Beneficial effects of melatonin on nicotine-induced vasculopathy
Abstract
Abstract: Tobacco smoking is responsible for death of many people each year and increases the risk of developing numerous disorders, particularly cardiovascular disease and cancer. Among the components of cigarette smoke, nicotine is known to excert proatherosclerotic, prothrombotic and proangiogenic effects on vascular endothelial cells. The current study was designed to investigate the mechanisms by which nicotine induces endothelial dysfunction and further to examine whether melatonin protects against nicotine-induced vasculopathy. Four groups of male rats (controls, melatonin-treated, nicotine treated [100 μg/mL in drinking water], and nicotine plus melatonin [5 mg/kg/day] treated) were used in this study. After 28 days all the animals were killed by decapitation and the aorta was removed. We evaluated the hydroxyproline content, and the different expression of proteins involved in several types of stress (ERK1/2), in fibrosis (TGF-β1, NF-κB) and in recruitment of circulating leukocytes onto the vessel wall, including intercellular adhesion molecule-1 (ICAM-1) and vascular cellular adhesion molecule-1 (VCAM-1). These metabolic pathways are important in the development of nicotine-induced atherosclerosis and hypertension. Our results show that nicotine induces marked structural and functional alterations in the aorta. Nicotine receptor binding results in activation and phosphorylation of ERK 1/2. This enzyme, in turn, activates both TGF-β1 and NF-κB; they stimulate respectively the synthesis of type I collagen, responsible of fibrosis, and moreover ICAM-1, VCAM-1 and reactive oxygen species. Based on these findings, melatonin is able to minimize the negative effects of nicotine by blocking the activation of ERK and the other signalling pathways in which this enzyme is involved.
Introduction
Cigarette smoking is today one of the most important risk factors for cardiovascular disease and premature death in developed world [1–3]. In fact, it promotes atherosclerosis and is associated with an increased risk of cardiac death, myocardial infarction, angina pectoris, peripheral vascular disease and stroke [4]. Cigarette smoking especially accelerates atherosclerosis in the coronary arteries, the aorta, the carotid and cerebral arteries, and the large arteries in the peripheral circulation [5].
Among the components of cigarette smoke, nicotine is known to excert proatherosclerotic, prothrombotic and proangiogenic effects on vascular endothelial cells, due to excessive lipid metabolism, vascular cell damage, as well as hypertension and thrombosis [6–8]. Several studies have demonstrated that nicotine is responsible for the structural and functional alterations in blood vessels such as impaired endothelium-dependent vasodilation, increased intimal thickening, hyperplasia, atherosclerotic plaques and aneurysmal dilation [9]. Concomitantly, different pro-inflammatory mediators such as proinflammatory cytokines [10], cellular adhesion molecules including intercellular adhesion molecule-1 (ICAM-1), vascular cellular adhesion molecule-1 (VCAM-1) [11] and reactive oxygen species (ROS) [12] are also produced.
The mitogen-activated protein kinases (MAPKs) are a family of evolutionally-conserved molecules playing a critical role in cell signalling and gene expression. The MAPK family includes three major members: extracellular signal regulated kinase (ERK), p38 and c-Jun N terminal kinase (JNK), representing three different signalling cascades. The MAPKs are activated by phosphorylation and transduce a broad range of extracellular stimuli into diverse intracellular responses by both transcriptional and nontranscriptional regulation [13]. Among these survival pathways, ERK1/2 is thought to be the major signaling pathway involved in nicotine-induced cell proliferation, which is also considered a precursor event in tumorigenesis [14]. Moreover, nicotine and tobacco-derived nitrosamines have been shown to exhibit their pathobiologic effects in part by the activation of the nicotinic acetylcholine (ACh) receptors (NAChRs), particularly α7-NAChR subtype, localized at the cell membrane. Nicotine binding to α7NAChR leads to activation of calcium channels, leading to the depolarization of cell membranes and promoting a number of deleterious metabolic pathways [8].
In previous studies, it was demonstrated that the melatonin and its metabolites can detoxify free radicals and their derivatives [15–19]. Beneficial antioxidant effects of melatonin have been recently shown in clinical settings for several chronic diseases, including patients with rheumatoid arthritis or diabetes, elderly patients with primary essential hypertension and females suffering from infertility [20–23]. This suggests the possible role of melatonin as a disease-modifying therapeutic agent in different pathological conditions.
In view of these observations, the current study was designed to clarify the mechanisms by which nicotine induces endothelial dysfunction and further to examine whether melatonin protects against nicotine-induced vasculopathy.
Materials and methods
Animal care and experimental treatments
Fourty male Wistar rats (200–250 g) were housed in a room with constant temperature of 22°C and a 12 hr alternating light–dark cycle. Protocols were approved by the Italian Ministry of Health and complied with ‘Guiding Principles in the Use of Animals in Toxicology’. Rats were divided into four groups (each of 10 animals) and treated for 28 days. Nicotine was orally administrated at the dose of 100 μg/mL in drinking water according to Lindenblatt et al. (2007) (Sigma-Aldrich, Milan, Italy); melatonin was diluted in water at the dose of 5 mg/kg/day (Nathura srl, Reggio Emilia, Italy) and orally (intraesophageal application) administrated. Group I was the control; group II was treated with melatonin; group III was treated with nicotine; group IV was treated both with nicotine and melatonin. After the 28 days all the animals were killed by decapitation and the thoracic aorta was carefully removed. One part of each sample was frozen at −20°C for the measure of hydroxyproline, whereas the other one was fixed in 4% paraformaldehyde and embedded in paraffin wax according to standard procedures for the other analyses.
Histopathological examination
Paraffin-embedded slices were serially sectioned at 5 μm. These sections were deparaffinized, rehydrated and then stained with hematoxylin-eosin, Sirius Red staining and Verhoeff-Van Gieson stain [24]. After Sirius Red staining, collagen fibers were detected by polarized light microscopy (Olympus, Hamburg, Germany); under these conditions, type I collagen fibers appear yellow-red, whereas type III collagen fibers appear green.
Determination of hydroxyproline
The collagen content was estimated by the measure of hydroxyproline according to the method described by Woessner [25]. Extracted samples (10 mg for sample) were hydrolyzed with 6 N HCl (3 mL/10 mg of dry tissue) at 110°C for 24 hr. After the hydrolysis, all the hydrolyzates were evaporated to dryness and the precipitates were dissolved in 3 mL of redistilled water. After neutralization by 1 N NaOH, from the tubes 0.2 mL of sample were taken and diluted to 2 mL of final volume with redistilled water. Hydroxyproline was oxidized to pyrrole by 1.25 mL of 0.05 m chloramine T in a citrate buffer pH 6.0, then shaken for 5 min and incubated for 20 min at 20°C. In order to remove the excess of chloramine T, 1.25 mL of 3.15 m perchloric acid was added. After 5 min the samples were treated with 1 mL of 20% P-dimethylaminobenzaldheyde and incubated at 60°C in a water bath for 20 min. The optical density was measured at 560 nm on a spectrophotometer.
Immunohistochemical localization of ERK1,2 and p-ERK1,2
Sections were deparaffinized, rehydrated and immerse in 3% hydrogen peroxide (H2O2) in methanol for 30 min to quench endogenous peroxidase. All the samples were then incubated in 1% bovine serum albumin (BSA) for 1 hr at room temperature. Sections were incubated with rabbit primary antibodies against ERK1,2 (1:100, code DB 012-1 n,s; DB Biotech, Slovakia) or p-ERK1,2 (1:100, code DB 013-1 n,s; DB Biotech) for 24 hr at 4°C. Sections were washed in tris buffered saline (TBS), then sequentially incubated with specific biotinylated secondary antibodies and avidin-biotin peroxidase complex (code PK6101; Vector Labs, Burlingame, CA, USA). The reaction products of all sections was visualized using hydrogen peroxide and diaminobenzidine (DAB) as chromogen. Finally, the samples were counter-stained with hematoxylin, dehydrated and mounted. The immunohistochemistry control was performed by omitting the primary antibody and in presence of isotype matched IgGs. The immunohistochemical data were evaluated by an observer blinded to the treatments. For quantitative evaluation, staining intensity was measured as integrated optical density (IOD) in five samples for each experimental group. Digitally fixed images of the slices at 40× magnification were analyzed using an optical microscope (Olympus) equipped with a camera and with an image analyzer (Image Pro Plus, Milan, Italy) software working on the computer. The IOD was calculated for arbitrary areas, measuring ten fields with the same area (0.04 mm2/field) for each sample. Data were pooled to represent a mean value and a statistical analysis was applied to compare the results obtained from different experimental groups.
Western blot analysis of TGF-β1 and NF-κB
Tissues were homogenized in a buffer system according to Laemmli [26] and centrifuged at 10,000 g at 4°C for 15 min. Protein concentration was determined on supernatant with BSA serving as standard protein by absorption spectroscopy. Supernatants were analyzed by 10% SDS-PAGE and electro-transferred to a nitro-cellulose membrane (pore size 0.45 μm; BioRad, Hercules, CA, USA) by wet blotting (100 V for 1 hr). The membrane was blocked with 5% nonfat dry milk in Tris-buffered saline Tween-20 (TTBS). After washing with TTBS, proteins were exposed overnight at 4°C to primary antibodies: rabbit TGF-β1 (code sc-146) and mouse NF-κB (code sc-8414) (1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA). These were detected using a biotinylated secondary antibody (goat anti rabbit code E0432 and goat anti mouse code E0433 respectively; Dakopatts, Denmark) as appropriate and an avidin-peroxidase complex according to the manufacturers instructions (ABC kit; Dakopatts), with a solution of 0.05% DAB and 0.03% hydrogen peroxide. Densitometric analysis was performed on all samples using Analytical Imaging Station software (GEL-PRO ANALYZER; Media Cybernetics, Bethesda, MD, USA). TGF-β1 and NF-κB levels are expressed as arbitrary units (AU).
Immunofluorescence localization of VCAM-1 and ICAM-1
Sections were deparaffinized, rehydrated and incubated in 1% BSA for 2 hr at room temperature. Subsequently sections were incubated with primary antibodies against adhesion molecules mouse ICAM-1 (1:100, code 202401; BioLegend, San Diego, CA, USA) and rabbit VCAM-1 (1:100, code sc-8304; Santa Cruz Biotechnology) for 24 hr at 4°C. Samples were washed in phosphate buffered saline (PBS) and labeled using anti-mouse Alexa Fluor 546 (code A11030) and anti-rabbit Alexa Fluor 488 (code A11034) conjugated secondary antibodies (1:200; Invitrogen, UK) respectively for the primary antibodies above reported. Finally, the samples were counter-stained with 4′,6-diamidino-2-phenylindole (DAPI), mounted and observed with a confocal microscope (LSM 510 Zeiss, Germany). The immunofluorescent control was performed by omitting the primary antibody and in presence of isotype matched IgGs.
Statistical analysis
Results are expressed as mean ± S.E.M. Statistical significance of differences among the experimental groups was evaluated by analysis of variance (ANOVA) and Bonferroni test. The P-value <0.05 was considered as significant.
The mean is determined for each animal (n = 10) of each group using n = 6 samples for each animal in different groups.
Results
The morphological and biochemical results were similar in control and melatonin alone treated rats, so they are considered without distinction.
Histological examination of the aorta from nicotine-treated rats revealed marked damage with increased amounts of connective tissue, fibrosis and disorganization of the fibers when compared to control.
By morphological analysis under polarized light microscope (Sirius Red), the collagen fibers of groups treated with nicotine and those of control were, respectively, yellow/red (type I collagen indicating fibrosis) (Fig. 1A) and green (type III collagen, constitutive) (Fig. 1B) stained. The aorta of nicotine + melatonin treated animals was characterized by a small amount of green collagen fibers, similar to control (Fig. 1C).
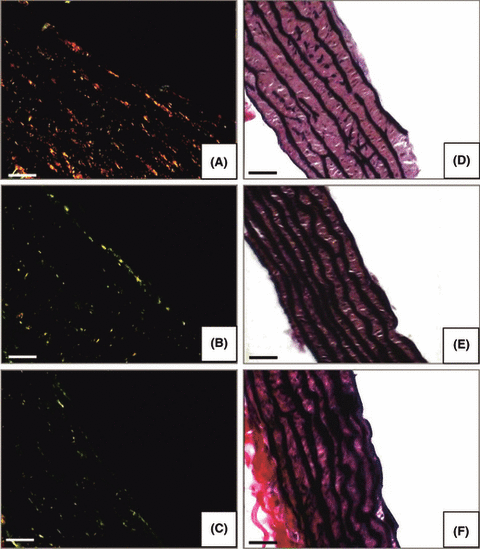
Photomicrographs showing aorta stained by Sirius Red (A,B,C) for collagen fibers and Verhoeff-Van Gieson staining (D,E,F) for elastic fibers. Nicotine (A,D); control (B,E); nicotine plus melatonin (C,F). (Bar = 50 μm).
Verhoeff-Van Gieson staining showed that nicotine treatment reduced the elastic properties of the aorta and the fibers appeared fragmented, discontinuous and visibly thinner compared to thick and regular elastic fibers (black color) of the aorta in control group (Fig. 1D,E). The aorta of animals treated with nicotine + melatonin, instead, had the same morphological pattern of control group (Fig. 1F).
Fig. 2 shows the collagen content of the aorta measured by the quantitative determination of hydroxyproline. Nicotine markedly increased the total collagen level in aorta of treated animals as compared with control, whereas the administration of melatonin in association with nicotine reduced the collagen content to levels of the controls.
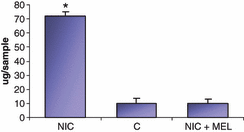
Graphic showing hydroxyproline content in the aorta of nicotine-treated animals (NIC), of control (C) and nicotine plus melatonin (NIC + MEL) groups. For each group (n = 10 animals) it has been used five samples. *P < 0.05 versus control.
The expression of ERK1,2 was significantly decreased in the aorta of nicotine-treated animals (Fig. 3A), whereas its phosphorylated form, p-ERK, increased in comparison with controls (Fig. 3B). Melatonin co-administration restored the expression of both enzymes to the levels in the control group (Fig. 3A,B).
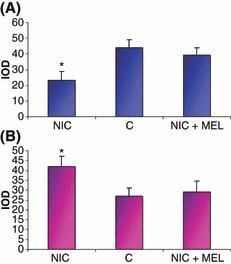
Quantitative analysis (IOD) of extracellular signal regulated kinase (ERK) (A) and p-ERK (B) immunostaining in the aorta of nicotine-treated animals (NIC), of control (C) and nicotine plus melatonin (NIC + MEL) groups *P < 0.05 versus control.
TGF-β1 immunoblotting showed that nicotine administration induced high levels of the protein, whereas basal levels were seen in control and in nicotine + melatonin-treated rats (Fig. 4A). The same analysis for NF-κB revealed a significant increase in the aorta of nicotine treated animals compared to control and to nicotine + melatonin groups (Fig. 4B). The densitometric reading of TGF-β1 and NF-κB expression is shown as histogram in Fig. 4C.
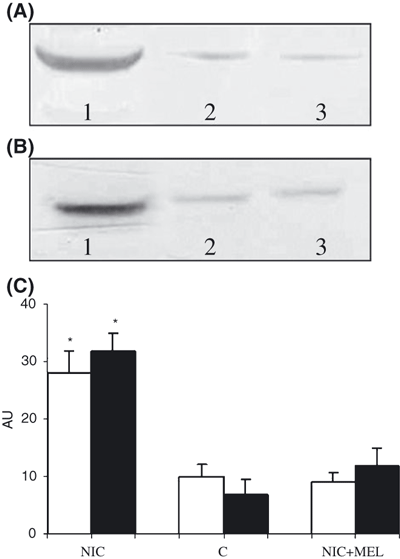
Western blot analysis of TGF-β1 (A) and NF-κB (B) in the aorta of nicotine-treated animals (NIC) (lane 1), of control (C) (lane 2) and nicotine plus melatonin (NIC + MEL) (lane 3) groups. (C) Histogram representing densitometric readings of TGF-β1 (white) and NF-κB (black) expressed as AU. *P < 0.05 versus control.
Immunofluorescence analysis revealed that nicotine induced the expression of both ICAM-1 and VCAM-1 in the aorta of treated animals. As depicted in Fig. 5, the red positivity was linked to ICAM presence, whereas the green positivity showed VCAM expression. Immunofluorescence for ICAM-1 and VCAM-1 was positive on endothelial cells (Fig. 5A). Slides from control and from nicotine + melatonin groups did not exhibit such positivity (Fig. 5B,C).
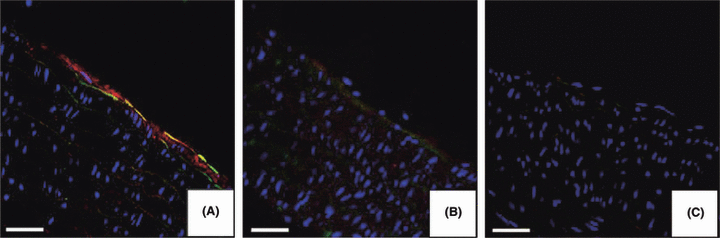
Immunofluorescence images of adhesion molecules intercellular adhesion molecule-1 (ICAM-1) (red) and vascular cellular adhesion molecule-1 (VCAM-1) (green) expression on the surface of endothelial cells in nicotine-treated animals (A), of control (B) and nicotine + melatonin (C) groups. Nuclei were stained with DAPI (blue). (Bar = 50 μm).
Discussion
Our results show that nicotine induces marked structural and functional alterations in the aorta. These data are consistent with those presented in previous papers demonstrating nicotine-induced damage in the tunica intima and tunica media. The morphological injuries were characterized by cell swelling, cytoplasmic vacuolisation and irregularity of the vessel luminal surface [5]. We found that administration of nicotine for 28 days is associated with important changes in cytoarchitecture of aorta including injury of endothelium, apparent loss of elastic properties, increase in the amount of connective tissue and fibrosis. Moreover, nicotine treatment increased hydroxyproline concentration and type I collagen; this may relate to a significant upregulation of collagen mRNA expression as demonstrated by Sekhon et al. [27] in fetal monkey lung. Consequently, the alteration of elastin/collagen ratio in the arterial wall increases the wall thickness, causes loss of elasticity and plays an important role in atherogenesis and hypertension as demonstrated in many papers [28, 29].
In regard to the functional alterations induced by nicotine treatment, we studied different proteins involved in several types of stress (ERK1/2), in fibrosis (TGF-β1, NF-κB) and in recruitment of circulating leukocytes onto the vessel wall, including ICAM-1 and VCAM-1. These metabolic pathways are important in the development of nicotine-induced atherosclerosis and hypertension [30–32].
We examined the expression of ERK1/2 and its activated form p-ERK1/2; a significant activation of ERK was apparent in nicotine-treated animals. This could be explained in light of its important role, as serine/threonine protein kinase, in a variety of extracellular stimuli [33]. Accumulated evidence indicates an involvement of this protein as a result of nicotine treatment [34–36]. In particular, a recent study carried out from Chowdhury et al. [35] suggested that acute or chronic exposure to nicotine via cigarette smoking resulted in a substained activation of ERK1/2 in isolated pancreatic acinar cells. Although ERK1/2 activation by nicotine was completely inhibited with specific kinase inhibitor, the inhibitor had no influence on the basal and stimulated secretory response induced by nicotine. Together, these results suggest that nicotine-induced proliferation of primary cells was correlated with ERK1/2 activation, which intimated that ERK1/2 pathway was responsible for the stimulation of primary cell proliferation, as also observed in AR42J cells [37].
We also evaluated the potential role of ERK1/2 protein, after nicotine treatment, in the regulation of type I collagen promoter activity (TGF-β1) via profibrogenic stimuli. Its involvement was apparent since we observed an increase in type I collagen and in TGF-β1 in the aorta of nicotine-treated animals. In line with this, Bhogal and Bona [32] showed that collagen synthesis is upregulated by profibrogenic growth factors and cytokines such as TGF-β1, IL-4 and IL-13 binding to their corresponding cell membrane receptors to fibroblasts. Thus, the ERK pathway is an important MAPK signalling route that is involved in regulating cell function.
In light of these observations, we considered ERK1/2 involvement in the stimulation of different proteins, including ICAM-1 and VCAM-1, which precede monocyte adhesion to the endothelium and the subendothelial accumulation of macrophages [38]. Our data indicate that ICAM and VCAM proteins were induced by the aorta endothelium of the nicotine-treated animals. These proteins belong to the Ig superfamily and their increase has been related to subsequent death from cardiovascular causes, suggesting a role of these molecules in the initiation and progression of atherosclerosis [39]. The mechanisms through which nicotine enhances the expression of the adhesion molecules have not been well characterized. Some authors have documented the involvement of MAPK signalling in nicotine-mediated adhesion molecule synthesis. One of the most important MAPK cascades is represented by ERK pathway and the activation of ERK is associated with nicotine treatment and adhesion molecule elevation mediated by NF-κB [32]. Other studies indicate that ERK might be primarily involved in the VCAM-1 overexpression induced by TNF-α and it might partially mediate the TNF-α-induced ICAM-1 expression [40]. The increase of the ERK and NF-κB expression in the aorta of nicotine-treated rats supports their possible role in the mechanism of nicotine-induced adhesion molecule expression. Fig. 6 summarizes the putative mechanism by which nicotine may induce endothelium dysfunction that serves as a nidus for atheroma formation.
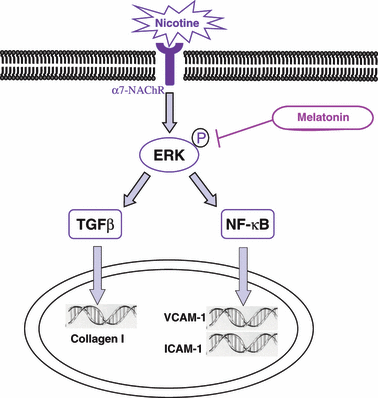
Hypothetical pathways involved in nicotine-induced endothelial dysfunction and in melatonin vascular protection. Nicotine receptor binding (may be α7-nicotinic acetylcholine receptor [NAChR]) results in activation of extracellular signal regulated kinase (ERK)1,2 with its phosphorylation. This enzyme, in turn, activates both TGF-β1 and NF-κB; they translocate into the nucleus where they stimulate respectively the synthesis of type I collagen responsible of fibrosis and intercellular adhesion molecule-1/vascular cellular adhesion molecule-1 (ICAM-1/VCAM-1) transcription. This latter promotes cell surface expression of the adhesion molecules. According to our data, melatonin can be able to minimize the negative effects of nicotine blocking the activation of ERK and the other signalling pathways in which this enzyme is involved.
The other objective of this study was to investigate the beneficial effects of melatonin in counteracting nicotine-induced endothelial damage and examine a potential biochemical pathway in which this indoleamine mediates its protective action. Melatonin was discovered to be a free radical scavenger in 1993 [41] with the indoleamine exhibiting high scavenging activity [42]. Our results show that melatonin treatment reduces: (i) the degree of histological aorta damage, (ii) hydroxyproline content, (iii) p-ERK1/2 expression, and (iv) proinflammatory protein production. The protective effect of melatonin largely accounted for the inhibition of ERK1/2 expression was demonstrated by Esposito et al. [13] during treatment of spinal cord injury in mice. In fact, these authors proposed that melatonin’s ability to reduce spinal cord injury is also related to a reduction in the MAPK signalling pathway. In this regard, it has been documented that the activation of ERK after nicotine treatment, as previously reported, which induces upregulation of TGF-β1 and adhesion molecule elevation mediated by NF-κB protein, is downregulated by melatonin administration. In this action, melatonin prevents the translocation of NF-κB to the nucleus and its binding to DNA [43–45], thereby reducing the upregulation of a variety of proinflammatory cytokines and inhibiting the production of adhesion molecules that promote the sticking of leukocytes to endothelial cells. By this means, melatonin attenuates transendothelial cell migration and edema which contribute to tissue damage [43].
It has been also proposed that melatonin is able to inhibit the activation of NF-κB which in turn induces the inhibition of iNOS and COX2 expression [44, 45]. It is important to remember that the activation of NF-κB is a pivotal event in proinflammatory signal transduction. In fact, it has been shown that NF-κB activation is increased in rats with colitis. This increase regulates transcriptional activity by binding to specific DNA sequences in promoter/enhancer regions of inflammation genes. Genes regulated by NF-κB include those encoding IL-2, IL-6, IL-8, VCAM-1, ICAM-1 and so forth [46]. The same Authors indicated that melatonin regulates proinflammatory cytokines through inhibition of NF-κB. So, the ability of melatonin to inhibit NF-κB could be double: (i) melatonin can inhibit ERK and then NF-κB according our present results, and (ii) melatonin directly can inhibit NF-κB. We think that the action of melatonin against nicotine toxicity is higher since it is able to stop the negative pathway at the beginning through ERK inhibition, which in turn does not induce NF-κB activation. Therefore, the final proposal mechanism by which melatonin protects against nicotine damage in the aorta is depicted in Fig. 6.
In conclusion, the results of these studies extend our knowledge of the mechanisms by which nicotine causes endothelial dysfunction and pro-atherosclerotic changes in the aorta, and provides a possible pathway by which melatonin exerts its protective effects.
Acknowledgements
Sincerely thanks to Nathura srl (Reggio Emilia, Italy) for courteously providing melatonin tablets and to Franchini Acciai S. p. A. for financial support. Thanks also to Miss Stefania Castrezzati for technical support.




