Kidney Transplantation for Systemic Sclerosis Improves Survival and may Modulate Disease Activity
Parts of this study were presented at the American Transplant Congress, Boston MA, on 18 May 2004.
Abstract
Systemic sclerosis (SS) may lead to sclerodema renal crisis, an unusual cause of end-stage renal disease (ESRD) with historically poor hemodialysis outcomes. Little information is available on outcomes after kidney transplantation. Information from the UNOS registry was obtained on SS patients in the United States, listed for kidney transplants between 1985–2002. We compared survival at 1 and 3 years in patients who received cadaveric transplants with patients who remained on the waiting list. Graft survival, cause of graft loss, frequency of early graft loss and pre- and post-transplant skin scores were analyzed. Two hundred and fifty-eight patients with SS were listed for transplantation. Survival was significantly prolonged in patients receiving transplants (p = 0.005). Graft survival at 1 and 3 years was 68% and 60%. Early graft loss was common. Skin scores improved in all four subjects at our center, with an average decline of 60.7% (p = 0.024). Kidney transplantation confers a survival benefit in ESRD due to SS. Transplantation may be associated with an improvement in systemic manifestations of disease. Despite suboptimal graft survival, kidney transplant should be considered the treatment of choice in ESRD due to SS.
Introduction
Systemic sclerosis (SS) is a rare but debilitating disease of unknown etiology, characterized by disordered collagen production, endothelial dysfunction and vasospasm. SS manifests clinically with disabling skin thickening, digital ischemia, gastrointestinal dysfunction, pulmonary hypertension or fibrosis and other organ involvement, including kidney disease (1). Renal involvement in SS most frequently presents as rapidly progressive renal failure with malignant hypertension and oliguria (scleroderma renal crisis, SRC). Renal involvement may also present as a slowly progressive chronic kidney disease characterized by proteinuria, hypertension and renal dysfunction (2). The introduction of angiotensin converting enzyme (ACE) inhibitors has tempered the poor renal and overall prognosis associated with SRC and often leads to reversal of the syndrome (3–5). However, up to 50% of patients may still require long-term dialysis or transplantation after the onset of SRC.
The care of patients with SS and kidney failure remains a challenging problem. The survival of these patients following the initiation of either hemodialysis or transplantation is worse when compared to the overall dialysis and transplant population, and it remains unclear which modality of renal replacement is superior. While dialysis is life-sustaining, outcomes have historically been poor relative to patients with other primary renal diseases (6,7). Scant evidence is available to support the choice of peritoneal dialysis over hemodialysis (8,9). Little is known regarding outcomes after kidney transplantation in SS. The published data is conflicting, with case reports and case series that describe successful transplants, numerous reports of recurrence and potential problems with cyclosporine in precipitating acute renal failure or recurrent disease in the form of thrombotic microangiopathy (10–16). Both European and US reports have demonstrated poorer graft survival than other primary renal diseases (6,17). Similarly, a descriptive report from United Network of Organ Sharing (UNOS) data found low rates of graft survival but reported survival and graft survival rates without accounting for censored data and did not have a control population of SS patients who were well enough to be listed for transplantation (18). Finally, the effect of kidney transplantation on systemic manifestations of the disease has not been well-quantified. These data are potentially very important, as more toxic therapies incorporating cyclophoshphamide, total body irradiation, and stem-cell rescue are being explored in SS (19).
Given the lack of comparative data examining the benefits of transplantation over dialysis in SS, we sought to (i) determine if a survival benefit existed for kidney transplantation compared to a similar population of SS patients on the waiting list, (ii) examine graft survival, (iii) determine if improvements had occurred since the introduction of newer immunosuppressants and (iv) determine the frequency and causes of early graft loss.
Methods
Information from the UNOS registry was obtained on all SS patients in the United States listed for kidney transplants between 1985–2002. Descriptive statistics were generated for patients placed on the waiting list. The primary outcome of interest was survival at 1 and 3 years in patients who received cadaveric transplants compared with patients who remained on the waiting list. Graft survival, cause of graft failures and early graft failures were analyzed. Survival analysis was terminated at 2500 days due to small numbers of patients remaining beyond that follow-up period. To discern if newer immunosuppressants (mycophenolate mofetil, tacrolimus, sirolimus, induction therapies) had changed outcomes after transplantation, graft and patient survival was compared before and after 1996.
Statistical analysis
Survival data was assessed by Kaplan-Meier analysis, and comparisons of survival were made by the log rank test. All analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC).
This study was approved by the Colorado Multiple Institutional Review Board (COMIRB).
Results
The characteristics, transplant status and overall graft and patient survival status of patients placed on the waiting list are described in Table 1. Of 258 patients, 78% of patients were female. The mean age at listing was 50.8 years, and mean age at transplant was 52.1 years. A total of 142 patients received transplants (55.1%), of which 115 were cadaveric and 27 from live donors. Patient survival, graft survival and causes of graft loss are described in Table 2. For patients receiving cadaveric transplants, survival at 1 and 3 years was 90.1% and 79.5% with graft survival at 1 and 3 years of 68.0% and 60.3%. Patient survival in those not receiving transplants was 81.1% and 54.6% and 1 and 3 years. Common causes of graft failure were death with functioning graft (30 patients), chronic rejection (12), acute rejection (7), recurrence (3) and thrombosis (3). Similarly, causes of early graft loss (<90 days) was due to death with functioning graft (8 patients), acute rejection (5), thrombosis (3), or other (2). Six causes of graft loss and three causes of early graft loss were from unknown causes.
| Gender (N, %) | |
| Female | 201 (78%) |
| Age at listing (mean ± SD) | 51 ± 9 |
| Age at transplant (mean ± SD) | 52 ± 10 |
| Number transplanted (N, %) | 142 (55.1%) |
| Transplant or list status (N, %) | |
| Cadaveric transplant | 115 (44.2%) |
| Living donor transplant | 27 (10.5%) |
| Still waiting | 64 (24.8%) |
| Died | 29 (11.2%) |
| Other | 24 (9.3%) |
| Graft status (cadaveric transplant, N = 115) | |
| Functioning | 52 (45.2%) |
| Patient status (cadaveric transplant) | |
| Alive | 79 (68.7%) |
| 1 year | 3 year | |
|---|---|---|
| Patient survival: transplant | 90.1% | 79.5% |
| Patient survival: waiting list | 81.1% | 54.6% |
| Graft survival | 68.0% | 60.3% |
| Cause of graft loss (N = 63 losses) | ||
| Death with functinong graft | 30 | |
| Chronic rejection | 12 | |
| Acute rejection | 7 | |
| Thrombosis | 3 | |
| Recurrent disease | 3 | |
| Primary non-function (PNF) | 1 | |
| Other (ATN, infection) | 1 | |
| Unknown/not reported | 6 | |
| Causes of early graft loss | ||
| (N = 21, within 90 days of transplantation) | ||
| Death with functioning graft | 8 | |
| Acute rejection | 5 | |
| Other (thrombosis = 3, PNF, hepatitis) | 5 | |
| Unknown/not reported | 3 | |
The Kaplan Meier survival curves for the comparison of transplanted patients and those remaining on the waiting list are displayed in Figure 1. Compared with patients receiving cadaveric transplants, those who remained on the waiting list had a significant decrease in overall survival (p = 0.005 by the log rank test). In the analysis of the effect of newer therapies on outcomes, there were 54 transplants prior to 1996 and 61 transplants occurring after that time. Neither graft survival nor patient survival were statistically or qualitatively different between these eras. Figure 2 represents the distribution of graft loss after kidney transplantation. Thirty-three percent of all graft losses (21/66) occurred within 90 days of transplantation, with 53% occurring within 1 year of transplantation. Median number of days to graft failure was 201 days.
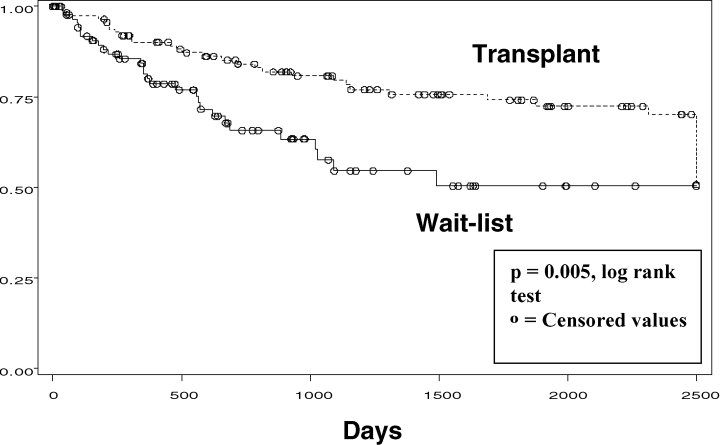
Patient survival in cadaveric transplants versus remaining on wait-list. Kidney transplant confers a survival advantage in systemic sclerosis.
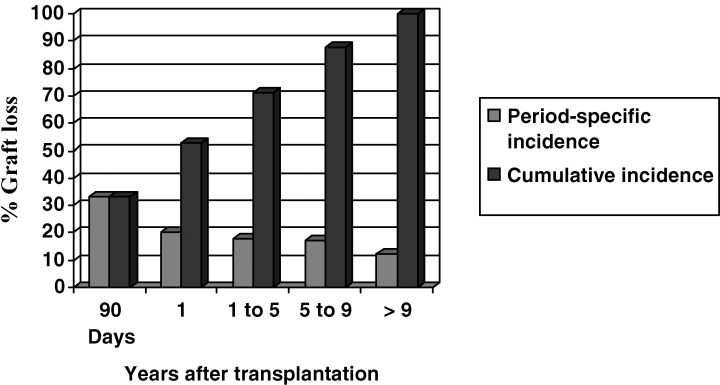
Distribution of graft loss after kidney transplant in systemic sclerosis. A high percentage of graft loss occurred within 90 days (33%) or the first year (53%).
Discussion
In patients with end-stage renal disease (ESRD) from causes other than SS, kidney transplantation provides a survival benefit when compared to patients remaining on hemodialysis (20). Dialysis in SS has historically been associated with poor outcomes, with 5-year survival in Europe of 20% and 3-year survival in the United States of 33% (6,7). However, more recent US Renal Data System reports on survival may indicate an improvement in prognosis with dialysis in the United States, with 3-year survival up to 60% for the period between 1980 and 2000 (21). Reports of poor graft survival and complications after kidney transplantation for SS rendered it impossible to extrapolate the survival benefit of transplantation in other kidney diseases to SS patients. This is the first study to demonstrate a survival benefit of kidney transplantation when compared to dialysis in SS patients.
While our study demonstrated a survival benefit, the results are somewhat provocative, as graft survival at 1 year was relatively poor when compared to the general transplant population. Given the poor 1-year graft survival (68%), it is difficult to discern the mechanism of the survival benefit from transplantation. While improved renal function in the narrow majority of patients may be responsible, other possibilities exist. Clinical trials of agents including antithymocyte globulin, corticosteroids, cyclophosphamide, cyclosporine and mycophenolate mofetil have variably demonstrated efficacy in modulating disease activity in SS (22–25). It is thus possible that combination immunosuppressive therapy used in kidney transplantation, usually consisting of a calcineurin inhibitor, antiproliferative and corticosteroid with or without antibody induction, had an important role in improving the systemic manifestations of disease. The hypothesis that feto-maternal microchimerism is involved in the pathogenesis of SS and other autoimmune diseases may also have relevance (26). Introduction of a donor organ, including competent T cells, may somehow modify the pre-transplant milieu of microchimerism and influence either the alloimmune or autoimmune response (27).
To evaluate the hypothesis that kidney transplantation could be associated with a modification of systemic disease activity, we analyzed modified Rodnan skin scores pre- and post-transplant in SS patients at our center who had received kidney transplants. The modified Rodnan skin score is a validated measure of disease activity in SS and is the most commonly reported measure to assess responses to therapy and predict progression and mortality (28). Skin scores were available before and after transplant in four of the six SS patients at our center and were compared using the paired Student's t-test (Figure 3). Each of the patients had a decline in skin scores, with an average reduction of 61% (p = 0.024). Clinical outcomes were mixed: two patients had early recurrent disease in the form of thrombotic microangiopathy; one of these two patients died and one received a second transplant with a current Cr of 0.9. Another patient developed chronic allograft nephropathy and returned to hemodialysis 7 years after her transplant. The other three have functioning allografts. Our single-center data are obviously limited by the small number of patients but provide some background for further investigations into the effects of transplant on systemic disease.
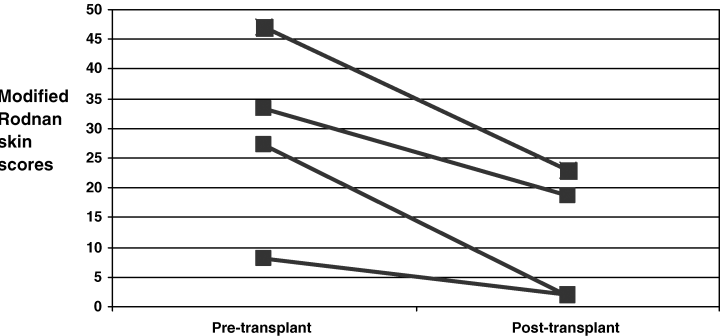
Modified Rodnan skin scores after kidney transplantation. There was a significant improvement (p = 0.024) in mean Rodnan skin score after transplantation (N = 4).
Another intriguing finding in our study is that graft and patient survival have not improved over the last decade, which contrasts with the general kidney transplant population's gains in survival (29). Despite the introduction of mycophenolate mofetil, tacrolimus, sirolimus, induction agents and improved post-transplant care, graft and patient survival have not improved in SS. While patients appear to benefit from kidney transplantation, perhaps the underlying disease continues to dominate the prognosis. Also, our findings on causes and frequency of early graft loss suggest that SS patients are particularly susceptible to early death, acute rejection and thrombosis. At our center, the two patients with graft loss were transplanted early after the onset of SS and experienced early occurrence of thrombotic microangiopathy. Thus, our local experience and this study each demonstrate that in SS, a heavy burden of death and early graft loss attenuates the benefits of transplantation in the first year after transplantation. While our study does not suggest mechanisms for early loss, activity of underlying disease, endothelial dysfunction and immune system dysfunction and are the most likely candidates.
Our conclusions regarding improved survival are limited by the assumption that patients receiving transplants are similar to those remaining on the waiting list. In an attempt to strengthen the comparison, we excluded patients who received living donor transplants from the analysis because populations who have live donors available may be different than populations who wait for cadaveric transplants. However, we cannot rule out the possibility of bias due to physician selection or the presence of common HLA antigens affecting likelihood of transplantation. Theoretically, this could allow healthier patients to receive transplants.
In summary, we have demonstrated that despite suboptimal outcomes in graft survival and frequent occurrence of early graft loss, kidney transplantation provides a survival benefit in ESRD due to SS. Further study is indicated to determine how to improve early graft loss due to patient death, acute rejection, thrombosis and recurrence. It will also be important to utilize multicenter data to assess the effect of transplantation on systemic disease manifestations.
Acknowledgments
Drs. Gibney and Fischer were supported by institutional training grant T32-DK007135-30. Dr. Parikh was supported by grant K23-DK064689-01 from the National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD.




