Melatonin inhibits human fibroblast-like synoviocyte proliferation via extracellular signal-regulated protein kinase/P21CIP1/P27KIP1 pathways
Abstract
Abstract: The excessive proliferation and migration of synoviocytes are well-characterized phenomena that play key roles in the pathophysiology of rheumatoid arthritis (RA). Melatonin has been shown to have potent anti-proliferative effect in various cancer cells such as breast and prostate cancer cells. In this study, we examined the role of melatonin on synoviocyte proliferation in primary cultured human fibroblast-like synoviocytes (FLSs) by analyzing protein expression of P21CIP1 (P21) and P27KIP1 (P27), the cyclin-dependent kinase inhibitors that are important in cell cycle control, and the phosphorylation of mitogen-activated protein kinases (MAPKs). RA-FLS proliferation was determined by a [3H]-thymidine incorporation assay. Western blot analysis was applied to examine the underlying mechanisms of melatonin’s effect. Melatonin inhibited RA-FLS proliferation in a dose-dependent manner. It reduced proliferation of passage 2 FLSs by 25% at 10 μm and by nearly 40% at 100 μm concentrations. The inhibitory effect of melatonin on RA-FLS proliferation was also observed in passages 4 and 6. Melatonin upregulated the expression levels of P21 and P27 dose-dependently (24 hr), induced the phosphorylation of extracellular signal-regulated protein kinase (ERK) time-dependently (10 μm), but did not affect phosphorylation of P38 in RA-FLSs. In addition, the expression of P21 and P27 triggered by melatonin was inhibited by the pretreatment of the ERK inhibitor, PD98059 (10 μm). The anti-proliferative action of melatonin in RA-FLSs was also blocked by PD98059. Taken together, these results suggest that melatonin exerts the inhibitory effect of the proliferation of RA-FLSs through the activation of P21 and P27 mediated by ERK. Hence we suggest that melatonin could be used as a therapeutic agent for the treatment of RA.
Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disease characterized by inflammatory cell infiltration, synovial tissue hyperplasia, and a progressive destruction of cartilage and bone [1]. Growing evidence suggests that, in addition to macrophages and T cells, activated fibroblast-like synoviocytes (FLSs) described as transformed or tumor-like in appearance, or simply activated, play a major role in both initiating and driving RA [2]. Particularly, on the basis of the well-known phenomenon that RA-FLSs can grow very fast over multiple passages in vitro, it is presumed that the massive synovial hyperplasia characteristic for RA is due to the proliferation of RA-FLSs [3–5]. Indeed, key regulators of cellular proliferation such as the proto-oncogene c-myc, are overexpressed in RA [4]. In addition, it was shown that gene transfer with the p16INK4a senescence gene not only inhibited the growth of RA-FLSs in vitro, but also ameliorated the course of rat adjuvant arthritis [6]. Therefore, the control of proliferation of RA-FLSs is one of the therapeutic strategies for the treatment of RA. Indeed, the histone deacetylase inhibitor, trichostatin A (TSA) was recently identified as a potential anti-arthritic agent by a mechanism inhibiting the proliferation of RA-FLSs, inducing the expression of P21CIP (P21) and P16INK4a [7, 8].
Melatonin (N-acetyl-5-methoxytryptamine) is synthesized by the pineal gland and is best known for its role as a transmitter of information about diurnal and seasonal time, favoring adaptation to environmental situations [9]. In addition to this classical role, melatonin exhibits remarkable functional versatility such as anti-aging effects [10], anti-inflammatory effect [11–13], immunomodulatory action [14, 15], and anti-oxidant activity as a free-radical scavenger [16, 17]. Melatonin also has anti-proliferative effects on cancer cells [18–21]. In human prostate cancer cells, 22Rv1, the anti-proliferative effect of melatonin was exerted on P27KIP1 (P27) gene transcription and protein upregulation mediated through stimulation of PKA and/or PKC [20]. In human breast cancer cells, MCF-7, melatonin reduces the cell proliferation by modulating cell-cycle length through the control of the P53-P21 pathway [21]. Additionally, Kim et al. reported that melatonin, in high concentrations, via modulation of melatonin receptors/ERK/PI3K/AKT/PKC/NF-κB pathways, reduced human umbilical vein endothelial cell (HUVECs) proliferation [22].
Considering these actions of melatonin, we expected that melatonin may suppress the proliferation of RA-FLSs, which normally leads to synovial hyperplasia. Hence we investigated the effect of melatonin on the proliferation of primary cultured human RA-FLSs, by analyzing the changes in the protein levels of key modulators of cell cycle, P21 and P27, and the phosphorylation of mitogen-activated protein kinases (MAPKs; ERK and P38).
Materials and methods
Isolation and culture of human RA-FLS
Synovial tissue samples were obtained at the time of total knee joint replacement from 15 patients with longstanding RA (mean age, 65.0 ± 21.3 years; mean disease duration, ≥10 years). RA patients had active disease, as evidenced by pauciarticular joint involvement (≥3 tender and/or swollen joints) and an increase in acute-phase reactants (mean serum C-reactive protein level, 21 ± 16 mg/L). Eleven patients were positive for rheumatoid factor IgM. At the time of surgery, patients presented with symptomatic disease requiring treatment with nonsteroidal anti-inflammatory drugs or selective COX-2 inhibitors. Patients who had received intra-articular steroid injections were excluded. Synovial tissue samples, collected in sterile phosphate-buffered saline (PBS), were chopped and digested with collagenase for 2 hr at 37°C. After removing tissue debris by filtering through a 70-μm cell strainer (BD Falcon™, Bedford, MA, USA), cells were resuspended in growth media containing DMEM plus 4.5 g/L glucose, 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% fetal bovine serum (FBS) in a humidified, 37°C incubator with ambient oxygen and 5% CO2. Cells were maintained under subconfluent conditions and used between passages 2 and 6.
Cell proliferation assay
The effect of melatonin on proliferation of RA-FLSs was determined by [3H]-thymidine incorporation as previously described [22]. Briefly, RA-FLSs of passage 2, 4, and 6 were seeded at the rate of 2 × 104 cells/well in 24-well culture plates. The medium was then replaced with serum-free medium, and the cells were incubated for 24 hr. The medium was then replaced with serum-free medium containing 10 and 100 μm melatonin, and the cells were incubated for 24 hr. After incubation, 2 μCi/mL of [3H]-thymidine was added to the medium for 4 hr. The reactions were terminated by aspirating the medium, and the cultures were sequentially washed on ice with PBS containing 10% trichloroacetic acid and ethanol/ether (1:1, v/v). Acid-insoluble [3H]-thymidine was extracted into 500 μL of 0.5 m NaOH/well, and this solution was mixed with 3 mL of scintillation cocktail (Packard Bioscience, Meriden, CT, USA) and quantified using a liquid scintillation counter (Beckman, Düsseldorf, Germany).
Western blot analysis
Cells were lysed in radioimmunoprecipitation assay buffer containing protease inhibitors and centrifuged at 12,000 g at 4°C for 15 min. The protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Equal amounts of protein (50 μg) was separated on sodium dodecyl sulfate (SDS)-polyacrylamide gels and transferred onto a nitrocellulose membrane (Schleicher & Schuell, Postfach, Germany). After blocking with 5% skim milk, membranes were probed with anti-P21, anti-P27, anti-phospho-ERK, and anti-ACTB (Cell Signaling Technology, Beverly, MA, USA) overnight at 4°C. Peroxidase-conjugate anti-mouse or anti-rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used for enhanced chemiluminescence (ECL) detection (Amersham Pharmacia Biotech, Arlington Heights, IL, USA).
Statistical analysis
Results are expressed as mean ± standard error of the mean (S.E.M.). The data were analyzed by one-way ANOVA, followed by Dunnett’s post hoc analysis, using the SPSS software (release 13.0; SPSS Inc., Chicago, IL, USA). Differences were considered statistically significant at P < 0.05.
Results
Melatonin significantly inhibited the proliferation of RA-FLSs at concentrations of 10 and 100 μm (Fig. 1). As shown in Fig. 1A, melatonin reduced [3H]-thymidine incorporations of passage 2 RA-FLSs by 25% at 10 μm and by nearly 40% at 100 μm concentrations, compared with control. The inhibitory effect of melatonin on proliferation of RA-FLSs was also observed in passages 4 and 6 RA-FLSs (Fig. 1B, C).
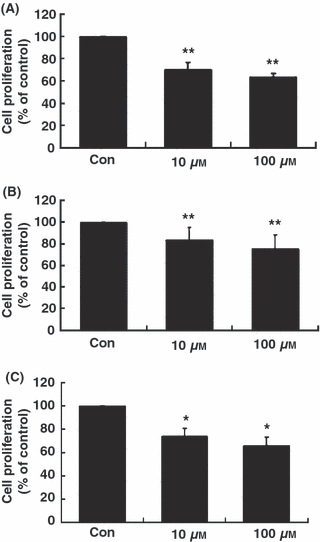
Anti-proliferative effects of melatonin in primary cultured human fibroblast-like synoviocytes (FLSs). RA-FLSs (2 × 104 cells/well in 24-well culture plates) of passage 2 (A), passage 4 (B) and passage 6 (C) were incubated for 24 hr with melatonin at concentrations of 0 (control), 10, and 100 μm. Cell proliferation was evaluated using [3H]-thymidine incorporation assay. Data are as means ± S.E.M. of three independent experiments. Con, control (no treatment). *P < 0.05 and **P < 0.01 compared with the control (Con).
To determine the mechanism of the anti-proliferative effects of melatonin, we examined the ability of melatonin to alter protein expression of P21 and P27 in RA-FLSs. Melatonin (24 hr) increased protein expression of P21 and P27 in a dose-dependent manner (Fig. 2A). The expression of P21 was gradually upregulated at a concentration of more than 1 μm, and that of P27 was increased dose-dependently from a concentration of 0.01 μm. We next investigated whether melatonin induced the phosphorylation of MAPKs (ERK and P38). The effect of melatonin (10 μm) on the phosphorylation of MAPKs was measured up to 6 hr. Melatonin induced the phosphorylation of ERK with peak expression observed at 0.2 hr (Fig. 2B), but did not affect that of P38 (data not shown).
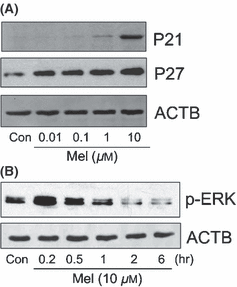
Effect of melatonin on P21CIP1 (P21), P27KIP1 (P27) and extracellular signal-regulated protein kinase (ERK) in rheumatoid arthritis (RA)-fibroblast-like synoviocytes (FLSs). To determine the expression of P21 and P27, RA-FLSs were treated with melatonin at concentrations of 0, 0.01, 0.1, 1, and 10 μm for 24 hr (A). To detect the phosphorylation of ERK, RA-FLSs were treated with or without melatonin (10 μm) for the indicated times (0.2, 0.5, 1, 2 or 6 hr) (B). The effect of melatonin on P21, P27 and ERK were assessed by western blot analysis. ACTB was detected as an internal control. Three independent experiments were performed. Con, control (no treatment); Mel, melatonin.
To examine whether the activation of ERK plays a role in mediating melatonin-induced inhibition of proliferation of RA-FLSs, we used the ERK inhibitor, PD98059. PD98059 was pretreated at a concentration of 10 μm, 1 hr prior to melatonin treatment. In the presence of PD98059, the phosphorylation of ERK triggered by melatonin is completely abrogated in a time-dependent fashion (data not shown). The effect of PD98059 on the melatonin-induced expressions of P21 and P27 was detected after 24 hr of melatonin treatment (100 μm). As shown in Fig. 3, the melatonin-induced protein levels of P21 and P27 were blocked by pretreatment of PD98059. The inhibition of [3H]-thymidine incorporations observed in melatonin-treated RA-FLSs was also blocked by the pretreatment of PD98059. Melatonin (100 μm) inhibited the proliferation of RA-FLSs passage 2, whereas the pretreatment of PD98059 (10 μm, 1 hr prior to melatonin) prevented the inhibition of proliferation by melatonin (Fig. 4).
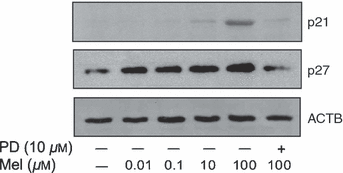
Effects of extracellular signal-regulated protein kinase (ERK) inhibitor, PD98059, on melatonin-triggered expressions of P21CIP1 (P21), and P27KIP1 (P27) in rheumatoid arthritis (RA)-fibroblast like synoviocytes (FLSs). RA-FLSs pretreated for 1 with PD98059 (10 μm) were treated with melatonin (100 μm) for 24 hr. The effect of PD98059 on P21, and P27 in melatonin-treated RA-FLSs were assessed by western blot analysis. ACTB was detected as an internal control. Independent experiment was performed three times. PD, PD98059; Mel, melatonin.
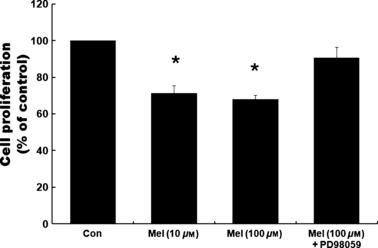
Effects of extracellular signal-regulated protein kinase (ERK) inhibitor, PD98059, on melatonin-induced anti-proliferation in rheumatoid arthritis (RA)-fibroblast-like synoviocytes (FLSs). FLSs (2 × 104 cells/well in 24-well culture plates) of passage 2 (A), passage 4 (B) and passage 6 (C) pretreated for 1 with PD98059 (10 μm) were treated with or without melatonin (10 and 100 μm) for 24 hr. Cell proliferation was evaluated using [3H]-thymidine incorporation assay. Data are as mean ± S.E.M. of three independent experiments. Con, control (no treatment). *P < 0.05 and **P < 0.01 compared with the control (Con).
Discussion
The central role of RA-FLS in regulating inflammation and joint destruction is becoming clearer in the pathogenesis of RA [23, 24]. RA-FLSs are key effectors in the destruction of the connective tissue of cartilage and tendon [2], and once they arrive in the bone, they activate osteoclasts to enhance bone erosion [25]. Identification of candidate chemicals that can inhibit the invasion of RA-FLSs through cartilage and into bone may contribute to the improvement of RA. Considering these roles of RA-FLSs on the pathogenesis of RA, melatonin may be a potent candidate chemical for treatment of RA; it has been shown not only to have significant anti-inflammatory effects [11–13], but also anti-proliferative effects on various cancer cells [18–21]. Indeed, in our study, melatonin inhibited the proliferation, reducing the incorporation of [3H]-thymidine in melatonin-treated RA-FLSs. We examined the role of melatonin on synoviocyte proliferation using primary cultured human RA-FLSs, by analyzing the changes in the levels of P21/P27 proteins and the phosphorylation of MAPKs (ERK and P38).
Cyclin-dependent kinase inhibitors (CKIs), P21 and P27, which belong to the CIP1/KIP1 family, inhibit all cyclin/CDK complexes and have been found to be associated with the arrest in the growth of both normal and malignant cells [26, 27]. Additionally, MAPK signaling has been shown to induce growth arrest through P21 and P27 [28–31]. Particularly, the ERK and P38 pathways have recently been reported to cooperate to cause sustained G1 cell cycle arrest requiring P21 expression [31]. In our study, melatonin (24 hr) increased the expressions of P21 and P27 dose-dependently in RA-FLS. In addition, it induced the phosphorylation of ERK with peak expression observed at 0.2 hr (10 μm), but did not affect that of P38 (data not shown). These results indicate that melatonin inhibited the proliferation of RA-FLS through the upregulation of P21 and P27, and the phosphorylation of ERK.
Exploring further, the ERK-specific inhibitor, PD98059, was employed in an attempt to determine the signaling pathway involved in the melatonin-induced expressions of P21 and P27 in RA-FLSs. Interestingly, the protein expressions of P21 and P27 triggered by melatonin were abrogated by the inhibition of ERK. The inhibition of proliferation of RA-FLSs by melatonin was also blocked by PD98059 pretreatment. These results suggest that ERK plays a critical role in mediating melatonin-induced inhibition of proliferation of RA-FLSs through the activation of P21 and P27.
Several investigators, however, discourage the use of melatonin in the treatment of RA. Maestroni et al. [32] opposed using melatonin in RA patients, because there are receptors for melatonin on synovial macrophages [33, 34] which promote the release of some T-helper-1 (Th-1)-type pro-inflammatory cytokines such as interleukin (IL)-12 [35]. Indeed, it has been pointed out that the higher blood concentrations of melatonin in RA patients, especially in the early mornings, may help to explain the morning stiffness and joint swelling experienced by patients [36, 37]. In contrast, Forrest et al. [38] reported that melatonin showed beneficial effects in a clinical study in which it was used (10 mg daily for 6 months) as an add-on therapy for treating RA. Although pro-inflammatory actions such as increases in erythrocyte sedimentation rate (ESR) and neopterin were evident, no significant differences between placebo and melatonin groups either in the assessments of patients’ symptoms or in the concentrations of the proinflammatory cytokines, IL-1b, IL-6 or tumor necrosis factor (TNF)-α, were seen [38]. Additionally, a daily dose of 10 mg melatonin showed a slowly developing antioxidant profile in patients with RA. Recently, Korkmaz [39] also suggested that the increased blood melatonin levels may be a compensatory response to the inflammation of RA, and it should be an effective add-on therapy in the treatment of RA.
Further studies of the molecular mechanisms mediating the anti-arthritic activity of melatonin will be needed on both its anti-inflammatory and anti-proliferative effect in RA-FLS and/or other cells related with the pathology of RA. This study showed that melatonin exerts the inhibitory effect of the proliferation of RA-FLSs through the activation of ERK/P21/P27 pathways. The synovial tissue hyperplasia directly invades the adjacent tissue like bone and cartilage, and induce pro-inflammatory cytokine like TNF-α, IL-1β, and IL-6 [1, 2]. Therefore, the inhibition of synovial proliferation is an important therapeutic modality in the treatment of RA. Hence we suggest that the anti-proliferative effect of melatonin could be useful for the treatment of RA.
Acknowledgment
This work was supported by the KOSEF grant funded by the Korea government (MEST) (R11-2008-036-01001-0).




