Evidence of immune system melatonin production by two pineal melatonin deficient mice, C57BL/6 and Swiss strains
Abstract
Abstract: We evaluated two pineal melatonin deficient mice described in the literature, i.e., C57BL/6 and Swiss mice, as animal models for studying the immunomodulatory action of melatonin. Plasma melatonin levels in C57BL/6 and Swiss strains were detectable, but lower than levels in control C3H/HENHSD mice. Since these strains are suppose to be pineal melatonin deficient an extrapineal melatonin synthesis may contribute to plasma levels. Regarding cells and tissues from the immune system, all of them were found to synthesize melatonin although at low levels. N-acetyltransferase (AANAT) mRNA was also amplified in order to analyze the alternative splicing between exons 3–4 described for pineal C57BL/6 mice which generates an inclusion of a pseudoexon of 102 bp. For the pineal gland, both the wild type and the mutant isoforms were present in all mice strains although in different proportions. We observed a predominant wild type AANAT mature RNA in thymus, spleen and bone marrow cells. Peripheral blood mononuclear cells (PBMC) culture shown an evident AANAT amplification in all strains studied. Although the bands detected were less intense in melatonin deficient mice, the amplification almost reached the control cell intensity after stimulation with phytohemaglutinin (PHA). In summary, melatonin detection and AANAT mRNA expression in inbred and outbred mice clearly indicate that different cells and tissues from the immune system are able to synthesize melatonin. Thus, the pineal defect seems not to be generalized to all tissues, suggesting that other cells may compensate the low pineal melatonin production contributing to the measurable plasma melatonin level.
Introduction
Melatonin is produced in the pineal gland by N-acetylation of serotonin by N-acetyltransferase (AANAT) and by O-methylation by hydroxyindole-O-methyltransferase (HIOMT). Circulating melatonin levels increase at night in essentially all vertebrates due to an elevation in melatonin synthesis in the pineal gland [1]. This elevated production reflects a large increase in the activity of the AANAT that has been considered the rate-limiting in melatonin synthesis. However, there are situations in which AANAT activity does not correlate with melatonin production [2]. In contrast to the most vertebrates, it has been reported that many strains of inbred mice appear to produce very little or no melatonin [3–5]. This melatonin defect may be mediated by a defective AANAT enzyme due to a point mutation in the enzyme sequence in C57BL/6 mouse strain [6]. This group described an altered splicing with the result of an inclusion of a 102 base pair pseudo-exon between exons three and four generating a stop codon in the mature mRNA. Moreover, two additional isoforms has been identified in this mouse strain, a 89 base pair insertion between exons one and two, and a 62 base pair deletion from exons three and four [7].
Pineal melatonin is involved in the regulation of circadian rhythms and seasonal changes [8], also shows antioxidant [9–11], oncostatic [12], and antiaging [13] properties. Moreover, this neurohormone has been shown to play a fundamental role in neuroimmunomodulation [14, 15]. In vivo studies show that melatonin exerts immunoenhancing properties as well regulates thymic peptides production in rat [16–18]. In vitro studies performed with human immune cells show that melatonin acts by activating cytokine production in T-cell and monocytes, increasing IL-2, IL-1, IL-6, tumor necrosis factor (TNF), reactive oxygen species (ROS), and nitric oxide (NO) levels [14, 19, 20], and enhances monocytes IL-12 production driving to a Th1 cell phenotype [21].
Although melatonin was considered exclusive to the pineal gland, melatonin extrapineal sources have been reported including the retina, Harderian gland [22] and enterochromaffin cells [23]. Regarding the immune system, melatonin has been localized in thymus and in mast cells, natural killer (NK) cells, eosinophilic leukocytes, platelets, and endothelial cells [24]. In addition, high concentrations of the neurohormone and the presence of the enzymatic machinery involved in its synthesis have been described in human, mouse, and rat bone marrow [25, 26] and rat and human thymus [27]. Besides, cultured human lymphocytes has been reported to synthesize and release large amounts of melatonin [28] that is biologically active involving both membrane and nuclear receptors [29].
Multiple sites of endogenous melatonin production may make difficult to determine the actions of melatonin from a specific group of cells. Thus, some in vitro studies have been controversial due to contradictory results that could be explained, at least in part, by endogenous melatonin production by cultured cells [28, 29]. The use of pineal melatonin deficient mice may be a good model given the low pineal melatonin synthesis detected in these mice. As a consequence, the objective of this study was to determine melatonin production in two deficient melatonin strains, one imbred, C57BL/6, and another outbred, Swiss mice, in several cells and tissues of the immune system. With this aim, we studied the melatonin content and different isoforms of mature RNA expression for the AANAT [6, 7]. The two isoforms, in variable proportions, were observed not only in pineal but also in the tissues and cells studied. Surprisingly, although pineal melatonin content in these two deficient mice strains was very low compared with the control group (C3H/HENHSD mice strain), differences were less evident when plasma melatonin levels was estimated, suggesting an extrapineal melatonin synthesis that might compensate for the pineal deficiency.
Materials and methods
Animals and tissues samples
Tissues from two melatonin deficient mice strains, C57BL/6J and Swiss, and a control strain, C3H/HENHSD, were obtained. All mice strains were purchased by Harlan Ibérica, S.L (Barcelona, Spain) and housed under controlled photoperiods (12 hr light: 12 hr darkness; lights were turned off daily from 20:00 to 08:00 hr) and maintained in our animal facilities with food and water ad libitum for 1 wk. Animals were sacrificed by decapitation between 10:00–14:00 hr and blood samples, thymuses, bone marrow cells, spleens and pineals were obtained. Blood samples were either centrifuged for 20 min at 1500× g and collected sera were frozen at −20°C until melatonin determination, or used for peripheral blood mononuclear cells (PBMC) isolation. Bone marrow cells were centrifuged 10 min at 1500 rpm and frozen until determinations. Thymus, spleen and pineal samples were quickly removed and frozen on solid CO2 and stored at −80°C until their use for RNA extraction or melatonin determination. The experiments were conducted in accordance with the Spanish Government Guide and the European Community Guide for Animal Care.
PBMC isolation and culture
Peripheral blood mononuclear cells were obtained by centrifugation over 1.077 g/mL Ficoll-Hypaque gradient (Seromed Biochrom KG, Berlin, Germany) [30]. Cells were washed in saline and finally resuspended in RPMI 1640 medium and cultured (2 × 106 cells/mL) in 12-well flat-bottom culture plates in medium supplemented with 25 mm HEPES, 10% fetal calf serum (FCS), 2 mm L-glutamine, 100 U/mL penicilin, and 100 μg/mL streptomycin (Sigma-Aldrich, Dorset, UK). During culture, cells were stimulated or not with 8 μg/mL of phytohemagglutinin (PHA) for 48 hr. Cell viability was determined by trypan blue exclusion test. After incubation at 37°C in 5% CO2 humidified atmosphere, cell free culture supernatants were collected, filtered, and stored at −20°C for melatonin determinations. PBMC precipitated were frozen at −80°C, until enzyme activity determination and RNA extraction.
RNA extraction and first-strand cDNA synthesis
Total RNA was extracted from the tissues samples by a modification of Chomczynski and Sacchi′s method [31], using TriPure Isolation Reagent (Roche, Mannheim, Germany) as denaturing solution and appropriate chloroform volume. After cell lysis and RNA extraction, RNA was precipitated with isopropanol, and the pellet was washed in 75% ethanol. The RNA samples were recovered by centrifugation at 7500× g for 5 min and dried. Then, each RNA pellet was raised in 50 μL of RNAse-free water and quantified spectrophotometrically at 260 nm. Each sample was treated with of DNAsa I (Roche) at 37°C during 30 min (2 U/10 μg RNA) in a final volume of 60 μL. Thereafter, 1 mL of TriPure Isolation Reagent was added and the whole protocol was repeated.
RNA from 2 × 106 PBMC/mL was extracted by a commercial kit: High Pure RNA Isolation Kit (Roche) following product instruction. RNA was eluted in 50 μL of elution buffer and quantified spectrophotometrically at 260 nm. The final volume of 45 μL RNA was evaporated (Gyrovap, Howe and Co, UK) and resuspended in 20 μL final volume before retrotranscription.
Reverse transcription
1 to 3 μg of RNA (tissues or PBMC respectively) were transcribed reversely in a final volume of 40 μL to obtain single-stranded cDNA using the following method: RNA was preincubated in 19 μL of RNAse-free water at 85°C for 10 min to denature it, and then rapidly chilled on ice. Then, 21 μL of a mixture formed by 1× RT buffer, dithiotheritol 20 mm (DTT), 2′-deoxyribonucleoside-5′-triphosphates (0.5 mm of each dNTP: dATP, dGTP, dCTP and dTTP), 40 units of Recombinant RNasin Ribonuclease Inhibitor, 0.5 μg Oligo (dT)15 Primer and 200 units Moloney Murine Leukemia Virus Reverse Transcriptase (M-MLV RT) were added (all reagents from Promega, Madison, WI, USA). The reverse transcription (RT) reaction was carried out for 60 min at 42°C and heated at 94°C for 5 min to terminate the RT reaction.
Polymerase chain reaction (PCR)
Serotonin AANAT was amplified by nested RT-PCR. The cDNA was amplified in a reaction containing 5 μL of RT product as template DNA, 1X PCR buffer, 1.5 mm of MgCl2, 0.4 mm each deoxynucleotide, 2.5 units Taq-DNA Polymerase (Roche) and 1 μm sense and antisense primers, all this in a final volume of 25 μL. Nested PCR was performed from1 μL of first PCR product.
The localization of the primers in corresponding genes is presented in Fig. 1. Exons 3–4 of mouse AANAT were amplified by primers N2L (5′-CCTAACCCTGTGTCCAGAGC-3′) and N2R (5′-GCATCCTCACACATGAGCAC-3′) in the first round and primers N22L (5′-CGCTGTGGGACAAGGAGA-3′) and N22R (5′-TCTCCACAGGAGGACAGAGC-3′) in the second round of PCR. Primers for the first PCR of exons 1-2 of mouse AANAT were N1L (5′-TTGCAGTCAGGAGTCTCAGC-3′) and N1R (5′-CCTCAGGTTTCAGGGAGTTG-3′). Primers for the second round were N11L (5′-GCTTCTCCTAGTCCCAGCAC-3′) and N11R (5′-AGGGGTTCCCCAGCTTCA-3′).
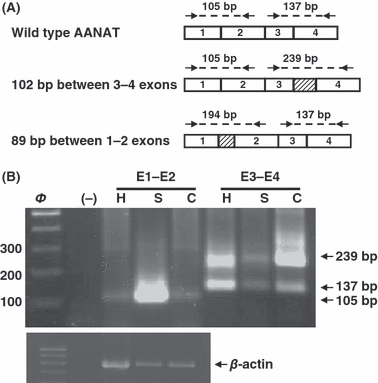
N-acetyltransferase (AANAT) expression in the pineal gland (A) PCR design for alternative splicing detection between exons 1 and 2 and exons 3 and 4. Primers are represented by arrows. (B) AANAT amplification of exons 1–2 (lines 3–5) and 3–4 (lines 6–8) in the pineal gland from C57BL/6 (C), Swiss (S) and C3H/HENHSD (H) mice strain. Negative control was included in the second line (−). β-actin was used as reference gene.
The template was initially denatured for 3 min at 95°C followed by two different programs optimized for: AANAT exons 3–4 (35-cycle program with 30 s of denaturation at 95°C, 45 s annealing at 59°C, 1 min extension at 72°C), and AANAT exons 1–2 (35-cycle program with 30 s of denaturation at 95°C, 45 s annealing at 58°C, 1 min extension at 72°C), all programs terminated by an extension of 6 min at 72°C. For each PCR run a negative control was systematically added, in which water replaced cDNA.
Before primer designing we took in consideration the alternative splicing between exon 3–4 described for pineal C57BL/6 mice [6] wtih the inclusion of a pseudoexon of 102 bp. Besides, insertion of 89 bp between exons 1 and 2 described by Slominski in several tissues was considered for primer design around exon 1–2 [7]. Primers design is shown in Fig. 1A.
NAT activity assay
N-acetyltransferase activity was determined by the Champney et al. method [32]. From all mice strains 4 × 106 PBMC either stimulated or not with PHA were homogenated at 4°C in 100 μL of 0.05 m phosphate-buffered saline buffer (PBS), pH 6.8, using a cell sonicator (Sonics and Materials Inc., Danbury, CT, USA). Twenty microliters of this homogenate were mixed with 10 μL of PBS containing 40 nCi [1–14C] acetylcoenzyme A and 5.6 mm tryptamine. The reaction was carried out for 20 min at 37°C, and was stopped by the addition of 100 μL 0.2 m sodium borate buffer, pH 10, and 1 mL chroloform at 4°C. The N-acetyltryptamine produced was extracted with chloroform and its radioactivity was measured by liquid scintillation spectometry with a beta counter. NAT activity was expressed as nmol N-acetyltryptamine produced/mg protein/h. Protein content was measured following the Bradford protocol [33].
Melatonin determinations
Melatonin levels were determined by a competitive enzyme immunoassay kit (Immuno Biological Laboratories, Hamburg, Germany) according to manufacturer’s intructions. Pineal glands were homogenated in 500 μL of PBS in a sonicator (Sonics and Materials Inc.) and centrifuged at 16000× g for 3 min. Pineal, thymus, spleen and bone marrow homogenate supernatants, plasma and culture supernatants were used for melatonin determinations.
Melatonin from 500 μL of the samples, standards and controls was extracted (90–100% yield recovery) using C18 reversed phase columns (IBL-Hamburg, Germany) and methanol elution. The dried extracts (after evaporating methanol) were stored at −20°C for up to 48 hr. Melatonin levels were measured in duplicate using 96 well microtiter plates coated with captured antibody goat anti-rabbit Ig. Each microtiter plate was filled either with 50 μL blank reagent, extracted calibrators, extracted samples or extracted standard solutions (containing 0, 3, 10, 30, 100 or 300 pg/mL of melatonin). Then, 50 μL of melatonin biotin and 50 μL of rabbit-antiserum were added into each well, shaken carefully, sealed with adhesive foil and incubated overnight (14–20 hr) at 2–8°C. After washing three times with 250 μL diluted assay buffer, 150 μL of anti-biotin conjugate to alkaline phosphatase was added into each well and incubated for 2 hr at room temperature. The reaction was developed using p-nitrophenyl phosphate and optical densities were determined at 450 nm in an automatic microplate reader. The sensitivity of the melatonin assay was 3.0 pg/mL. Both the intra- and inter-assay coefficients of variation (CV) were less than 10%.
Statistical data analysis
Data were statistically analyzed using an ANOVA variance analysis follow by a Bonferroni Multiple Comparison Test. Results are expressed as mean ± standard error of the mean (S.E.M.). P values of <0.05 were considered statistically significant.
Results
AANAT amplification was performed using primers for 3–4 exons and 1–2 exons. As shown in Fig. 1B, we found AANAT expression in the pineal gland of each mice strain. A unique 105 bp band corresponding to the wild type enzyme was observed when 1–2 exons were amplified. This band was clearly more intense in the Swiss mice when compare with the other strains. Amplification of 3–4 exons showed the presence of two bands, one of them of 137 bp corresponding to the wild type, and the other one of 239 bp corresponding to the mutant isoform with 102 bp insertion. This band pattern was observed not only in the two melatonin deficient strains but also in the C3H/HENHSD control mice. The relatively amount of each isoform expression was similar in the C3H/HENHSD and Swiss strains, although much less intense in this last one. Regarding C57BL/6 mice, as expected, the mutant AANAT isoform was predominantly over the wild type one. β-actin expression was used as housekeeping-control.
We observed AANAT expression in all tissues studied of each mice strain (Fig. 2). In Fig. 2A a unique band of 105 bp (wild type) was observed when 1–2 exons were amplified. This band was poorly amplified except in bone marrow cells from C3H/HENHSD. AANAT expression of pineal gland was used as a positive control.
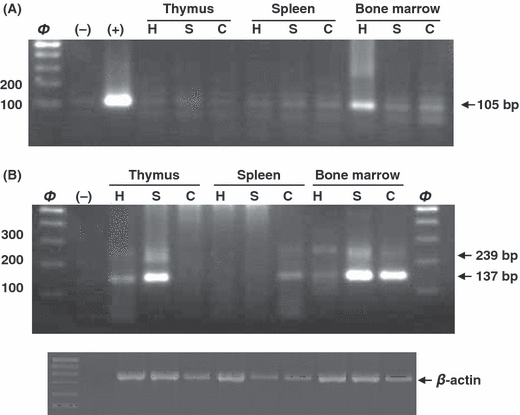
N-acetyltransferase expression in thymus, spleen and bone marrow cells. (A) AANAT amplification of exons 1–2 from thymus (lines 4–6), spleen (lines 7–9) and bone marrow (lines 10–12) of C57BL/6 (C), Swiss (S) and C3H/HENHSD (H) mice strains. Negative (−) and (+) positive controls were included in the second and third line respectively. (B) AANAT amplification of exons 3–4 from thymus (lines 3–5), spleen (lines 6–8) and bone marrow (lines 9–11) of the mice strains studied. Negative control was included in the second line (−). β-actin was used as reference gene.
As shown in Fig. 2B, the amplification pattern for 3–4 exons was not homogeneous, and both bands corresponding to the wild type (137 bp) and the mutant one (239 bp) always appeared in our experiments. The C57BL/6 mice strain described as melatonin deficient also revealed two isoforms in both spleen and bone marrow. Opposite to the results described in the pineal gland, the wild type isoform was predominantly over the mutant one, being this more evident in bone marrow cells. Similarly, a predominant wild type band was observed analysing AANAT expression in both thymus and bone marrow cells from Swiss mice. No amplification was observed when studying RNA from spleen in these mice strain. RNA from control mice C3H/HENHSD was also amplified in samples from both thymus and bone marrow cells. As occurs in the pineal gland, the mutant isoform was also confirmed in this mice strain although showed less intensity compared with the wild type one. β-actin expression was used as housekeeping control.
Pineal melatonin levels were significantly higher in control C3H/HENHSD mice compared with the deficient ones (Table 1, one-way ANOVA test, P < 0.001). However, unexpected results were found when plasma samples were analysed. Thus, although melatonin levels were higher in control C3H/HENHSD mice, these differences were less evident regarding C57BL/6 and Swiss strains (Table 1, one-way ANOVA test, P < 0.05).
| Mice strain | |||
|---|---|---|---|
| C3H/HENHSD | C57BL/6 | Swiss | |
| Pineal | 90.6 | 15.75a | 5.57a |
| Plasma | 66.6 | 44.04b | 49.23b |
- a P < 0.001 versus C3H/HENHSD mice; bP < 0.05 versus C3H/HENHSD mice.
Melatonin levels were also measured in several tissues from C57BL/6 and C3H/HENHSD mice strains. As we can observe, in Table 2 melatonin levels measured in thymus, spleen and bone marrow cells homogenates were detectable and very similar in both mice strains.
| Mice strain | ||
|---|---|---|
| C3H/HENHSD | C57BL/6 | |
| Thymus | 2.56 | 3.00 |
| Spleen | 4.14 | 2.66 |
| Bone marrow cells | 4.66 | 4.8 |
Fig. 3A shown AANAT 1–2 exons amplification in PBMC from each mice strain. Cells from melatonin deficient mice (C57BL/6 and Swiss) were also stimulated with PHA for 48 hr. Amplification pattern revealed a wild type band in cells from C3H/HENHSD and Swiss mice strains. After stimulation with PHA, the band obtained for Swiss mice was more intense and an amplification product was detected in cells from C57BL/6 mice.
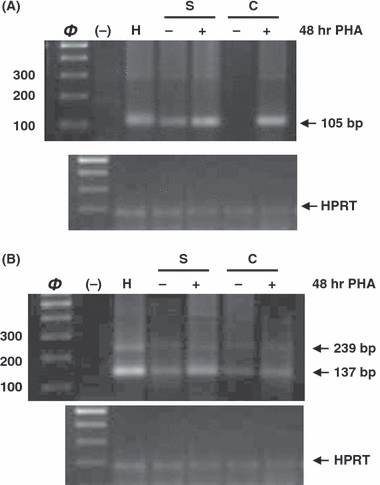
N-acetyltransferase expression in stimulated and unstimulated Peripheral blood mononuclear cells (PBMCs) from each mice strain. Cells from Swiss and C57BL mice were cultured for 48 hr in presence (stimulated) or absence (unstimulated) of PHA (8 μg/mL). (A) AANAT amplification of exons 1–2 of PBMC from C57BL/6 (C), Swiss (S) and C3H/HENHSD (H) mice strains. (B) AANAT amplification of exons 3–4 of PBMC from the same mice strains. Negative control was included in the second line (−). HPRT was used as reference gene.
In AANAT 3–4 exons expression study (Fig. 3B) two bands were again observed in unstimulated cells from each mice strain, being always more intense the wild type one. PHA stimulation of melatonin deficient mice strains increased the AANAT expression; however these values did not reach those obtained from control C3H/HENHSD cells. HPRT expression was used as housekeeping control.
Results from AANAT activity in PHA stimulated and unstimulated PBMC cells from each mice strain are shown in Fig. 4A. AANAT activity in unstimulated cells from C57BL/6 and Swiss mice were significatively more elevated compared with the control ones (one-way ANOVA test, P < 0.001, Bonferroni Multiple Comparison Test, P = 0.0005 Swiss and C57BL/6 mice versus C3H/HENHSD control mice). After 48 hr stimulation with PHA, AANAT activity significantly increased in all strains studied, being only significant in the Swiss mice (Bonferroni test P = 0.05).
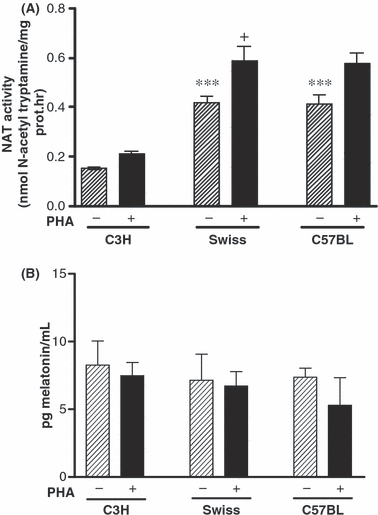
AANAT enzyme activity (A) and melatonin levels (B) in stimulated and unstimulated PBMCs from each mice strain. Cells from Swiss and C57BL/6 mice were cultured for 48 hr in presence (stimulated) or absence (unstimulated) of PHA (8 μg/mL). Data are expressed as mean ± S.E. of three different experiments performed in triplicate. (*) AANAT activity in unstimulated cells from each mice strain (one-way ANOVA test P < 0.001; Bonferroni test (***)P < 0.0005 C57BL/6 and Swiss mice versus C3H/HENHSD mice). (+) Significant differences on AANAT activity between stimulated and unstimulated cells were observed in Swiss mice (Bonferroni test, (+)P = 0.05).
Melatonin was detected in unstimulated PBMC culture supernatant (Fig. 4B) although no significant differences were found among mice strains. PHA stimulation did not significantly increase basal levels.
Discussion
The study of melatonin’s effects on the immune system lacks of a good model where melatonin production by immune system cells has been ablated. We evaluated two pineal melatonin deficient mice, C57BL/6 and Swiss mice, as possible models for studing the immunomodulatory melatonin action. We conclude that neither of them is ideal, as both deficient mice were able to produce melatonin in several cells and tissues from the immune system. Additionally, we postulate there are other extrapineal melatonin sources in these mice strains since plasma melatonin levels are not dramatically different from the control C3H/HENHSD strain.
Mice of the most inbred strains, except for same specified strains such as C3H/HENHSD and CBA, has been described to produce small amount of melatonin by pineal gland. In this way, it has been difficult to determined melatonin levels individually using traditional methods [4]. In the present report melatonin was detected in the two deficient mice strains studied, C57BL/6 and Swiss mice. These results agree with some recent reports postulating a low melatonin synthesis by inbred mice [25, 34]. More accurate laboratory techniques may be available for determining melatonin low levels in inbred mice that were not possible to detect in former reports. Unexpectedly, plasma melatonin levels in C57BL/6 and Swiss strains were only slighitly lower than in the control C3H/HENHSD one (34% and 26% respectively), while reduction in pineal melatonin content was more dramatic reaching the 94% diminution in the Swiss mice. These results suggest an extrapineal melatonin synthesis that might counterbalance the pineal melatonin deficiency. Thus, melatonin has been reported to be produced in many endocrine and nonendocrine cells and tissues [22–24]. Moreover, a rather high extrapineal melatonin production has been reported in C57BL/6 mice, not only in the immune system [25] but also in other tissues as skin and hair follicles [35, 36]. On the other hand, melatonin produced and released by Harderian gland is an important source of this hormone in rodents [37], and might be also a good candidate for this compensatory melatonin secretion. Nevertheless, C57BL/6 mice should not be considered totally pineal melatonin-deficient as has been reported to produce melatonin in their pineal gland [25] and the variations reported might depend on collection of a sampling, extraction of melatonin or methodology used for melatonin detection. Additionally, prolonged norepinephrine stimulation under short photoperiods revealed that pineal gland of so-called melatonin deficient mice is capable of producing measurable amounts of melatonin [38].
The C57BL/6 mouse strain is considered melatonin deficient because one or both of the enzymes in the serotonin to melatonin pathway seem to be severely compromised or undetectable [5]. However, the regulatory mechanism responsible of the day-night circadian rhythm seems to be unaffected when compared with melatonin proficient C3H/HENHSD mice [38]. The presence of defective AANAT enzyme has been reported to be due to a point mutation that deals with an alternative splicing generating a mature mRNA with a 102 base pair pseudo-exon inclusion between exons three and four that generate a stop codon [6]. Other alternative isoforms have been also described in several tissues [7]. In the present study we reported the expression of the different isoforms of mature RNA described in the literature for the AANAT in several cells and tissues of the immune system. Regarding the 102 bp inclusion described by Roseboom et al. [6], both isoforms, in variable proportions, were observed in all tissues and cells studied in the two melatonin deficient mice and also in the control one. Moreover, pineal AANAT amplification of 3–4 exons showed the presence of two bands corresponding to the wild type and the mutant isoform not only in the two melatonin deficient strains but also in the C3H/HENHSD control mice (Fig. 1). As we expected, the mutant AANAT isoform in C57BL/6 mice was predominantly over the wild type one, while in the control mice strain and in the Swiss mice the relative amount of each isoform expression was similar. Based on these results, the significance of this mutant isoform should be reconsidered. Thus, melatonin production by pineal gland was higher in C3H/HENHSD control mice than in the others; however, the mutant isoform is present in all strains studied. Supporting that, the point mutation described in C57BL/6 mice is not observed in other deficient inbred mice such as BALB/c and 129sv strains. Melatonin deficiency might also be explained by a defect in the last enzyme involved in melatonin synthesis, i.e., HIOMT. Thus, undetectable pineal HIOMT activity has been described in many mice strains [5].
The role of melatonin as an immunomodulatory hormone has been widely studied using in vivo models [15]. However most in vitro studies have shown contradictory results. Although many authors report in vitro a direct effect of melatonin as a positive modulator of lymphocyte proliferation and cytokine production [14], others have observed no effect of melatonin on lymphocytes activated or not with PHA, Con-A, or phorbol myristate acetate. Consequently, melatonin at low or high concentrations failed to activate human [39] or rat [40] lymphoid cell proliferation. Besides, in some cases, an inhibitory effect of melatonin on lymphocyte proliferation was noted as being coupled to IFN-γ and TNF-α production [41]. Recent results have reported that both resting and stimulated in vitro cultured human lymphocytes release large amounts of melatonin, that is synthesized by the cells [28]. In the present work, PBMC culture showed AANAT amplification in all strains studied. Although the bands detected were less intense in the melatonin deficient mice the amplification almost reached the control cell intensity after stimulation with PHA. It is important to note that a unique band observed for the 1–2 exons and the predominant wild type band found when exons 3–4 were analyzed. These results do not support the use of these mice strains for in vitro immunomodulator role of melatonin study.
In so-called melatonin deficient mice, measurable melatonin levels were detected in thymus and spleen as well in bone marrow cells. Conti et al. [25] reported a melatonin production by bone marrow cells in C57BL/6 mice even higher (15% increase) than the C3H/HENHSD control mice, similar to our findings. Also, melatonin levels in bone marrow cells and thymus from the deficient strain C57BL/6 mice were slightly higher when compared with control mice. These findings add to the controversy over the assumed melatonin deficiency in inbred strains. AANAT expression results in these tissues agree with the detectable melatonin levels.
In summary, melatonin detection in both inbred and outbred mice clearly indicates that different cells and tissues from the immune system are able to synthesize melatonin. Moreover, the presence of a clear and predominant wild type AANAT mature RNA for the 3–4 exons in thymus, spleen as well as bone marrow and PBMC cells supports this affirmation. We hypothesize that melatonin released from these cells acts in a paracrine or autocrine way, as previously described form human culture PBMC [28]. On the other hand, we postulate the existence of another alternative enzyme alteration in the melatonin pathway to the point mutation in the AANAT gene previously described [6]. Thus, the mutant isoform is present in C3H/HENHSD control mice, but melatonin production by pineal gland was quite higher compared with the other strains. Finally, C57BL/6 and Swiss mice shown a pineal melatonin synthesis deficiency, although this defect may be not be generalized to all tissues. In this sense, other tissues, such as Harderian gland, may compensate the low pineal melatonin production contributing to the measurable plasma melatonin levels. Nevertheless, further experiments should be performed aimed to elucidating plasma melatonin source, and melatonin effect in these mice strains.
Acknowledgements
This work was supported by grants from the Ministerio de Sanidad FIS (06/0091) and Red-FIS (RD06/0013/0001) and Grupo Excelencia del Plan Andaluz de Investigación (P06-CTS-01604).




