Steroid Sparing with Tacrolimus and Mycophenolate Mofetil in Renal Transplantation
Abstract
Evidence suggests that steroid sparing in renal transplantation is associated with good outcomes, although there are limited data regarding steroid sparing in Tacrolimus and Mycophenolate Mofetil (MMF)-based regimes. In this study we describe the use of these agents in 101 consecutive patients undergoing renal transplantation using only a 7-day course of prednisolone.
Median follow-up was 33 months (range 18–44). Patient and graft survival at 1 year were 100% and 98%, respectively. The acute rejection rate at both 6 and 12 months was 19%, with two episodes beyond 12 months. Anti-CD25 monoclonal antibody (anti-CD25 mAb) was administered to 25 patients at high immunological risk: a trend toward a lower rejection rate was seen in these patients compared with those at lower risk but not receiving induction therapy (8% vs. 22%; p = 0.11). Two patients experienced recurrent rejection. Of the twenty-three rejection episodes in total, 26% showed vascular involvement. Allograft function was preserved at 12 months with a mean creatinine of 144 μmol/L and mean estimated glomerular filtration rate (GFR) of 55 mL/min. At 12 months, the incidence of post-transplant diabetes mellitus was 3.5%.
This steroid-sparing regime is associated with excellent patient and graft outcomes, and a low incidence of side effects.
Introduction
Cardiovascular mortality remains the commonest cause of death in renal transplant recipients and impacts on graft survival due to death with graft function (1,2). It is an unfortunate paradox that the use of corticosteroids, which have played a pivotal role in the treatment of acute rejection episodes and maintenance immunosuppression since the early days of transplantation (3), are associated with worsening of cardiovascular risk profiles. Steroid therapy leads to post-transplant diabetes mellitus (PTDM), hypertension, hyperlipidemia and weight gain, which impart a greater risk of cardiovascular disease to the transplant recipient (4,5), and may also have adverse effects on graft survival independent of losses due to patient death (6–8). Other steroid-related complications such as bone loss, cataracts, growth retardation in children and an increased susceptibility to infection lead to an even greater impact of steroids on patient outcome and satisfaction (4).
In an attempt to limit such steroid-related side effects, a number of studies have investigated the outcomes of immunosuppression regimes without steroids. Unfortunately, early attempts at steroid withdrawal (weaning and subsequent cessation of steroids, usually 3–6 months after transplantation) were met with an increase in rejection, a reduction in graft function and an increase in graft loss when performed with maintenance immunosuppression consisting of non-microemulsion Cyclosporin with or without azathioprine (9–11). Even in modern immunosuppression eras, steroid withdrawal has been associated with an increase in rejection, particularly in high-risk patients, leading to adverse outcomes in Neoral and Mycophenolate Mofetil (MMF)-based regimes (12), although recent reports suggest that steroid withdrawal regimes using Tacrolimus maintenance immunosuppression may be associated with acceptable rejection rates (13).
Steroid-sparing regimes (transplantation without the use of steroids, or with rapid elimination of steroids—within the first week post-transplantation) have certain advantages over steroid withdrawal. Firstly, steroid-related side effects occurring early post-transplantation, and which may not reverse with subsequent steroid withdrawal, can be minimized (4,14). Secondly, the steroid withdrawal syndrome—myalgia, arthralgia and non-specific malaise—which can result from the weaning of longer-term steroid therapy have not been described in steroid-sparing regimes. Finally, steroid sparing has been associated with acceptable rejection rates and graft outcomes in recent studies of Neoral and MMF-based regimes with an incidence of acute rejection at 1 year of <25%, good patient and allograft survival, a low incidence of PTDM (7% or less) and limited weight gains (4 kg) in pilot studies and a recent controlled trial (15–17).
Despite the increased use of Tacrolimus in standard triple-therapy regimes, its use in steroid-sparing protocols has been less widely reported, although such regimes would seem particularly attractive given the incidence of PTDM seen in some early Tacrolimus-based regimes of up to 22% (18). Studies of Tacrolimus monotherapy regimes without steroids have shown rejection rates of around 30% despite exclusion from the study of patients who were highly sensitized or who received poorly matched grafts (19). The addition of MMF to Tacrolimus showed 100% patient and graft survivals, with a rejection rate of 26% at a mean follow-up of 7.4 months in a non-selected group of 27 patients undergoing renal transplantation without induction antibody therapy (20). No PTDM was observed. A further study showed 100% 1-year patient and graft survival in 20 patients undergoing simultaneous pancreas-kidney transplantation with Tacrolimus and MMF (and antilymphocyte globulin [ALG] induction), with only one episode of acute rejection (21). More recently, the use of an extended course of the anti-CD25 monoclonal antibody (anti-CD25 mAb) induction agent, Daclizumab, with Tacrolimus and MMF resulted in excellent outcomes in an observational study of 57 pediatric renal transplant recipients, with a rejection rate at 1 year of 6% and a good safety profile (22).
Here we report in detail the outcome of a Tacrolimus, MMF and steroid-sparing regime with longer follow-up than previously reported, in 101 renal transplant recipients, half of whom were non-Caucasoid. In this study anti-CD25 mAb induction was administered to a selected group of recipients at high immunological risk, and the use of antilymphocyte antibody (ALG), which has been associated with an increase in adverse events was avoided (23).
Methods
Patients
One hundred and one consecutive patients undergoing renal transplantation at our institution between July 2000 and September 2002 were studied. Patient demographics are shown in Table 1. No patients were excluded from analysis.
| Mean age (years) | 40 ± 9 (17–70) |
|---|---|
| Male:female | 64:37 |
| Mean HLA mismatch (antigens) | |
| Total | 2.2 |
| A locus | 0.9 |
| B locus | 0.8 |
| DR locus | 0.5 |
| Regrafts | 10 |
| PRA > 30% | 6 |
| Live donor recipients | 27 |
| Ethnicity | |
| Caucasian | 52 |
| Indo-Asian | 26 |
| Afro-Caribbean | 15 |
| Mid-Eastern | 4 |
| Oriental | 4 |
| Cause of ESRF | |
| Glomerulonephritis | 43 |
| Unknown cause | 19 |
| Diabetes | 15 |
| Polycystic kidney disease | 11 |
| Urological | 5 |
| Others | 8 |
| Mean cold ischemia time (h)* | 17 ± 4 |
- *Cadaver donor kidneys.
- HLA = human leucocyte antigen, PRA = panel reactive anti-HLA Antibody, ESRF = end stage renal failure.
- Figures in parenthesis represent range.
Immunosuppression
Tacrolimus and MMF: All patients received Tacrolimus (Prograf®; Fujisawa, IL), 0.15 mg/kg/day initially and then adjusted depending on levels, and MMF (Cellcept®; Roche, Nutley, NJ) initially 750 mg twice daily increasing to 2 g/day depending on white cell count.
Steroids: Our steroid protocol consisted of methylprednisolone (500 mg) intravenously pre-operatively, followed by prednisolone 1 mg/kg/day (maximum 60 mg/day), reduced to 0.5 mg/kg/day on day 4, and then discontinued after day 7. Steroid administration was continued in the event of an acute rejection episode in the first week (see below).
Anti-CD25 monoclonal antibodies: Anti-CD25 mAb induction as either Basiliximab (Simulect®; Novartis, Essex, UK), or Daclizumab (Zenapax®; Roche, Nutley, NJ) was also administered to those patients (n = 25) who were at higher immunological risk by virtue of any of the following: (i) complete mismatch at the DR locus, (ii) repeat transplantation or (iii) HLA antibody sensitization with a panel reactivity >30%. Nine patients received Basiliximab at a dose of 20 mg on the day of transplantation and repeated on day 4; sixteen patients received Daclizumab in a simplified regime of 2 mg/kg on the day of transplantation and repeated on day 14.
Prophylaxis
Antiviral prophylaxis was administered for 3 months post-transplantation as Ganciclovir, with dose adjusted to allograft function. Pneumocystis Carinii prophylaxis was Co-trimoxazole, 960 mg three times weekly, with inhaled pentamidine being reserved for cases of patient intolerance. Isoniazid (150 mg/day) and pyridoxine (50 mg/week) were used in all Indo-Asian patients, and those with a past history of tuberculosis. Amphotericin lozenges or Nystatin mouthwashes were used to prevent oral candidiasis. Proton pump inhibitors were used to prevent gastro-duodenal ulceration.
Patient monitoring
Tacrolimus levels: Tacrolimus levels were measured as a 12-h trough (IMx® Tacrolimus II; Abbott laboratories, Abbott Park, IL). Tacrolimus dosage was adjusted to achieve trough levels of 10–15 ng/mL during the first year post-transplantation, and 8–10 ng/mL thereafter.
Allograft dysfunction: An unexplained rise in serum creatinine of 15% or greater was investigated as follows: Tacrolimus trough level monitoring, renal ultrasonography, magnetic resonance angiography of the renal arteries with or without formal angiography and percutaneous renal biopsy with grading according to the Banff 1997 classification (24).
Delayed graft function (DGF) was defined as the need for dialysis within the first week post-transplantation. The immunosuppressive strategy was not altered in those patients with DGF: Tacrolimus administration was not delayed, and the same Tacrolimus levels were targeted as for patients with immediate graft function; DGF was not an indication for the use of induction therapy. All patients not dialysis-independent by day 7 post-transplantation were biopsied to exclude early rejection before steroids were withdrawn.
Allograft rejection: All rejection was diagnosed by biopsy. Acute rejection was treated with a 500 mg pulse of intravenous (i.v.) methylprednisolone on three consecutive days with optimization of Tacrolimus and MMF dosages and levels. Oral prednisolone was continued if rejection occurred within the first week, or re-introduced if occurring after steroid cessation. Prednisolone was re-introduced at 20 mg/day, reducing to a maintenance dose of 10 mg/day by 3 months.
Post-transplant diabetes mellitus: PTDM was defined as the requirement for insulin or oral hypoglycemic agents at any time post-transplantation, in the absence of evidence for diabetes pre-transplantation (assessed by regular checks of blood sugar and HbA1c on dialysis).
Statistical analysis: Statview for Windows (SAS Inc., Cary, NC, 1998) was used for analysis. Categorical variables were related by means of Pearson's Chi square test, Student's t-test or Mann-Whitney U-test being used for comparison of parametric and non-parametric characteristics, respectively. Rejection-free survival was established using Kaplan-Meier analysis. Results are shown as mean ± standard deviation unless otherwise stated. All p values are 2-tailed with a value of <0.05 being considered significant.
Results
Patient and graft survival
All patients were followed for at least 18 months (range 18–44 months; median 33 months). Patient survival at 1 year was 100% and graft survival was 98%. One graft was lost due to renal vein thrombosis at day 5, and the second graft loss after 8 months was due to repeated rejection in the context of non-compliance (as manifested by failure of clinic attendance and failure to achieve therapeutic Tacrolimus levels except under direct supervision). No graft losses occurred in patients with delayed graft function (n = 24).
By the end of follow-up overall patient survival was 99%, with a single death due to myocardial infarction 16 months post-transplantation. Therefore, overall survival with a functioning graft was 97%.
Acute rejection
The 3, 6 and 12 month acute rejection rates were 16% (16/101), 19% (19/101) and 19% (19/101), respectively. No rejection was seen between 6 and 12 months. Two other patients experienced their first rejection episode beyond 1 year, and so a total of 21 patients experienced a rejection episode by the end of follow-up. Kaplan-Meier analysis of rejection-free survival is shown in Figure 1. Only 2 patients experienced two rejection episodes (one in the context of non-compliance with therapy). Therefore, in all there were twenty-three rejection episodes in 21 patients.
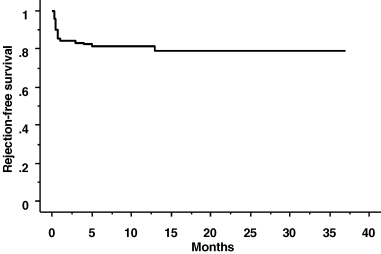
Rejection-free survival.
Seventy-four percent (17/23) of rejection episodes showed interstitial infiltrates and tubulitis, without evidence of vascular involvement (Banff ‘borderline’, IA or IB), with the remaining six biopsies (26%) showing mild vascular infiltrates with or without tubulitis (Banff IIA). No moderate or severe vascular rejection (Banff IIB or III) was seen. The median time to the first rejection episode was 15 days (range: 5 days–13 months). Only a single patient experienced rejection in the first week post-transplantation, that is, before steroid cessation.
Twenty-four patients had delayed graft function. Three patients developed acute rejection within their period of DGF, and three others following recovery from dialysis-dependency. The incidence of rejection in patients with DGF therefore was 25% (6/24), which was no different to the 17% incidence of rejection in patients with immediate graft function (13/77; p < 1.0). The median time to rejection was 12 days in the DGF group.
The incidence of rejection at both 6 and 12 months in the 25 patients who received anti-CD25 mAb was 8% (2/25), compared with a rate of 22% (17/76) in those not receiving anti-CD25 mAb. Although lower, this was not statistically significant (p = 0.11). Of the two rejection episodes in the induction therapy group, one occurred at day 5 in a patient given Basiliximab, and the other at day 10 in a patient given Daclizumab.
All but two episodes of rejection responded to i.v. corticosteroid administration and the re-institution of oral prednisolone. One non-compliant patient experienced recurrent rejection as described above, and lost the graft. The second patient developed vascular rejection at 30 days, unresponsive to methylprednisolone and optimization of Tacrolimus and MMF. Plasma exchange followed by a course of anti-CD20 mAb (Rituximab; MabThera®, Roche) led to clinical resolution with stable creatinine of 160 μmol/L at 12 months post-transplantation.
Most of the patients (85%) displayed adequate Tacrolimus exposure with median levels in the first 3 months >10 ng/mL, and 75% of patients achieved this therapeutic target by 72 h post-transplantation. No association was seen between mean Tacrolimus levels or periods of sub-therapeutic administration with rejection episodes.
Mean Tacrolimus and MMF doses and levels are shown in Table 2.
| Month of follow–up | <3 | 4–6 | 7–9 | 10–12 | 13–15 | 16–18 |
| Number of patients | 100 | 100 | 99 | 99 | 99 | 98 |
| Mean Tacrolimus dose (mg/day) | 10.4 (4.5) | 10.3 (4.4) | 10.3 (4.3) | 10.0 (4.1) | 9.8 (4.0) | 9.4 (3.9) |
| Mean Tacrolimus level (ng/mL) | 11.8 (3.8) | 11.5 (3.8) | 11.2 (3.4) | 10.9 (3.4) | 10.1 (3.2) | 9.6 (3.1) |
| Mean MMF dose (g/day) | 1.7 (0.7) | 1.6 (0.7) | 1.6 (0.6) | 1.5 (0.6) | 1.5 (0.7) | 1.5 (0.7) |
- Figures in parentheses represent standard deviation.
Graft function
Graft function was stable during the period of follow-up with a mean serum creatinine and glomerular filtration rate (GFR as estimated by the Cockcroft-Gault equation [25]) at 6, 12 and 18 months of 147(±40), 144(±42) and 150(±45) μmol/L and 54(±16), 55(±18) and 52(±19) mL/min, respectively. Only 12 individuals experienced a rise in creatinine of >10 μmol/L between months 6 and 12.
Allograft function was similar in groups stratified for acute rejection, DGF, and the use of anti-CD25 mAb (Table 3.)
| Rejection | No rejection | DGF | IGF | CD25 mAb Induction | No CD25 mAb Induction | |
|---|---|---|---|---|---|---|
| Mean creatinine (μmol/L) | ||||||
| 3 months | 154 (48) | 146 (42) | 150 (45) | 147 (43) | 146 (42) | 152 (46) |
| 6 months | 152 (47) | 145 (41) | 149 (44) | 146 (43) | 146 (42) | 149 (46) |
| 9 months | 151 (47) | 145 (41) | 150 (44) | 145 (42) | 144 (41) | 152 (47) |
| 12 months | 151 (47) | 143 (40) | 147 (43) | 143 (41) | 142 (40) | 147 (45) |
| 15 months | 153 (48) | 146 (41) | 151 (43) | 146 (41) | 146 (41) | 152 (46) |
| 18 months | 154 (48) | 148 (43) | 152 (44) | 149 (43) | 148 (43) | 153 (47) |
| Mean GFR (mL/min) | ||||||
| 3 months | 51 (19) | 53 (17) | 51 (18) | 55 (18) | 54 (17) | 51 (17) |
| 6 months | 51 (18) | 55 (17) | 52 (18) | 55 (17) | 55 (16) | 51 (16) |
| 9 months | 52 (18) | 55 (16) | 52 (17) | 56 (17) | 55 (16) | 52 (16) |
| 12 months | 52 (18) | 56 (16) | 53 (17) | 56 (16) | 57 (17) | 53 (17) |
| 15 months | 53 (19) | 55 (17) | 52 (18) | 55 (16) | 55 (17) | 52 (18) |
| 18 months | 51 (20) | 53 (18) | 51 (19) | 54 (17) | 54 (18) | 51 (19) |
- DGF = delayed graft function, IGF = immediate graft function.
- Figures in parentheses represent standard deviation.
- p = not significant for all comparisons.
Post-transplant diabetes mellitus
Of 86 patients not diabetic pre-transplantation, 3 developed PTDM within the first year (3.5%). Two patients required insulin, the others needing only oral hypoglycemic agents for adequate diabetic control. Two other patients developed PTDM beyond the first post-transplant year. Mean HbA1c and random clinic glucose levels for those patients not previously diabetic, or rendered diabetic post-transplantation, are shown in Table 4. With the exception of the 5 patients who developed PTDM, no individual displayed a random blood glucose level of 10 mmol/L or greater at any time during follow-up.
| Month of follow-up | 3 | 6 | 9 | 12 | 15 | 18 |
| Number of patients | 100 | 100 | 99 | 99 | 99 | 98 |
| Weight (kg) | 68.5 (11) | 70.2 (11) | 71.3 (12) | 72.0* (12) | 72.2* (12) | 72.3* (12) |
| Systolic blood pressure (mmHg) | 127 (13) | 130 (14) | 124 (13) | 133 (17) | 130 (16) | 129 (16) |
| Diastolic blood pressure (mmHg) | 77 (7) | 80 (8) | 79 (8) | 79 (9) | 77 (8) | 76 (8) |
| Number of anti-hypertensive agents | 1.3 (1.2) | 1.5 (1.3) | 1.5 (1.4) | 1.6 (1.3) | 1.8 (1.6) | 1.6 (1.5) |
| HbA1c** | 5.3 (0.8) | 5.4 (0.7) | 5.2 (0.7) | 5.3 (0.7) | 5.4 (0.8) | 5.4 (0.8) |
| Random blood glucose (mmol/L)** | 5.8 (1.0) | 5.8 (0.9) | 5.7 (1.0) | 5.7 (1.1) | 5.8 (1.1) | 5.8 (1.0) |
| Total serum cholesterol (mmol/L) | 4.3 (1.3) | 4.2 (1.3) | 4.4 (1.2) | 4.3 (1.1) | 4.2 (0.8) | 4.2 (0.9) |
| Percentage of patients on statin therapy | 40 | 48 | 50 | 50 | 52 | 53 |
- *p = 0.03 compared with baseline weight of 69.0 kg.
- **Only in non-diabetic patients.
- Results expressed as mean, with standard deviation in parentheses.
Post-transplant weight gain
Patient weight gains are shown in Table 4. Compared with a baseline mean weight of 69.0 kg, no significant increase in weight was observed until 12 months post-transplantation (weight gain 3.0 kg)
Other cardiovascular risk factors
Mean values for other cardiovascular risk variables are shown in Table 4.
Adverse events
Infection: Three episodes of tissue-invasive cytomegalovirus (CMV) were observed (gastrointestinal in all cases), all occurring after ganciclovir prophylaxis had ceased (days 125, 145 and 167). Two patients had experienced steroid-responsive rejection prior to the episode of CMV; no cases of CMV were observed in the group given anti-CD25 mAb. Four patients, one of whom received anti-CD25 mAb, developed drug-resistant urinary tract infections requiring admission for i.v. antibiotic therapy. Three patients, one of whom received anti-CD25 mAb, developed histologically proven BK polyoma virus nephropathy and were treated with a reduction in immunosuppression and Cidofovir (Vistide®; Pharmacia, Central Milton Keynes, UK). Currently, all grafts continue to function.
Malignancy: One patient was found to have a ductal carcinoma of the breast at 3 months post-transplantation. This was likely to have been present pre-transplantation but not clinically evident at the pre-transplant work-up. This patient had not received anti-CD25 mAb induction. There were no cases of post-transplant lymphoproliferative disease.
Other: Leukopenia (total white cell count <4.0 × 109/L on two successive occasions) was noted in 38 patients (38%), with temporary reduction in MMF dosage needed in 27 patients. No rejection episodes occurred during such dosage reduction. Eight patients developed gastrointestinal symptoms related to MMF treatment, which settled with reduction in daily dosage or dosing interval. No avascular necrosis was seen.
Discussion
Steroid sparing in adult renal transplantation with Tacrolimus and MMF without ALG induction has not been previously reported in detail. Our study shows that this regime is associated with excellent short-term patient and graft survivals (100% and 98%, respectively at 1 year), and a low rejection rate of 19% in the first year. The rejection rate is similar to that seen with Tacrolimus and MMF in steroid-based regimes (26), and has been achieved without recourse to induction ALG and its attendant risks. We also observed a low incidence of PTDM (3.5%) and minimal weight gains (mean: 3 kg) in the first year.
It is likely that with the advent of more potent immunosuppressive agents, the alloimmune response can be controlled without the need for maintenance steroids, hitherto a mainstay of renal transplantation. Using MMF in steroid-sparing protocols may be of particular benefit, as steroid administration reduces levels of Mycophenolic Acid, the active metabolite of MMF (27). Additionally, the rapid attainment of therapeutic Tacrolimus levels (within 72 h in the majority of patients) may allow an ‘umbrella of safety’ under which steroids can be safely discontinued. Of note, we saw no excess of vascular rejection (6/23 episodes), late rejection (6 episodes occurred beyond 90 days) or multiple rejection episodes (2 patients), which are particularly damaging to renal grafts (28,29).
The use of anti-CD25 mAb in patients at higher immunological risk was associated with a reduction in rejection rates at 1 year of over 50% (8% vs. 22% in the ‘standard risk’ patient not receiving induction therapy). Although not reaching statistical significance, it nevertheless suggests these agents may have an important role in high-risk patients. The 8% rejection rate is similar to that seen in the study by Sarwal et al. (22), where a rate of 6% at 1 year was seen when using Daclizumab in pediatric patients, another group at higher risk of rejection. Our current study therefore extends these observations on the use of anti-CD25 mAb to the adult arena. Our protocol for Daclizumab administration (which was the agent used in 16 of the 25 patients [64%] receiving induction) differs from the protocol used in Sarwal et al.'s study, and involved the simplified administration of a dose of 2 mg/kg on just two occasions (total dose 4 mg/kg), rather than an extended administration of a total of 10 mg/kg over 6 months (22). We saw no cases of CMV, no excess of urinary tract infection or polyoma virus nephropathy and no cases of malignancy (in the short-term) in patients given anti-CD25 mAb. The good safety profile of this mode of induction therapy in these studies points to a role for these agents in all patients undergoing steroid-sparing immunosuppression, and questions the need for ALG induction.
Early studies of steroid withdrawal showed a decline in graft function even in the absence of clinically recognized rejection episodes (10), and so it was reassuring to see stable graft function throughout the study duration. Serum creatinine at 6 months post-transplantation greater than 150 μmol/L and a creatinine rise (as little as 10 μmol/L) between 6 and 12 months are associated with graft loss (30,31), and it was therefore encouraging to observe a mean creatinine of 147 μmol/L at 6 months, with only 12 patients experiencing rise in creatinine over 10 μmol/L by 12 months. Furthermore, an elevated serum creatinine at 1 year is predictive of cardiovascular death (32), and so it is reassuring that attempts to limit patient morbidity and mortality with this steroid-sparing regime have not been accompanied by exacerbation of other risk factors for poor patient outcome (i.e., compromised graft function). There was no significant difference in graft function between recipients experiencing and not experiencing rejection episodes, which may be due to the reduction in the severity of rejection in Tacrolimus and MMF-based regimes (25) and a consequent reversal of the rejection episode with treatment (33). It should however be noted that one of the graft losses was due to a repeated rejection episode. It is also encouraging to note that no grafts with DGF were lost during the study and that renal function in patients with and without DGF was equivalent, despite no modification of the immunosuppressive regime such as delayed introduction of Tacrolimus or the use of additional induction agents. This confirms the benefits of Tacrolimus and MMF in DGF (25) and provides evidence for the safety of managing DGF in the fashion described.
Although longer-term follow-up is necessary, in the short-term beneficial effects were seen in terms of risk factor modification by steroid sparing, in particular an incidence of PTDM of only 3.5% at 1 year when defined as the requirement for insulin or oral hypoglycemic agents post-transplantation, a definition, which has been associated with an increase in mortality in the largest studies to date (5). This is far lower than the incidence in most Tacrolimus-based studies using steroids (34). In addition, maximum blood pressure (mean: 133/80) and cholesterol (4.4 mmol/L) were well within suggested guidelines (35), and weight gains were small (3.0 kg in the first year).
Although early reports of steroid-sparing protocols utilized Tacrolimus levels similar to or higher than those in this study (20,21), it is noteworthy that recently reported protocols have targeted lower levels of only 5–7 ng/mL beyond 3 months (22). Although the dosing strategy used in our study has succeeded in controlling rejection, it is likely that lower Tacrolimus levels may be associated with improvements in short- and long-term graft function, better blood pressure and lipid control and a further reduction in post-transplant diabetes.
In summary, renal transplantation with Tacrolimus and MMF is possible without concomitant corticosteroid therapy or ALG induction, is associated with excellent short-term patient and graft survival and a rejection rate comparable to that utilizing a triple-therapy regime. It is hoped the low incidence of PTDM, low weight gains and good blood pressure and lipid control will reduce the long-term cardiovascular morbidity of this high-risk population.




