Combined ‘En Bloc’ Liver and Pancreas Transplantation in Patients with Liver Disease and Type 1 Diabetes Mellitus
Abstract
Liver disease alters the glucose metabolism and may cause diabetes, but this condition is potentially reversible with liver transplantation (LTx). Type 1 diabetes mellitus may be coincidentally present in a LTx candidate and immunosuppressive drugs will aggravate diabetes and make its management more difficult for posttransplant. In addition, diabetes negatively influences outcome after LTx. Therefore, the question arises as to why not transplanting the pancreas in addition to the liver in selected patients suffering from both liver disease and Type 1 diabetes. We report two cases of en bloc combined liver and pancreatic transplantation, a technique originally described a decade ago in the treatment of upper abdominal malignancies but rarely used for the treatment of combined liver disease and Type 1 diabetes. Both recipients are currently liver disease-free and insulin-free more than 2 and 4 years posttransplant, respectively. Surgical, medical and immunological aspects of combined liver-pancreas transplantation are discussed in the light of the existing relevant literature.
Introduction
Impairment of glucose metabolism is a common feature in liver transplant (LTx) candidates and this is due to increased insulin resistance caused by liver disease (1,2). This condition is potentially reversible by LTx although immunosuppressive drugs are diabetogenic on their own (1,3–6). Type 1 diabetes mellitus can also be present in LTx candidates. In those patients, liver replacement will not correct glucose metabolism and persistant diabetes may negatively affect the long-term results of LTx (5). Indeed, we and others showed earlier that insulin-dependent diabetes mellitus in LTx candidates is a risk factor for posttransplant complications and poorer outcomes (7,8). In addition, daily management of diabetes will be particularly challenging in transplant recipients receiving diabetogenic drugs such as corticosteroids and calcineurin-inhibitors (5). Finally, secondary diabetic lesions will continue to progress after successful LTx and their evolution may even be accelerated by the use of immunosuppressive drugs (3,4). There is therefore a strong rationale to replace the pancreas in addition to the liver in selected patients suffering from both liver failure and Type 1 diabetes. Surprisingly, however, only very few cases of combined pancreas and LTx have been performed for this indication and the information available on this procedure is extremely scarce (11). Therefore, we report two cases of combined pancreas and LTx and we discuss the indications, surgical, medical and immunological aspects of this transplant procedure.
Case Report 1
AP is a 26-year-old female (blood group A+; 53 kg; 156 cm) with a medical history of Type 1 diabetes (basal and post-glucagon C peptide: not detected) since age 12. At age 19, she presented with pruritis, jaundice and pronounced xanthelasma of the eye lids. Laboratory findings demonstrated elevated cholestasis and hyperlipidemia. An endoscopic retrograde cholangiography revealed multiple strictures of the intra- and extrahepatic bile ducts consistent with the diagnosis of primary sclerosing cholangitis (PSC). A liver biopsy confirmed the diagnosis of PSC. At age 25, her clinical and biochemical condition deteriorated with refractory pruritis, aggravating cholestasis, extremely high serum cholesterol levels (2230 mg/dL), and repeated episodes of cholangitis and colitis. Her renal function was impaired with a creatinine clearance of 24 mL/min and mild proteinuria (<150 mg/24 h). Due to the risk of bleeding, no renal biopsy was performed. Glycosylated hemoglobin levels were above normal values and she had retinopathy and neuropathy.
She received a combined liver and pancreas transplant from an 18-year-old 50 kg female donor in May 2000. A kidney was not transplanted because renal dysfunction was probably not only caused by diabetes, but also by the liver disease and therefore was potentially reversible. The operative procedure started with a bilateral subcostal incision and a median extension to the xyphoid. Hepatectomy was performed and the en bloc liver-duodeno-pancreatic graft was transplanted orthotopically (1, 2 and 3). Supra- and infrahepatic caval anastomoses were first performed followed by a piggy-back anastomosis of the native portal vein onto the grafted portal vein. Next, a circular donor aortic patch including both the celiac trunk and the superior mesenteric artery was anastomosed end-to-end to a donor aortic tube that had been previously implanted on the receptor infrarenal aorta. Finally, an anterocolic side-to-side duodeno-jejunostomy was performed for exocrine pancreatic and biliary drainage. The procedure was uneventful and required three units of packed red cells replacement and was completed within 8 h. Cold ischemia time was 5 h 50 min. Pathological examination of the native liver proved the presence of PSC and also showed the existence of an incidental small central mixed cholangio-hepatocellular carcinoma (1 cm diameter). Initial immunosuppression included induction with ATG (1 mg/kg; 7 days) and maintenance treatment with tacrolimus (target through levels 10 ng/mL), mycophenolate mofetil (2 × 1 g/day), and low-dose steroids. The postoperative course was uneventful and the patient was discharged 13 days posttransplantation with normal liver function tests, normalized serum cholesterol levels and was free of insulin therapy. At 26 months, she developed one episode of late acute liver rejection due to noncompliance and withdrawal of immunosuppression. This rejection episode responded fully to steroid therapy and reestablishment of maintenance immunosuppression. The patient is now 4 years posttransplant, fully physically and socially rehabilitated. Liver function is normal. She remains insulin-free and has a normal glycosylated hemoglobin (5.6%). Fasting C-peptide values prior to and after glucagon stimulation are now within normal range. The diabetic retinopathy remains stable and her neuropathy has improved. Kidney function has normalized.
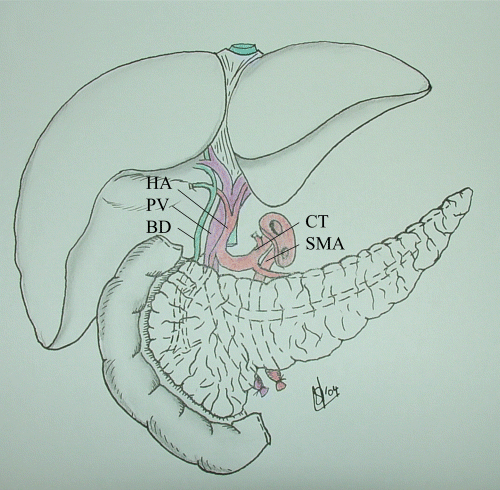
The liver-pancreas graft. The liver and pancreas are procured en bloc with a donor aortic patch including both the celiac trunk (CT) and the superior mesenteric artery (SMA). The liver hilum is left untouched and includes the hepatic artery (HA), the bile duct (BD) and the portal vein (PV).
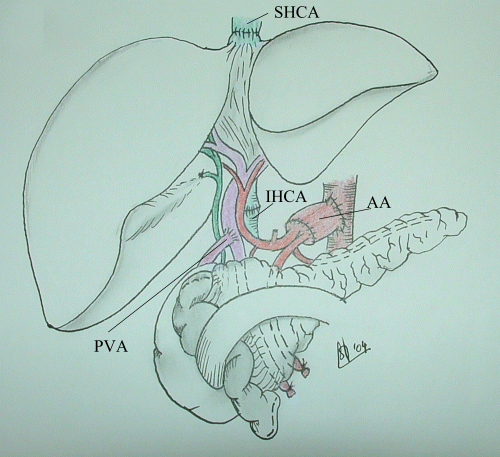
Illustration of the vascular implantation of the liver-pancreas graft: suprahepatic cava anastomosis (SHCA), infrahepatic cava anastomosis (IHCA), arterial anastomosis (AA) through an interposition aortic conduit, and portal vein anastomosis (PVA) of the recipient native portal vein end-to-side on the posterior aspect of the donor portal vein.
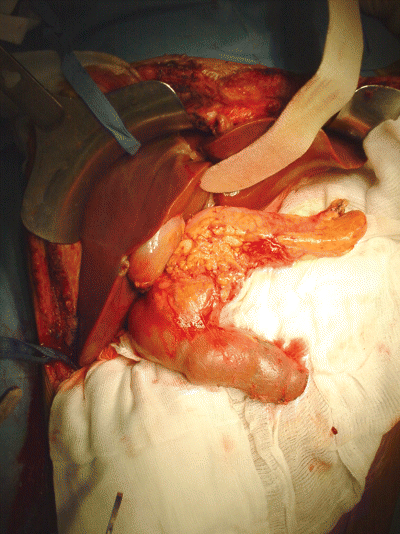
En bloc liver and pancreas graft after reperfusion in vivo. The operation will then be completed by performing a cholecystectomy, shortening the distal segment of the duodenum and performing a side-to-side duodeno-jejunostomy for the biliary drainage of the graft.
Case Report 2
IL is a 50-year-old male (blood group A+; 58 kg; 163 cm) who presents with a 2-month history of rapidly evolving severe cholestasis (bilirubine > 30 mg/dL and alkaline phosphatase > 2000 U/L). A biopsy shows an acute necrotizing hepatitis, presumably drug-induced. Patient had received mirtazapine that was consequently stopped. Independently of his liver disease, this patient has a 10-year history of Type 1 diabetes (basal and post-glucagon C peptide: not detected) and now presents with diabetes-related complications, mainly neuropathy and peripheral vasculopathy, and hypercholesterolemia. Clinically, a component of nonalcoholic steatohepatitis (NASH) cannot be excluded given the co-existence of diabetes and hyperlipemia in a patient with progressively deteriorating liver function. The patient is placed on an urgent list for LTx because of the rapidly deteriorating clinical condition and the evolution toward subacute liver failure with development of grade 2 encephalopathy. Because diabetes may have played a role in the onset and/or deterioration of liver disease, and given the expected worse outcome of LTx in patients with IDDM, we elected to replace the pancreas in addition to the liver.
The procedure takes place on February 2002 and a technique identical to that used in case 1 is used (1-3). The donor was a 38-year-old 70 kg male. Cold ischemia time was 4 h 48 min. Surgery was uneventful and required no transfusion of blood products and was completed within 6 h. Initial immunosuppression included induction with anti-IL-2 receptor antibodies (basiliximab 2 × 20 mg) and maintenance treatment with tacrolimus (10 ng/mL), mycophenolate mofetil (2 × 1 g/day), and low-dose steroids. An episode of mild cellular rejection of the liver responded to a single course of intravenous steroids. Patient suffered posttransplant from a severe but spontaneously resolving episode of confusion. He was discharged on postoperative day 28. He is currently more than 2 years posttransplantation and physically rehabilitated and insulin-free. Glycosylated hemoglobin is now within normal range. Fasting C-peptide prior to and after glucagon stimulation are within normal range. Kidney function is normal.
Discussion
A decade ago, there were reports of ‘multiorgan’ or ‘cluster’ transplantations including the liver and the pancreas. These cluster operations were mostly performed following massive abdominal evisceration, including the pancreas, for otherwise nonresectable upper abdominal malignancies. With the exception of neuroendocrine tumors, the results have been poor mostly due to tumor recurrence, and this procedure has rarely been performed (9,10).
A more appropriate indication for combined liver and pancreas replacement is in LTx candidates who coincidentally suffer from Type 1 diabetes. Our two case reports show that combined organ replacement—in addition to correcting liver disease—allows to achieve insulin-independence in these patients. Surprisingly, we could identify only eight patients in the literature who received a liver-pancreas graft for combined liver disease and diabetes (11–15).
Data from the International Pancreas Transplant Registry indicate that most cases of combined liver-pancreas transplantation have been done for abdominal malignancies or—for technical reasons—when the liver-small bowel bloc was transplanted (16).
PSC has been described in association with various autoimmune diseases and among them ulcerative colitis, Sjögren's syndrome and Type 1 diabetes (17–20). Although this association PSC/Type 1 diabetes is not exceptional, there are only two cases reported (including one reported here) of combined liver pancreatic graft for that indication (11). We are aware of a third case recently performed at another transplant centre in Belgium (personal communication, Dirk Ysebaert, Antwerp, Belgium).
A frequent association between diabetes and liver disease is the one seen in NASH (21,22). Diabetes —in addition to obesity and hyperlipemia—is thought to play a key role in the development of NASH (21,22). NASH is becoming an increasing indication for LTx and we and others have seen relapses of NASH after transplantation (23,24 and unpublished observations). The fact that we could not actively exclude NASH in our second case was an additional reason to replace the pancreas in addition to the liver.
Finally, patients suffering from cystic fibrosis may present with both exocrine and endocrine pancreatic insufficiency—in addition to liver disease and severe portal hypertension—justifying combined liver and pancreas replacement in selected cases (15).
There are several reasons to add the pancreas to the liver in LTx candidates with Type 1 diabetes. First, we showed earlier that pretransplant insulin-dependent diabetes mellitus negatively influences the long-term survival after LTx (7). Similar data were recently reported by Yoo et al., using the database from the United Network for Organ Sharing (UNOS), they compared 1629 patients with insulin-dependent diabetes mellitus with 17 974 patients without insulin-dependent diabetes mellitus and found that the 5-year survival rate for the diabetic patients was approximately 40% lower than for nondiabetics (8).
Secondly, LTx candidates with early diabetic nephropathy are at risk of developing overt renal failure once they are exposed to calcineurin-inhibitors (3–5). On the contrary, Fioretto showed that pancreas transplantation done at an early stage (in patients with Type 1 diabetes) can prevent further progression of renal disease and even reverse diabetic nephropathy (25). The classical argument against application of early isolated pancreas transplantation in nonuremic Type 1 diabetes is that ‘chronic immunosuppression is not necessarily better than diabetes itself.’ But in a LTx candidate who will necessarily be obligated to immunosuppression, there should be no reason not to render the patient insulin-free via combined pancreatic replacement, unless the patient is physically not fit enough to tolerate liver transplant surgery.
Finally from a quality of life standpoint, there is an important incentive to transplant the pancreas in addition to the liver in LTx candidates with Type 1 diabetes. Indeed, corticosteroid and calcineurin-inhibitors will aggravate diabetes and make its management more complex after isolated LTx (3–5).
Technically, there are two ways to perform a combined liver-pancreas transplantation.
The first possibility is to perform a standard orthotopic LTx followed by a standard heterotopic implantation of the pancreas allograft in the right lower quadrant onto the iliac vessels (11). Pancreatic exocrine drainage can be done either in the bladder with the advantage that amylasuria can be used as a marker of pancreas rejection (26), or into the ileum. The most important advantage of this technique is that the liver graft will not be in danger in case of pancreas-related complications such as pancreatitis, thrombosis and infection.
The second technique described herein and that is inspired from the ‘cluster cases’ described by Starzl et al., is to perform an orthotopic en bloc implantation of the liver graft accompanied by the pancreas, both organs being left in continuity and being procured with their entire arterial blood supply including the donor celiac axis and superior mesenteric artery (9,10). The native recipient venous portal drainage is connected via an end-to-side anastomosis into the graft portal vein. The exocrine pancreatic and biliary drainage is enabled by anastomosing the donor duodenum into the recipient jejunum. Compared to the separate implantation, the main advantage of this technique is its rapidity and simplicity since it involves only three or four large vascular anastomoses and one duodeno-jejunostomy and no separate biliary anastomosis. Additional advantage of the en bloc technique is the physiological position of the pancreas with a natural venous drainage into the donor liver graft. Indeed, there is some evidence that portally drained pancreatic grafts provide a better metabolic control (27). Esthetically, the en bloc procedure can be done through one single subcostal incision, something that may be of importance in younger recipients.
A variation of the en bloc technique is when the duodenum is not procured and the exocrine drainage of the pancreas is via a mucosa-to-mucosa anastomosis between the gastric antrum and the pancreatic duct, a technique that—we believe—exposes to a higher risk of pancreatic fistula (12,28).
To limit the additional morbidity inherent in a combined liver and pancreas transplant procedure, it is important to select high-quality organs and—in case the en bloc technique is used—to avoid larger donors given the relatively large size of an en bloc graft.
Equally determinant for the success in the combined procedure is the careful selection of the patients and avoids transplanting patients debilitated by a long-standing disease history.
What are the immunological consequences of associating a pancreas to a liver graft? First, a pancreas allograft is more immunogenic than a liver allograft (29). Therefore, one cannot exclude that addition of the pancreas to the liver may make the cluster graft more immunogenic. For that reason, and similar to the practice usually recommended in pancreas transplantation, we added an induction course (antithymocyte immunoglobulines in the first case and anti-IL-2 receptor antibodies in the second case) to compensate for the possibly increased risk of rejection. However, a well-established benefit of transplanting the liver in combination with another organ is the ability of the liver to protect accompanying organs against rejection (30–32). This protective effect has been documented experimentally after combined liver plus pancreas transplantation in rodents (33,34). Clinically, the immunoprotective effect of the liver allograft on the concommitantly transplanted pancreas and intestine (in multivisceral and liver/intestine recipients) was clearly demonstrated by Abu-Elmagd et al. (35,36). At the time of this writing, no pancreatic rejection was seen in our cases. Finally, a last immunological particularity of multiorgan grafts is that they convey a large amount of immuno-competent lymphoid cells which can potentially mount a Graft-versus-Host disease (GvHD) in an immuno-compromised mismatched recipient (37). Experimentally, combined liver and pancreatic grafts have been shown to cause GvHD and that possibility should not be ignored in recipients of combined liver and pancreas grafts (37,38).
An alternative to the combined liver and vascularized pancreas transplantation is the combined transplantation of the liver and islets. The pancreas is procured and separated from the liver and processed into islets using standard isolation techniques. The islet preparation is then infused in the recipient portal vein after liver reperfusion. This strategy was first applied by the Pittsburgh team in their series of cluster transplantation for malignancies (39). The rationale was to avoid the inclusion of the solid pancreas in the multivisceral graft and yet maintaining these recipients euglycemic (39). Liver and islet transplantation has also been done for nonmalignant indications in patients with liver disease and diabetes (40). One concern with this strategy is the previously described occurrence of portal vein thrombosis caused by infusion of the islets in a freshly reperfused and edematous liver (41).
Patients with liver disease generally present with some degree of impairment of the glucose metabolism and this is due to increased peripheral insulin resistance. This condition is reversible with LTx alone although immunosuppressive drugs can cause diabetes posttransplant on their own (1,2,5). Addition of beta cells by pancreas or islet cell transplantation is theoretically of little benefit and—given the severe shortage of pancreata—is not justified in LTx patients with cirrhosis-associated diabetes mellitus or with Type 2 diabetes. A few patients with liver disease may present with diabetes that is not secondary to liver disease and that has evolved into absent or extremely low C peptide production and no glucagon response. Some of these patients may reveal not controllable despite weight loss. The possibility of combined liver and pancreas transplantation in these patients should be cautiously discussed on an individual basis.
In summary, we report two successful cases of combined liver-pancreas transplantation in patients with liver disease and Type 1 diabetes. The en bloc technique described here is reproducible and does not complicate neither the difficulty of the LTx operation nor the posttransplant evolution. This technique is more rapid to perform and more physiological than the separate implantation of both organs. Type 1 diabetes in association with terminal liver disease should be treated by combined liver and pancreas replacement since euglycemia and insulin-independence achieved in these patients simplify the posttransplant management, improve the quality of life and may positively impact on the long-term outcome.
Acknowledgments
We thank Dr. Rainer Gruessner (University of Minnesota) for critical reading of this manuscript. We are grateful to Mrs Lydia Coolen for editorial assistance and to Mr Frank Van Gelder for the illustrations. Jacques Pirenne is holder of a CAF chair for abdominal transplant surgery.




