Stented versus Nonstented Extravesical Ureteroneocystostomy in Renal Transplantation: A Metaanalysis
This paper was presented at the meeting of the American Society of Transplant Surgeons, Scottsdale, AZ, 2004.
Abstract
Stenting of the extravesical ureteroneocystostomy in renal transplantation is controversial. This study is a metaanalysis of 49 published studies over 30 years time in which the extravesical technique was used. Stented and nonstented anastomoses were compared. One-hundred six articles published between 1973 and 2002 were reviewed and 49 met criteria for inclusion. Articles were required to list original, numeric, previously unpublished data and to report or to describe the use of an extravesical ureteroneocystostomy, with or without stent. Data were analyzed within separate groups, (1) randomized, controlled trials and (2) case series. Data were included from five randomized, controlled trials and 44 case series. In the controlled trials group, there were urologic complications in 6 of 407 stented (1.5%), and 35 of 389 nonstented subjects (9.0%) (p < 0.0001, OR 0.24, 95% CI 0.10–0.57). In the case-series group, there were urologic complications in 137 of 4245 stented (3.2%) and 433 of 9077 nonstented subjects (4.8%) (p = 0.007, OR 0.58, 95% CI 0.39–0.86). Renal transplants with stented extravesical ureteroneocystostomy have a significantly lower urologic complication rate than those with nonstented anastomoses. All five randomized, controlled trials individually found stented anastomoses to have a lower complication rate and this was confirmed by metaanalysis of these trials and of case-series data.
Introduction
Extravesical ureteroneocystostomy has become the standard for reestablishment of urinary tract continuity in renal transplantation given its technical ease and low complication rate. Evolution to this technique has covered some 50 years of surgical progress. The ureteral anastomosis of kidney transplantation has historically been plagued by high complication rates, with early studies reporting mortality as high as 50% in those patients developing a urinary leak (1–3). Other commonly reported urinary complications include ureteral necrosis, stenosis and obstruction, clinically significant posttransplant hematuria, vesicoureteral reflux and infection (4,5). Many of these complications are now managed nonoperatively through image-guided procedures and cystoscopy, but they remain a frequent cause of reoperation, graft loss and postoperative morbidity (6,7).
The extravesical ureteroneocystostomy is commonly referred to as a Lich-Gregoir anastomosis (8,9), though the theory behind this anastomosis likely began as early as 1896 with Witzel (10). Variations have been suggested by others including Sampson (11), Woodruff (12), Konnack (13), McDonald (14) and Barry (15). The salient features of this anastomosis include a direct anastomosis between the donor ureter and the anterior or lateral wall of the recipient bladder without the use of a separate cystotomy. The bladder is entered through a bladder wall myotomy with a small perforation being placed in the bladder mucosa for direct suturing of the ureter to the bladder mucosa. The remaining portion of the bladder wall from the myotomy is then reapproximated over the ureter for an ‘anti-reflux’ tunnel. Benefits of this technique include less ureteral length and preserved ureteral blood supply, minimal bladder manipulation, no separate cystotomy and relative technical ease.
The practice of routine stenting of this anastomoses is controversial. Early reports described ureterocystostomy over a pediatric feeding tube with more recent papers describing use of silastic ‘double pigtail’ or ‘double J’ stent (16). Proposed benefits to a stented anastomosis include continuous decompression of the ureter to avoid anastomotic tension, maintenance of the ureter in a more linear alignment to avoid kinking and protection from ureteral narrowing or postoperative lumenal obstruction due to edema or external compression (17). Opponents of this practice complain that the stent can actually lead to obstruction through occlusion of the ureteral lumen or dislodgement and migration (18). They also counter that the stent can exacerbate long-term stricturing of the anastomosis, can erode through the lumen causing hematuria and can increase postoperative infection risk and ureteral calcification as well as postoperative pain (19–21). Additionally, presence of the stent results in the additional costs and risks of an invasive postoperative procedure for stent removal (by cystoscopy).
Five randomized, controlled trials have been published in which stented and nonstented extravesical anastomoses are compared directly (17,20–23). All of these studies have demonstrated lower urologic complication rates for the stented anastomosis, though these results have not always been statistically significant. Additionally, more than 40 case series have been published in which data are available regarding urologic complication rates of stented and nonstented extravesical ureteroneocystostomy (16,24–65). This study is a metaanalysis of available published reports in which urologic complication rates are reported after extravesical ureteroneocystostomy in renal transplantation. Data were pooled separately for the (1) randomized, controlled trials and (2) case series to compare stented versus nonstented anastomoses.
Materials and Methods
The performance of a metaanalysis requires meticulous review of the available literature to identify appropriate studies for inclusion. Those studies describing similar methods, interventions and endpoints can have their results combined statistically to arrive at a single outcome. This process of combining studies provides the researcher with greater power to identify significant differences and provides a methodical framework for review of a topic. The primary drawback to this technique is that the results of the metaanalysis are subject to the limitations of each of the included studies. This problem is more of a concern in the metaanalysis of observational data than randomized, controlled trials.
Published articles for this metaanalysis were identified for review using a Medline search for renal/kidney transplant reports and associated complications. All listed references from the identified articles were then reviewed to identify additional appropriate peer-reviewed articles. Inclusion criteria included those articles published during the 30-year time period 1973–2002. Studies were required to report original, previously unpublished data including a numerical listing of included transplants and urologic complications. The article was required to provide a description of the urinary reconstruction, and cases were considered to have had an extravesical ureteroneocystostomy if the procedure was described as such, or if the authors referred to a ‘Lich,’‘Lich-Gregoir,’‘Barry’ or ‘extravesical’ anastomosis. Anastomoses were categorized as ‘stented’ if they were specifically described as such; they were otherwise considered to have been ‘nonstented’ (less than 50 of the 13 000 transplants not specifically described). Articles were excluded if they did not report original, numeric data, included data previously published, or were not published in a peer-reviewed journal. Cases were excluded if it was not possible from the report to determine the type of reconstruction or if the complications were not reported in a stratified manner according to the type of ureteral anastomosis.
One-hundred six articles were reviewed from 17 journals with 49 meeting criteria for inclusion and 57 articles being excluded. Sixteen articles, which potentially could have contained appropriate case-series data were in lesser known or discontinued journals and could not be pulled for review. The primary reason for exclusion was non-use of an extravesical technique. Stented and nonstented cases were compared in two separate gropings, (1) randomized, controlled trials and (2) case-series studies. These two types of study design were felt to be of sufficient dissimilarity to warrant the individualized analysis of each of these types of data.
The most commonly reported complications were leak/ureteral necrosis, obstruction/stricture and persistent hematuria. For analysis, patients were considered to have suffered a urologic complication if they were reported to have any of these findings. Patients with postoperative urinary infections or stent-related issues were not considered to have urinary complications if these events did not lead to leak, obstruction or significant hematuria. Lymphoceles and other postoperative fluid collections were not considered to be urologic complications unless they were demonstrated to have a urologic etiology or to lead to a urologic complication. Follow-up time ranged from 1 month to several years. Most stents were ‘J-J’ or ‘pigtail’ stents though other stents were described (most commonly a pediatric feeding tube). Stents remained in place between 5 days and 12 weeks, though the majority were removed between 3 and 6 weeks after transplantation. Extravesical drainage was variable and not well reported. Differentiation was not made in the analysis between cadaveric and living donor transplants as this was not consistently reported. There were a limited number of case series in which the types of complications were not individually reported within the body of the report. The numeric values from these studies were included in the pooled case-series portion of the metaanalysis as reported by the primary authors, though the complication type could not be differentiated.
Group comparisons were made using SAS version 8.2 (Cary, North Carolina, USA) statistical software package. The Cochran–Mantel–Haenszel test was utilized for comparison of the randomized trial data given the relative uniformity of these studies. For the cohort (case-series) data, most of the studies did not involve patients in both the stented and nonstented groups, so Cochran–Mantel–Haenszel could not be performed. The group comparisons for this data were performed using generalized estimating equation (GEE) methodology applied to binary data to account for correlations within a study when both groups are present.
Results
Five randomized, controlled trials were found which compared stented and nonstented groups. The studies were similar in their design, entry and exclusionary criteria, technique for intervention and complication measures. The five studies were conducted in five different countries, all outside of the United States. The 44 studies included in the case-series group were much more disparate. The majority of these included single series data from either stented or nonstented patients. A variety of stent types and sizes were used and total study sample sizes ranged from 8 to greater than 1000 subjects. Urologic complications were routinely reported within one of three categories: obstruction/stricture, leak/necrosis and significant hematuria.
In the randomized trials group, there were urologic complications in 6 of 407 stented (1.5%), and 35 of 389 nonstented subjects (9.0%) (p < 0.0001, OR 0.24, 95% CI 0.10–0.57) (Table 1, Figure 1). In the case-series group, there were urologic complications in 137 of 4245 stented (3.2%) and 433 of 9077 nonstented subjects (4.8%) (p = 0.007, OR 0.58, 95% CI 0.39–0.86) (Tables 2 and 3).
| Study | Year | Country | Number stented | Number of complications | % | Number not stented | Number of complications | % |
|---|---|---|---|---|---|---|---|---|
| Dominguez et al. | 2000 | Canada | 143 | 5 | 3.5 | 137 | 9 | 6.6 |
| Kumar et al. | 1998 | India | 57 | 0 | 0 | 43 | 3 | 7.0 |
| Benoit et al. | 1996 | France | 97 | 1 | 1 | 97 | 10 | 10.3 |
| Bassir et al. | 1995 | Iran | 35 | 0 | 0 | 37 | 3 | 8.1 |
| Pleass et al. | 1995 | England | 75 | 0 | 0 | 75 | 10 | 13.3 |
| Total | 407 | 6 | 1.5 | 389 | 35 | 9.0 p < 0.0001* |
- *Cochran–Mantel–Haenszel test. Odds ratio 0.24, 95% CI 0.10–0.57. Number needed to stent to avoid one complication is 14.
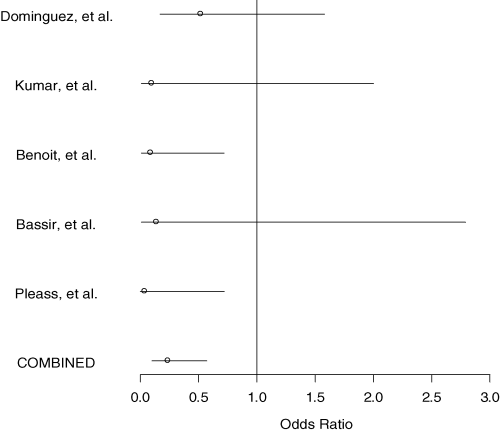
Odds ratios for urologic complication with stent usage versus nonusage in five randomized trials used in metaanalysis (overall OR = 0.24, 95% CI 0.10–0.57).
| Study | Year | Country | Number stented | Number of complications | % | Number not stented | Number of complications | % |
|---|---|---|---|---|---|---|---|---|
| Lasaponara et al. | 2002 | Italy | 58 | 2 | 3.4% | × | × | N/A |
| Gogus et al. | 2002 | Turkey | × | × | N/A | 433 | 19 | 4.2% |
| Briones-Mardones et al. | 2001 | Spain | 28 | 1 | 3.6% | 28 | 6 | 21.4% |
| Kmetec et al. | 2001 | Slovania | 129 | 0 | 0% | 0 | 0 | N/A |
| Khauli et al. | 2001 | USA | 300 | 6 | 2% | 0 | 0 | N/A |
| Nane et al. | 2000 | Turkey | × | × | N/A | 241 | 12 | 5% |
| Lasaponara et al. | 2000 | Italy | 112 | 0 | 0 | 0 | 0 | N/A |
| Bassiri et al. | 2000 | Iran | 644 | 11 | 1.7% | 232 | 10 | 4.3% |
| Masahiko et al. | 2000 | Japan | × | × | N/A | 225 | 7 | 3.1% |
| Blanchet et al. | 2000 | France | 415 | 19 | 4.6% | 350 | 36 | 10.3% |
| Ahmad et al. | 1999 | Iran | 100 | 2 | 2% | 460 | 28 | 6% |
| Shamsa et al. | 1999 | Iran | 257 | 8 | 3.1% | 0 | 0 | N/A |
| Samhan et al. | 1999 | Kuwait | × | × | N/A | 151 | 10 | 6.6% |
| Salomon et al. | 1999 | France | × | × | N/A | 570 | 16 | 2.8% |
| Delin et al. | 1998 | China | 80 | 2 | 2.5% | 125 | 8 | 6.4% |
| Junjie et al. | 1998 | China | 505 | 26 | 5.2% | 95 | 8 | 8.4% |
| Mahdavi et al. | 1997 | Iran | 140 | 0 | 0% | 40 | 4 | 10% |
| Butterworth et al. | 1997 | England | 108 | 2 | 1.8% | 0 | 0 | N/A |
| Rizvi et al. | 1996 | Pakistan | × | × | N/A | 148 | 5 | 3.4% |
| Hakim et al. | 1994 | USA (MN) | × | × | N/A | 773 | 49 | 6.3% |
| Nicol et al. | 1993 | Australia | 358 | 9 | 2.6% | 0 | 0 | N/A |
| Gibbons et al. | 1992 | USA | × | × | N/A | 1000 | 26 | 2.6% |
| Satwekar et al. | 1992 | India | × | × | N/A | 50 | 3 | 6.0% |
| Khauli et al. | 1991 | USA | 60 | 0 | 0% | 0 | 0 | N/A |
| Thrasher et al. | 1990 | USA | 160 | 6 | 3.7% | 0 | 0 | N/A |
| Sert et al. | 1990 | Turkey | × | × | N/A | 350 | 10 | 2.9% |
| Sumrani et al. | 1989 | USA (NY) | × | × | N/A | 1097 | 27 | 2.5% |
| Gedroyc et al. | 1988 | England | 265 | 7 | 2.6% | × | × | N/A |
| Shah et al. | 1988 | India | 0 | 0 | N/A | 125 | 5 | 4% |
| Hickey et al. | 1988 | Ireland | 454 | 28 | 6.2% | × | × | N/A |
| Ohl et al. | 1988 | USA (MI) | × | × | N/A | 808 | 23 | 2.8% |
| Santiago-Delpin et al. | 1986 | Puerto Rico | × | × | N/A | 23 | 3 | 13.0% |
| Hefty et al. | 1985 | Saudi Arabia | × | × | N/A | 43 | 1 | 2.3% |
| Cos et al. | 1985 | USA (DC) | × | × | N/A | 184 | 11 | 6.0% |
| Kartheuser et al. | 1985 | Belgium | × | × | N/A | 690 | 23 | 3.3% |
| Waltke et al. | 1982 | USA (WI) | 72 | 8 | 11.1% | × | × | N/A |
| Wasnick et al. | 1981 | USA (NY) | × | × | N/A | 183 | 8 | 4.4% |
| McDonald et al. | 1979 | USA (LA) | × | × | N/A | 78 | 1 | 1.3% |
| Mehta et al. | 1979 | Ireland | × | × | N/A | 32 | 7 | 21.8% |
| Meech et al. | 1979 | Australia | × | × | N/A | 266 | 40 | 15.0% |
| Hooghe et al. | 1977 | Belgium | × | × | N/A | 133 | 10 | 7.5% |
| Colfry et al. | 1974 | USA (LA) | × | × | N/A | 6 | 2 | 33.3% |
| Campos-Freire et al. | 1974 | Brazil | × | × | N/A | 88 | 10 | 11.4 |
| Clunie et al. | 1974 | Australia | × | × | N/A | 50 | 5 | 10.0% |
| Total | 4245 | 137 | 3.2% | 9077 | 433 | 4.8% p = 0.007* |
- *Group comparison performed using generalized estimating equation (GEE) methodology applied to binary data. OR 0.58, 95% CI 0.39–0.86.
- Number needed to stent to avoid one complication is 47.
| Complications | No of complications | Total | ||
|---|---|---|---|---|
| Randomized trials | ||||
| Stent | 6 (1.5%) | 401 | 407 | p < 0.0001* |
| No stent | 35 (9.0%) | 354 | 389 | |
| 41 | 755 | 796 | ||
| Case series | ||||
| Stent | 137 (3.2%) | 4108 | 4245 | p = 0.007** |
| No stent | 433 (4.8%) | 8644 | 9077 | |
| 570 | 12 752 | 13 322 | ||
- *Cochran–Mantel–Haenszel test. Odds ratio 0.24, 95% CI 0.10–0.57. Number needed to stent to avoid one complication is 14.
- **Group comparison performed using generalized estimating equation (GEE) methodology applied to binary data.
- OR 0.58, 95% CI 0.39–0.86. Number needed to stent to avoid one complication is 47.
All five randomized, controlled trials found stented anastomoses to have a lower complication rate, with three of these five reporting a statistically significant difference. Only seven of the case series provided a control group within the same report, generally an historical control at the same institution, and all seven of these reported lower complication rates in the stented group. A temporal subgroup analysis was completed within the case-series group, and stented and nonstented transplants were compared with the following results: (1973–1982) 11.1% versus 9.9% (p = 0.62, OR 1.13 95% CI 0.69–1.87), (1983–1992) 4.4% versus 3.0% (p = 0.14, OR 1.47 95% CI 0.88–2.45) and (1993–2002) 2.7% versus 5.6% (p < 0.05, OR 0.39 95% CI 0.25–0.60) (Table 4). Seventy-five percent of all stented transplants in the case-series group were reported in the 1993–2002 group compared to only 43% of nonstented transplants. All five of the randomized, controlled trials were reported between 1995 and 2000. Table 5 contains a listing of the complications within each randomized trial.
| Number of papers | Stent | No stent | p-value* | OR, 95% CI | NNT** | |
|---|---|---|---|---|---|---|
| Case series | ||||||
| 1993–2002 | 21 | 2.7% | 5.6% | <0.05 | 0.39, 0.25–0.60 | 24 |
| 1983–1992 | 14 | 4.4% | 3.0% | 0.14 | 1.47, 0.88–2.45 | |
| 1973–1982 | 9 | 11.1% | 9.9% | 0.62 | 1.13, 0.69–1.87 | |
| Overall | 44 | 3.2% | 4.8% | 0.007 | 0.58, 0.39–0.86 | 47 |
| Randomized trials | ||||||
| 1995–2000 | 5 | 1.5% | 9.0% | <0.0001 | 0.24, 0.10–0.57 | 14 |
- *Case-series data analyzed using generalized estimating equation (GEE) methodology applied to binary data.
- Randomized trials analyzed using Cochran–Mantel–Haenszel equation.
- **NNT = Number needed to treat (stent) to avoid one urologic complication.
| Study | Year | Country | Number of complications | Complications | Number of complications | Complications |
|---|---|---|---|---|---|---|
| Dominguez et al. | 2000 | Canada | 5 | 2 leak | 9 | 6 leak |
| 3 obstruction | 3 obstruction | |||||
| Kumar et al. | 1998 | India | 0 | 3 | 2 leak | |
| 1 obstruction | ||||||
| Benoit et al. | 1996 | France | 1 | 1 leak | 10 | 6 leak |
| 4 obstruction | ||||||
| Bassir et al. | 1995 | Iran | 0 | 3 | 1 leak | |
| 2 obstruction | ||||||
| Pleass et al. | 1995 | England | 0 | 10 | 5 leak | |
| 5 obstruction | ||||||
Discussion
Renal transplants with a stented extravesical ureteroneocystostomy have a significantly lower urologic complication rate than those with a nonstented anastomosis. All five randomized, controlled trials reviewed in this study found stented anastomoses to have a lower complication rate and this was confirmed by metaanalysis. The metaanalysis also demonstrates a lower complication rate from the pooled data of 44 reported case series. Though recent reports have demonstrated only small or insignificant differences between stented and nonstented anastomoses, the pooled data are convincing that the placement of a stent does provide some protection against the most common urologic complications of leak/necrosis, obstruction/stricture and significant hematuria in the extravesical ureteroneocystostomy.
Interestingly, a greater absolute difference in complication rates was seen in the randomized, controlled trials. These studies are carefully designed and regulated and are an epidemiologically superior mechanism for comparing two interventions. The stented transplants in these studies had a complication rate of only 1.5%, which may serve as the standard against which other studies are compared. Randomized, controlled trials are the epidemiologic gold standard for comparison of two interventions and provide Type I evidence, compared to cohort studies and case series which provide Type IV evidence. The cohort studies and case series do have value in that these reports often contain much larger subject numbers than can be accommodated in a controlled trial.
The clinical significance of these results must now be determined. Dominguez and colleagues, in publishing their randomized trial, reported complication rates of 3.5% and 6.6% in the stented and nonstented groups, respectively (22). With this difference, they calculated that 33 patients would require routine stenting to avoid one ureteral complication. They advocate, instead, selective stenting ‘in patients with preoperative or intraoperative risk factors for ureteric complications.’ It should be noted that in the Dominguez study, 6 of the 137 patients initially randomized to the nonstented group were, in fact, stented intraoperatively ‘for reasons that in the surgeon's opinion mandated stent placement.’ These reasons included difficult anastomoses (n = 3), twisted ureter (n = 2) and intraoperative leak (n = 1). Therefore, this trial actually compared mandatory stenting versus selective stenting, and the mandatory stenting still had a better outcome (absolute decrease of 3%).
The present metaanalysis finds an absolute difference in complication rates to be 7.5% (1.5% for stented subjects and 9.0% for nonstented subjects), which is highly significant with a number needed to stent of 14. If selective stenting were to be adopted, further study would be required to determine those patients at risk for complication who would benefit from stenting, but this group presumably includes those who have had previous pelvic or bladder surgery, patients with abnormal or small bladders or intraoperative technical difficulty with the anastomosis. Additionally, potential candidates for stenting include those patients with significant comorbidities such as obesity, multiple abdominal surgeries or previous peritonitis. Donor kidney characteristics such as a double ureter, a shortened or injured ureter or prolonged ischemia time may influence the choice for ureteral stent placement. Results of this metaanalysis, however, would certainly support those surgeons choosing to adopt routine stenting in all renal transplant recipients.
The case-series data were reviewed in three subgroups by decade (1973–1982, 1983–1992 and 1993–2002). Data from the first two decades fail to show a significant difference between stented and nonstented subjects, while the most recent decade more closely mirrors the results of the pooled data from the randomized trials, stented anastomoses having a significantly protective effect against urologic complication. Obviously, there were many changes in care throughout this 30-year time period including pre-and postoperative care, immunosuppression and transplant volume. It is important to note, then, that the most recent case-series data does closely resemble the randomized trial data, supporting and somewhat validating the results of these studies and also supporting the findings of this metaanalysis. The most clinically relevant data for today's transplant surgeon is that from the last decade which will be most reflective of current clinical practice.
Therefore, the true reduction in complication rate by stent usage may be closer to the 9.0% versus 1.5% for nonstented and stented anastomoses, respectively, as seen from the pooled data of the metaanalysis. Such a large difference would support the adoption of routine stenting in all patients and research would fall more to determining patients who could potentially be excluded from stenting and the avoidance of stent-related complications. Twenty-two of the 49 studies included in this metaanalysis reported use of a ureteral stent. A question yet to be addressed is the ideal type and size of ureteral stent. Stents reported in our review were most commonly ‘double J’ or ‘double pigtail’ stents ranging from 6F to 8F in size and 12 to 16 cm in length. Silastic double-J stents are readily available, easy to place and are relatively inexpensive as they are frequently used by urologists. They are also easily removed by cystoscopy and have a low rate of migration, calcification and breakage. The only other stent described was a pediatric feeding tube, which ranged in size between 6F and 10F.
The length of time posttransplant for which a ureteral stent should remain in place is unknown. In this study, the time period varied between 7 days to 12 weeks in the controlled trials group and 3 days to 12 weeks in the case-series group (of 16 total studies which reported this information). Junjie and colleagues (39) addressed this question when they reported consecutive groups of patients, the first group had no stent (8.7% complication rate), the second had stent removal at 3–4 weeks posttransplant (7.7% complication rate) and the third had stent removal at 5–7 days posttransplant (4.3% complication rate). The differences were not significant and the authors concluded that there was no particular benefit to early stent removal as most of the complications in the early removal group appeared after removal of the stent. Thirteen of the 16 studies that reported timing of stent removal stated that the stent was removed between 3 and 12 weeks posttransplant. Some surgeons elected to coordinate stent removal by cystoscopy with the ligation of an arteriovenous fistula or removal of a peritoneal dialysis catheter. Other surgeons report attaching the ureteral stent to the foley catheter so that the stent is removed at the time of removal of the bladder catheter. Occasionally, stents require removal prior to the anticipated time interval because of persistent infection, hematuria, dislodgement/migration or patient discomfort. Complications regarding the use of stents have been well summarized previously (6). These complications are now relatively rare and most can be managed simply by stent removal.
Results of this metaanalysis must be viewed with an understanding of the inherent limitations of the metaanalysis process. A metaanalysis pools data from studies with similar inclusion and exclusion criteria and a similar defined outcome. However, each of these studies has weaknesses and limitations unique to the individual study. The metaanalysis, therefore, incorporates a number of individual study limitations into one analysis which may limit the conclusions that can be drawn from the results. In general, the metaanalysis of randomized, controlled trials has more value than the pooling of data from cohort studies or case series because the stringent criteria established in a clinical trial tend to minimize bias, errors or statistical manipulation.
Results from this analysis certainly support the use of ureteral stents in the extravesical ureteroneocystostomy of renal transplantation. Whether this stenting is used on a routine basis or selectively in those patients at risk for ureteral complications remains to be decided. Also to be decided is the ideal stent and the timing of stent removal. Polyurethane ‘double J’ stents are the most frequently used because they are safe and readily available but it is unknown if outcomes could be improved upon by use of a different stent and varying the timing of stent removal based upon clinical factors. Regardless, dramatic improvements have been seen in the ureterovesical anastomosis for the past four decades. The widespread adoption of the extravesical anastomosis appears to have lowered routine urologic complication rates below 5% while being a technically easier and faster anastomosis to perform. This metaanalysis suggests that urologic complication rates could be in the 2–3% range with routine stent use.




