± 6% apoptotic nuclei. The TUNEL technique has been proven to be the most sensitive technique now available for detecting apoptosis in tissue samples (35,36). Yet, it has been suggested that the TUNEL method is overly sensitive (33). The percentage of apoptotic cells measured in our cat study is comparable with previous studies in both rat (19) and human (20) lungs showing that after 2 h of reperfusion 34% and 30% of cells, respectively, were apoptotic. Because caspase 3 activation is pivotal for executing the later stages of apoptosis, we also evaluated IR-induced changes in caspase 3 activity and expression and found significant increases after ischemia and reperfusion.
The concept that adenosine may function as a direct regulator of cell viability has recently been explored as the effects of adenosine analogs on cell death, either necrotic or apoptotic, have been noted (37). Of the four G proteins-coupled AR, the A3 receptor has been specifically implicated in modulation of cell survival. Results obtained by exposing both cardiomyocytes and astrocytes (24,37–39) to graded concentrations of selective A3AR agonists suggest induction of both cell protection and cell death, an opposite effect, which likely depends on the degree of receptor activation. In addition, the ability of the A3AR agonist to affect IR injury in various species, models and organs has also been previously demonstrated. In the brain, Von Lubitz et al. (40) reported that post-ischemic stimulation of adenosine A3 receptors with IB-MECA resulted in cerebroprotection. Similarly, in the heart, both in-vivo and ex-vivo studies demonstrated that the A3AR subtype elicited protection against infarction (8–11). In contrast, in the kidney, the highly selective A3AR agonist IB-MECA worsened renal IR injury (7). Previous studies reported that A2AR agonist and A1AR antagonist blocked IR-induced lung injury (5,6,41,42). The present results expand these previous observations and elucidate the role of A3AR. To our knowledge, this is the first study to identify a lung-protective and anti-apoptotic role of A3 receptors during IR in-vivo, using a selective agonist for these receptors. To confirm the specific involvement of the A3 receptor, the selective A3 receptor antagonist MRS-1191 completely counteracted A3 agonist-induced protection; both lung injury and apoptosis were inhibited by treatment with IB-MECA. The molecular mechanisms involved in the modulation of apoptosis by IB-MECA have not been elucidated. Reinforcement of cytoskeleton function leading to a better adherence of cells and hence a reduced susceptibility of cells to apoptosis has been suggested by in vitro studies in astroglial cells and cardiomyocytes (38–39). In addition, modulation of Bcl-2-like protein by the A3 receptor has been noted, raising the possibility that A3 receptor-mediated modulation of cell survival may occur through a specific action on the cytoskeleton, mediated by Bcl-2-like proteins (37).
Inhibition of the apoptotic response to IR may be very important. For example, in the mouse and rat heart, attenuation of IR-induced apoptosis (17) or administration of caspase 3 inhibitor during reperfusion (21, 22) significantly improved contractile recovery. Also, ablation of the proapoptotic Bax gene (43) or over-expression of the antiapoptotic Bcl-2 gene in transgenic or knockout mice (44) resulted in improved myocardial tolerance to IR. These studies suggested that apoptosis is an independent contributor to injury during ischemia and reperfusion. In the lung, however, deterioration in lung function correlated only with the extent of cell necrosis, and not with apoptosis (20). Further studies are therefore required to better define the implications of apoptosis in this setting.
IB-MECA is one of the most potent A3AR analogues identified with nearly 50-fold selectivity over A1 and A2aAR in the rat and dog (45, 46) and 13- to 21-fold in the rabbit (27,47). Nevertheless, it may be argued that IB-MECA may have induced lung protection not just by interacting with A3AR, but rather through nonspecific interactions with other AR. In the present study, the intravenous administration of IB-MECA did not produce any effect on heart rate or blood pressure suggesting that at the dose of 300 μg/kg, this agent does not interact with A1 or A2a receptors in cats. A recent study, however, showed that A3AR agonists may bind to A2aAR (48). This possibility was addressed using an A3AR-selective inhibitor, MRS-1191. MRS-1191, which was reported to be useful as A3 receptor antagonist across species (49), blocked the actions of IB-MECA against lung injury, supporting the hypothesis that this agent attenuated IR injury by interacting with the A3AR.
In summary, acute administration of a selective agonist at the A3 receptor subtype before or during the ischemic period or with reperfusion proved useful in counteracting IR lung injury through a mechanism that is likely mediated, in part, by attenuation of apoptosis. Thus, these agents may provide new fascinating perspectives in the prophylaxis and treatment of lung injury in patients undergoing lung transplantation, pulmonary thromboembolectomy, thrombolysis or cardiopulmonary bypass.
Acknowledgments
This study was supported by the Chief Scientist's, Ministry of Health Grant 4994, the Joint Research Fund of the Hebrew University and Hadassah Hospital and the Batsheva de Rothschild Fund, Israel Academy of Science.




