EBV Positive Primary Cutaneous CD30+ Large T-Cell Lymphoma in a Heart Transplanted Patient: Case Report
Abstract
Most post-transplant lymphoproliferative disorders (PTLDs) are of B-cell origin, whereas T-cell lymphomas rarely occur. We detail the clinicopathological features of the first case of Epstein–Barr virus (EBV)-associated primary cutaneous CD30+ anaplastic large cell lymphoma (ALCL) in the setting of heart transplant. A 71-year-old patient, 111 months after transplant, presented with multiple cutaneous lesions on the left thigh; histological and immunohistochemical examinations led to diagnosis of T-cell CD30+ ALCL. In situ hybridization demonstrated the presence of EBV-positive tumour cells. The patient received radiotherapy, but he relapsed at the same cutaneous site with loco-regional nodal spread. Chemotherapy was administered resulting in complete remission; four years later the patient is alive and well. Our findings indicate that primary cutaneous EBV+ CD30+ ALCLs should be included within the T-cell PTLDs spectrum; further studies are required to confirm whether they may be also considered, in transplantation settings, a distinct lymphoma subset with relatively favourable outcome.
Introduction
Post-transplantation lymphoproliferative disorders (PTLDs) are a potentially life-threatening complication of solid organ transplantation, ranging from early Epstein–Barr virus (EBV) driven policlonal polymorphic proliferations (resembling infectious mononucleosis) to EBV positive or EBV negative monomorphic tumours (1).
The recent World Health Organization (WHO) Classification of Tumours of Haematopoietic and Lymphoid Tissues has dedicated a specific paragraph to the spectrum of PTLDs, in which monomorphic tumours have been classified according to the classification scheme for B- and T-cell non-Hodgkin's lymphoma (NHL) (2). Most of monomorphic PTLDs are B-cell lymphomas whereas T-cell lymphomas are a rare occurrence (2).
Data from the Cincinnati Transplant Tumour Registry (CTTR) indicated that most monomorphic PTLDs are B-cell lymphomas whereas T-cell lymphomas account for about 14% of monomorphic PTLD (3). However, two recent PTLDs series have reported a lower incidence of T-cell lymphoma, 6, 5% (4/61) (4) and 4% (3/80), respectively (5).
The T-cell PTLDs span the whole spectrum of T-cell neoplasms including subcutaneous panniculitis-like T-cell lymphomas, hepatosplenic γ/δ T-cell lymphoma, NK/T-cell lymphomas and peripheral T-cell lymphomas unspecified (2). Sporadic cases of CD30+ anaplastic large cell lymphomas (ALCLs) of both nodal and extranodal type have been also reported (2,6,7), including a case of primary cutaneous CD30+ ALCL that arose in a kidney allograft (8), and a very small series of CD30+ ALCLs with primary cutaneous presentation but subsequent predominant visceral spread (9).
Here we describe in detail the clinical and pathological features of a case of primary cutaneous EBV positive CD30+ ALCL in a heart transplantation setting, which is charaterized by a very good response to chemotherapy and prolonged disease-free survival.
Case Report
September 1990
A 63-year-old man suffering from ischemic cardiomyopathy underwent orthotopic heart transplantation. Pre-transplant immunosuppression consisted of methylprednisolone (mPred: 500 mg after cardiopulmunary bypass and 125 mg i.v. q 12°× 3 doses). After transplant, the immunosuppressive regimen consisted of RATG (2.5 mg/kg/day i.v. for 5 days), Azathioprine (2 mg/kg/day), Cyclosporin A (5 mg/kg/day, adjusted to maintain plasma levels between 200–300 ng/mL), and Prednisone (0.2 mg/kg/day p.o.). On day 20, endomyocardic biopsy showed 1B degree of acute rejection, treated with increased immune suppression.
December 1999
One hundred and eleven months after the transplantation, the patient presented multiple reddish, sometimes ulcero-necrotic, cutaneous nodules and infiltrated plaques involving the upper posterior left thigh (Figure 1A); the ultrasonography documented that the subcutaneous tissues were infiltrated by a hypoecogenic lesion (Figure 1B), showing at colour Doppler examination, a significant intra and perilesional vascularisation (Figure 1C). One of the nodular skin lesions was biopsied for histological analysis. The specimen was formalin fixed and paraffin embedded; 3 μm thick paraffin sections were cut for routine histology, immunohistochemical and molecular analyses. The histological examination revealed a dense, non-epidermotropic, nodular and diffuse lymphoid infiltrate extending through the whole dermis and subcutis (Figure 2A).
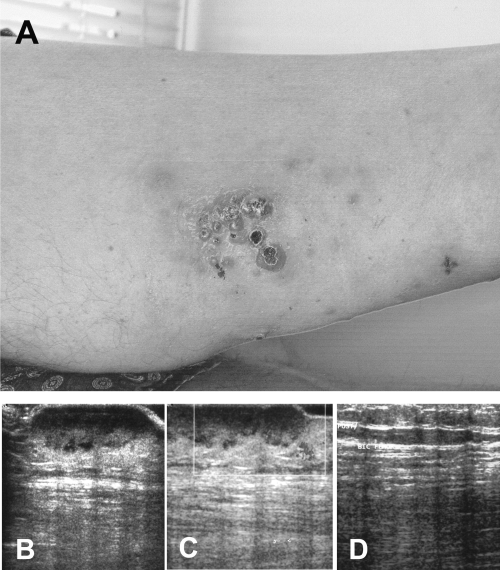
(A) The patient presented multiple reddish, sometimes ulcero-necrotic, cutaneous nodules and small infiltrated plaques at the upper posterior left thigh. (B) Ultrasonography documented that the lymphoma extensively infiltrated the subcutaneous tissues and it was characterised by a significant intra and perilesional vascularisation (colour Doppler examination, (C)). (D) The clinical remission (after chemotherapy) was confirmed by ultrasonography showing normalisation of the previously infiltrated subcutaneous areas.
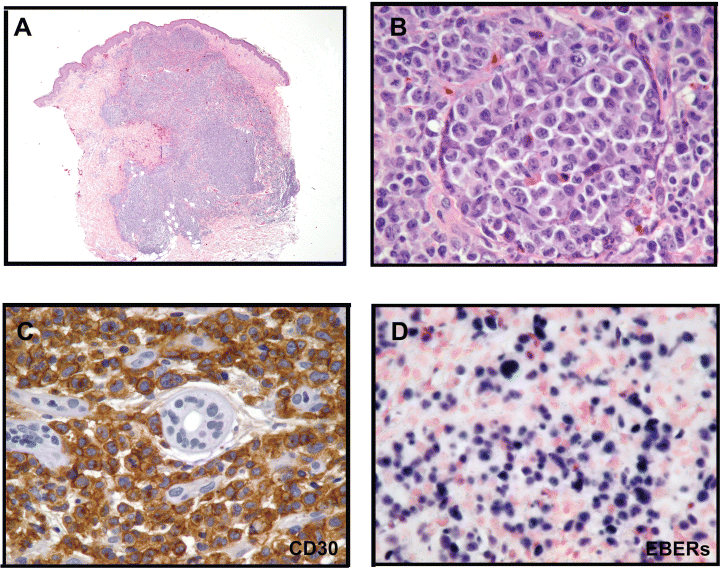
(A) Histology at low magnification: a dense, non-epidermotropic, nodular and diffuse lymphoid infiltrate extending through the whole dermis and subcutis (HE 20X). (B) The lymphoma cells were large, atypical with abundant slightly eosinophilic cytoplasm and had irregular pleomorphic to anaplastic nuclei, often with prominent multiple nucleoli (HE 400X). (C) More than 90% of the lymphoma cells intensely expressed the CD30 antigen (SABC method, 400X). (D) ISH demonstrated the presence of EBV transcripts within tumour cells (ISH 400X).
The lymphoid population consisted of large, atypical cells with abundant slightly eosinophilic cytoplasm and irregular pleomorphic to anaplastic nuclei, often with prominent multiple nucleoli (Figure 2B). Numerous atypical mitotic figures were found. A scanty reactive infiltrate (small lymphocytes, eosinophils and neutrophils) was present. The overlying epidermis was slightly atrophic and focally ulcerated.
At automated immunostainings (DakoCytomation Autostainer) (Table 1), the lymphoma cells stained for the CD45/LCA, the T-cell related antigens CD2, LAT and CD43; in addition they uniformly (more than 90%) expressed the CD30/Ki-1 (Figure 2C) and LN3/HLA-DR antigens. Cytotoxic associated-granules protein (TIA-1) and granzyme B were detected in less than 50% of the lymphoma cells; other cytotoxic and NK markers (perforin and CD56), and the ALK-protein were negative.
| Antibody; source | CD; Specificity | Lymphoma cells |
|---|---|---|
| B-cell markers | ||
| L26/CD20cy (D) | CD20; pan B | − |
| CD79a (D) | CD79a; pan B | − |
| BSAP/PAX5 polyclonal (SC) | NC; B-cell specific activator protein; pan B, Reed–Sternberg cells | − |
| T-cell markers | ||
| CD2 (N) | CD2; pan T | + |
| CD3 polyclonal (D) | CD3; pan T | − |
| CD4 (N) | CD4; helper T-cells; histiocytes/monocytes | − |
| CD5 (N) | CD5; pan T | − |
| CD7 (N) | CD7; pan T | − |
| CD8 (D) | CD8; cytotoxic T-cells | − |
| Linker for Activation of T-cells (LAT) (N) | NC: pan-T, mast cells, megakaryocytes | + |
| MT1 (B) | CD43; histiocytes/ monocytes, pan T, and myeloid cells | + |
| UCHL1 (D) | CD45RO; pan T; myelomonocytic cells | − |
| NK cell and cytotoxic markers | ||
| TIA-1 (I) | NC; cytotoxic granule-associated protein | −/+ |
| Perforin (N) | NC; cytotoxic T lymphocytes | − |
| Granzyme B (N) | NC; cytotoxic T lymphocytes and NK cells | −/+ |
| CD56 (Z) | CD56/ neural cell adhesion molecule-1; NK cells, neuroendocrine tumors | − |
| Histiocytic and activation markers | ||
| PGM1 (D) | CD68; histiocytes/ monocytes | − |
| BerH2 (D) | CD30; activated B and T Lymphocytes; Reed–Sternberg cells | + |
| LN3/HLA-DR (B) | NC; activated B and T lymphocytes | + |
| LeuM1 (BD) | CD15; myeloid cells; Reed–Sternberg cells | − |
| Mib-1/Ki67 (D) | NC; proliferation-associated nuclear antigen | 70% |
| Miscellaneous | ||
| CAM 5.2 (BD) | NC; cytokeratin | − |
| AE1-AE3 (BG) | NC; cytokeratin | − |
| EMA (D) | NC: epithelial membrane antigen; activated B and T lymphocytes | − |
| Vimentin (D) | NC; mesenchimal cells and lymphoid cells | + |
| LCA (D) | CD45; leucocyte common antigen | +/− |
| MSA (HHF35) (D) | NC; muscle specific actin | − |
| Desmin (Z) | NC; desmin | − |
| S-100 polyclonal (D) | NC; Langerhans cells; interdigitating dendritic cells; nerve sheath cells; melanocytes | − |
| ALK1 (D) | CD246; anaplastic lymphoma kinase | − |
| LMP-1 (D) | Epstein–Barr virus latent membrane protein-1 | − |
| EBNA-2 (D) | Epstein-Barr nuclear antigen-2 | − |
| EBERs | Epstein-Barr encoded RNAs | + |
- B, Biotest (Dreieich, Germany); BD, Becton-Dickinson (San Jose, CA); BG, BioGenex (San Ramon CA); D, DakoCytomation (Glostrup, Denmark); I, Immunotech (Marseille, France); N, Novocastra Laboratories Ltd. (Newcastle upon Tyne, UK); SC, SantaCruz Biotechnology Inc. (Santa Cruz CA); Z, Zymed (San Francisco CA).
- MW, microwave oven; NC, not clustered; PC, pressure cooker.
Polymerase chain reaction (PCR) molecular analyses documented the presence of a monoclonal TCRγ rearrangement in the cellular lesional infiltrate.
The EBV encoded early nuclear RNAs (EBERs) were detected in about 40% of tumour cells by means of in situ hybridization (ISH) technique (Figure 2D).
A diagnosis of T-cell monomorphic PTLD was based on these findings and the lesion was classified as primary cutaneous CD30+ ALCL (according to the WHO 2001 Lymphoma Classification); the presence of EBV was also reported.
Staging (including chest radiography, abdominal ultrasound, total body CT-scan and bone marrow biopsy) was negative for extracutaneous lymphoma localizations.
The patient was initially treated with external radiotherapy (6 MeV X-photons and 8 MeV electrons of linear accelerator) resulting in a partial lesional regression.
June 2000
Six months later, the lymphoma progressed and the patient presented with rapidly growing nodular (reddish) skin lesions involving both inferior extremities; a loco-regional (inguinal) lymphnode was enlarged. A “punch” biopsy was made on the relapsing lesion, that showed a diffuse lymphoma infiltration with morphological and immunomolecular features overlapping with those observed in the presenting lesion. The enlarged node was not biopsed.
The patient had a reduction in the immunosuppressive regimen (Cyclosporin A levels between 50–100 ng/mL; Azathioprine and Prednisone were stopped) and, because of EBV positivity, he was treated with high doses of i.v. IgG (IVIG, 400 mg/kg/day for 3 days and then once every 3 weeks) and Interferon-alfa (IFN-α: 3 × 106 U.I. s.c. every day for a week and then three times a week) (10,11).
Twenty days after such therapy, the skin lesions partially regressed and the inguinal lymphoadenopathy reduced in size.
September 2000
Two months later, the skin lesions progressively increased in size and focally ulcerated. The patient underwent CHOP chemotherapy (Cytoxan 400 mg/m2 i.v. on day 1, Vincristine 1.5 mg/m2 i.v. on day 1, Doxorubicine 50 mg/m2 i.v. on day 1 and Prednisone standard dosage 100 mg p.o. from day 1 to day 5). After three cycles, the skin lesions completely regressed; clinical remission was also documented by an ultrasonography examination that showed normalisation of the subcutaneous areas previously infitrated by the lymphoma (Figure 1D). However, due to the onset of general complications (including myelosuppression, enterocolitis and symptoms of ischemic heart failure) chemotherapy was stopped and the patient continued with monthly IVIG, together with IFN-α 3 × 106 U.I. s.c. weekly. Therapy lasted for one year and was stopped afterwards because of the absence of clinical and laboratory signs of disease. At the last follow-up (January 2004), the patient was alive and in complete remission, 49 months after the initial diagnosis of lymphoma.
Discussion
We report in detail the clinical and pathological features of a 71-year-old male patient who developed primary cutaneous EBV+ CD30+ ALCL 111 months after heart transplantation.
Most PTLDs are of B-cell origin, whereas T-cell PTLDs are infrequent (1,2) with only about 70 cases described since the 1980s (6). In comparison with B-PTLDs, T-PTLDs tend to occur later, are less likely to involve the allograft, and are less commonly associated with EBV positivity (2). T-cell-PTLDs span the whole spectrum of T-cell-neoplasms, including rare cases of CD30+ ALCL, NK-like T-cell lymphomas and γ/δ T-cell lymphomas (2–9).
In our series of 765 transplant recipients (heart, lungs and heart-lungs), 23 (3%) patients developed a PTLD, most of which had a B-cell phenotype. The incidence of T-PTLDs in our series was 8. Seven percent (2/23), in keeping with the frequencies (4–12%) reported in previous studies (3–5).
Interestingly, both our T-cell PTLDs were CD30+ ALCLs, one systemic (nodal) and the other primarily extranodal (cutaneous), respectively. In the literature only 10 cases of T-PTLD of ALCL type have been reported (6–9); four of them, all observed in kidney recipients, presented in the skin but only two can be considered primary cutaneous CD30+ ALCLs, the remaining probably representing cutaneous manifestation of a systemic lymphoma (8,9). To the best of our knowledge, the present study is the first description of primary cutaneous CD30+ ALCL in the setting of heart transplantation, and it is especially interesting since it expands the spectrum of EBV-related post-transplant lymphoma subtypes to include a primarily cutaneous form of CD30+ ALCL.
CD30+ ALCL is a well-established subtype of peripheral T-cell lymphoma that has been clinically subdivided into primary nodal (systemic) and primary extranodal (cutaneous) forms (12). Systemic CD30+ ALCL may be associated with a characteristic genetic abnormality, the translocation t(2;5), that results in the nucleophosmine-anaplastic lymphoma kinase (NPM-ALK) chimeric gene fusion and expression of the related protein product (12). It has been suggested that ALK positive systemic CD30+ ALCLs have a better prognosis than ALK negative cases (12). Primary cutaneous CD30+ ALCLs uniformly lack the t(2;5) and ALK expression (13). Despite this, they usually have a favourable prognosis and are highly responsive to external radiotherapy and cytotoxic chemotherapy (either alone or in combination) (14,15).
EBV, in conjunction with T-cell immunosuppression, has been implicated in the pathogenesis of the majority of PTLDs of B-cell origin (1,2) but limited information is available on the biological features of the T-cell PTLDs. Reduced immunosurveillance, chronic antigenic stimulation due to the graft, or a direct oncogenic effect of immunosuppressive drugs have all been suggested as possible mechanisms leading to T-cell lymphoma in transplant recipients (16).
In our case we have documented the presence of EBV within the lymphoma cells by means of ISH, in contrast with the lack of EBV in the previously reported cases of post-transplant primary cutaneous CD30+ ALCL (8). However, our finding is in keeping with the presence of clonal EBV genomes observed in a proportion (about 25%) of monomorphic T-PTLDs (16), including rare cases of systemic CD30+ ALCL (17,18).
Although EBV has been detected in certain subtypes of T-cell lymphoma, the exact mechanism of how EBV gets into T-cells is still obscure. Normally, T-cells express the EBV receptor CD21 at a rate 10-fold lower than B-cells, and it has been hypothesised that the CD21 expression on neoplastic T-cells may be transient or cell-cycle dependent (19). However, further biological studies will be necessary in order to better elucidate the EBV lymphomagenic role in T-cell malignancies in both immunocompetent and immunosuppressed patients.
Overall the mortality of PTLDs in solid organ recipients is approximately 60%, but with early diagnosis, prompt reduction of immunosuppression and careful administration of chemotherapy or radiation therapy, the prognosis for all types of PTLDs has improved (2).
In our patient, reduction in immunosuppression and subsequent radiotherapy resulted only in a partial regression of the lesion. For this reason the patient received CHOP therapy as scheduled for LNH treatment, but because of the onset of side effects, this was discontinued after only three cycles.
In spite of this, the patient responded well and complete disease remission was achieved; presently, he is alive and disease-free, 4 years after the diagnosis of lymphoma.
Such findings suggest that low-dose systemic chemotherapy could be successfully used in other patients with similar clinical features, who frequently present difficulties in clinical management and do not respond to reduction of immune suppression and local radiotherapy.
Of the two previously reported cases of primary cutaneous CD30+ ALCL arisen in transplant recipients, one died, with lymphoma, but in a setting of chronic renal failure, dialysis associated amyloidosis and progressive emphysema (9). The other had a favourable outcome similar to our patient (8), thus diverging from the poor prognosis which usually characterises most T-cell PTLDs (2,4,16,20).
No firm clinical conclusions can be drawn from sporadic observations. However, in spite of such limitations, our findings and other findings (8) indicate that: (1) primary cutaneous CD30+ ALCL must be included within the spectrum of T-cell PTLDs which may be EBV associated; (2) CD30+ primary cutaneous ALCL might represent, also in post-transplant recipients, a distinct lymphoma subset in which a relatively favourable outcome may be expected.
Acknowledgments
This study was supported in part by grants from the Italian Ministry for University Scientific Research and Technology (M.U.R.S.T.). Dr. Roberta Riboni is the recipient of a fellowship from I.R.C.C.S. Policlinico San Matteo, Pavia, Italy.




