Coexpression of the melatonin receptor 1 and nestin in human breast cancer specimens
Abstract
Abstract: Activation of the G-protein-coupled receptor (GPCR) for melatonin (MT1) suppresses breast cancer cell growth in experimental models. To elucidate whether MT1 might play a role in cancer cells positive for the stem cell marker nestin, we assessed paired carcinomatous (Ca) and adjacent noncancerous (NCa) samples from 42 patients with primary breast cancer for MT1 and nestin by double immunofluorescence staining and quantitative image analysis with Tissue-Quest® software. MT1 was located in luminal and myoepithelial cells in milk ducts and in tumor cells in 40/42 and 39/42 of NCa and Ca specimens, respectively, independent of hormone receptor and HER-2 status. Nestin was located together with MT1 in myoepithelial cells in 38 NCa specimens (total n = 42) and in 18 Ca specimens with intact milk ducts. Quantitative evaluation of selected 16 NCa and Ca samples revealed that MT1 levels were higher in invasive Ca sections than in NCa specimens in eight and lower in six cases. Specimens from higher tumor stages (TII/III) with a higher risk of relapse were associated with MT1/nestin co-staining in more than 10% of tumor cells, whereas a lack of co-staining correlated with lower tumor stages. Abundant expression of MT1 and, particularly, coexpression of MT1 with nestin in invading tumor cells in more advanced tumors suggest an important role for this GPCR in the pathogenesis of breast cancer.
Introduction
Melatonin acts via receptor-independent antioxidative [1, 2] and receptor-mediated mechanisms [3]. Previous studies showed that melatonin inhibits proliferation of breast cancer cell lines and prevents progression of malignant breast tumors in animal models [4]. These anticancer effects of melatonin are thought to be mediated at least in part by binding and activation of its high-affinity G-protein-coupled receptor (GPCR), the melatonin receptor (MT) 1 [5, 6]. This receptor has been detected in various human tissues [7]. Many studies used the estrogen-sensitive breast cancer cell line MCF-7 overexpressing MT1, and showed that MT1 activation suppresses estrogen receptor (ER)-induced transcription of genes important for cell proliferation and tumor progression, resulting in the inhibition of breast cancer growth [8, 9]. Therefore, MT1 activation induces a strong antiproliferative effect in ER-expressing breast tumors sensitive to circulating estrogens [10]. This is important for a large number of breast cancers, which express ER and that are responsive to anti-hormonal therapy.
The success of this therapy, however, is often temporary as tumors escape their estrogen responsiveness and growth progresses [11]. With respect to this escape from estrogen dependency, it is interesting that in an immunohistochemical study in patients with breast cancer, the expression of MT1 was found to be higher in less differentiated breast tumors, independent of the expression of ER and also of the progesterone receptor (PR) [12]. Therefore, MT1 expression seems to be important for additional mechanisms, which could influence tumor growth independent of estrogen signaling. For example, in breast cancer cell lines, MT1 signaling was shown to interfere with the transcriptional activity of the glucocorticoid receptor and the retinoid receptor involving different G-protein signaling pathways [13]. Moreover, the inhibitory effect of melatonin on the uptake of tumor growth promoting fatty acids and the suppression of fatty acid metabolism could be important in these tumors [14].
In line with these findings, other studies showed that the activation of MT1 by melatonin is indeed associated with an induction of cell differentiation of neuronal as well as non-neuronal cells and also of tumor cells. For example, it induces neurite outgrowths in neuroblastoma cells and causes neuroendocrine differentiation of prostate cancer cells [15, 16]. In MT1-transfected Chinese hamster ovary (CHO) cells, receptor activation by melatonin provokes changes in cellular properties, e.g. induction of filamentous structures, dependent on activation of the MEK1/2 ERK1/2 signaling pathway [17].
While these studies very well explain the anticancer effects of MT1 activated by melatonin, the high MT1 expression levels in cancer tissue, as found in the breast cancer study of Dillon et al. [12] are still not easy to explain and, therefore, another function of MT1 seems likely. Like other GPCRs, MT1 might have the ability to become activated independent of its ligand melatonin, thereby changing the fate of normal and transformed cells in a still unknown way [18, 19]. This might be of particular significance for tumor induction and progression and could occur in mature cells as well as in their progenitor/stem cells, as cells with a capacity of unlimited renewal, differentiation and motility [20].
Similar to normal stem cells, also in cancer, a small population of cells, termed cancer stem cells (CSC), possess the capability for self-renewal, asymmetric cell division and indefinite proliferative capacity. These tumor-initiating CSCs have been identified in a variety of hematological and solid malignancies, and were also found in breast cancer [21]. CSCs share various characteristics with normal stem cells, but they also contain a unique and disease-specific signature of proteins [22]. For example, nestin, an intermediate filament protein, was originally identified as a marker of neuroepithelial stem/progenitor cells in the brain, but was also later found in a cell population with CSC characteristics in many tumors, e.g. breast cancer [23–25]. It is assumed that nestin-expressing stem cells initiate a population of dedifferentiated cells capable of unlimited growth giving rise to highly undifferentiated tumors. This model for malignant transformation in the breast is supported by the finding that high nestin levels were associated with aggressive, poorly differentiated basal-like breast cancer [24]. Moreover, due to their nestin expression, endothelial cells in newly formed capillaries are thought to have developed from nestin-positive precursor cells, and their presence is associated with a highly aggressive phenotype of cancer [25].
Importantly, expression of nestin together with MT1 was found in neural stem cells [26], but a possible association of nestin together with MT1 has not yet been evaluated in breast cancer cells. Therefore, the present study was carried out to elucidate whether MT1 expression in different cells might correspond to that of nestin. We further investigated whether this possible coexpression might be associated with particular features of the tumors (grading, tumor stage and risk of relapse). Investigations were carried out in the cancerous (Ca) and the adjacent noncancerous (NCa) parts of breast tissue specimens from the same patient using a novel quantitative image analysis system for the evaluation of the immunofluorescence data.
Materials and methods
Patients
Forty-two patients with primary, invasive breast carcinoma, diagnosed and operated between 1998 and 2003 at the Kaiser-Franz-Josef Hospital, Vienna, were enrolled in the study. Written informed consent was obtained from all patients, and the study was approved by the ethics committee of the institution.
The mean age of patients at primary diagnosis was 56 ± 10 yr (range 29–76). Patients were classified as described by Königsberg et al. [27]. Staging was carried out at the primary diagnosis according to the guidelines of the International Union against Cancer (UICC). The observation time after the date of primary diagnosis to the date of last appointment or disease-associated death was 67 ± 19 months, ranging from 11 to 96 months. Based on data on patient age, tumor size, presence of tumor cells in local lymph nodes, tumor differentiation grade, HER2 status and peritumoral vascular invasion, patients were assigned to be at low, intermediate or high risk of local or distant relapse [28]. From eight patients with distant metastases, lungs were affected in 87.5%, liver in 62.5%, bone in 37.5% and brain in 25.0% of patients. Seven of 42 (16.7%) patients died from metastatic disease within the observation time.
All tissue specimens were obtained from patients prior to chemotherapy by surgical excision immediately after primary diagnosis and, in our study, parts not required for further routine diagnosis were used. All samples were routinely subjected to pathological examination. Twenty-nine patients had invasive ductal carcinoma, six invasive lobular carcinoma and seven patients had tumors with other histological features (e.g. apocrine, medullary or mucinous carcinoma). Grading was carried out according to Bloom and Richardson, with 4 G1, 21 G2 and 17 G3 tumors. ER (27 positive), PR (16 positive) and HER2 (19 positive) expression was routinely assessed using immunohistochemistry at the Institute of Bacteriology and Pathology, Kaiser-Franz-Josef-Hospital, Vienna. The evaluation of ER and PR status was carried out according to the method of Remmele and Stegner [29], and HER2 staining were regarded positive if >10% of cells showed distinct and complete membrane staining of HER2.
Antibodies
The rabbit polyclonal antibody against the human MT1 was purchased from Abcam, Cambridge, UK. A mouse monoclonal antibody (mAB) against nestin (clone 10C2) was obtained from Millipore (Temecula, CA, USA). Both primary antibodies were applied at a concentration of 2.5 μg/mL. The mouse mAB against CD10/CALLA (clone 56C6) and the mAB against α-SMA (clone 1A4) were both obtained from Labvision/Neomarkers (Fremont, CA, USA), and were applied at concentrations of 1:30 tissue culture supernatant and 0.25 μg/mL respectively.
As secondary antibodies, we applied Alexa Fluor®488 (green) and 568 (red) labeled goat anti-rabbit (488), and goat anti-mouse (568) antibodies at concentrations of 2 μg/mL, which were obtained from Molecular Probes (Eugene, OR, USA). For immunohistochemical detection of MT1, a goat anti-rabbit/mouse antibody conjugated to horseradish peroxidase labeled dextran (Envision®) was used as a secondary antibody and purchased from Dako Company (Glostrup, Denmark).
For negative control, the following immunoglobulins (Ig) were applied: rabbit anti-mouse IgG (Pierce, Rockford, IL, USA) and mouse myeloma IgG1 (Zymed Laboratories, South San Francisco, CA, USA).
Immunohistochemical detection of MT1
After surgical excision of the breast carcinoma and the surrounding nonmalignant resection margin, both tissues were routinely fixed in formalin (4%), dehydrated and embedded in paraffin. From tissue blocks, 1-μm sections were prepared, mounted on adhesive slides and dried for at least 1 hr at 65°C. Thereafter, sections were deparaffinized in xylene, and the endogenous enzyme activity was blocked with 3% hydrogen peroxide in methanol for 10 min at room temperature (RT). The sections were then processed through a graded series of alcohols and rehydrated in phosphate-buffered saline (PBS). After permeabilization in 0.1% Tween-20 in PBS for 10 min at RT, antigen retrieval was performed using the microwave technique in 10 mm citrate buffer (pH 6.0) for 10 min, followed by blocking with 10% bovine serum albumin (BSA) in PBS for 30 min. Thereafter, the sections were incubated with the MT1 antibody overnight at 4°C in a humidified chamber. After washing three times in PBS, the dextran polymer peroxidase Envision System was applied for 30 min at RT. The antigen was then visualized using the 3′,3-diaminobenzidine chromogen for 4 min. Cell nuclei were counterstained with hematoxylin (ChemMate®; Dako), rinsed with distilled water and mounted in Mowiol (Sigma-Aldrich GmbH, Steinheim, Germany).
Immunofluorescence staining
Antigen retrieval and blocking were performed as described in the immunohistochemistry experiments. For MT1 staining, the sections were incubated with the MT1 antibody overnight at 4°C. This step was followed by washing three times in PBS. Alexa Fluor® 488-conjugated goat anti-rabbit IgG was applied as secondary antibody for 1 hr at RT.
For MT1–nestin double staining, initially, staining was performed with an anti-nestin mAB for 1 hr (RT). This step was followed by washing in PBS 3 × 5 min, and the staining was completed by incubation with Alexa Fluor® 568 goat anti-mouse IgG for 1 hr at RT. Thereafter, slides were washed in PBS and blocked again with 10% BSA in PBS for 30 min before staining with the second primary antibody against MT1 (overnight, 4°C) was performed. As a secondary antibody, Alexa Fluor® 488-conjugated goat anti-rabbit IgG (1 hr at RT) was used.
For CD10 single staining, heat-induced antigen retrieval in 1 mm EDTA (pH 8.0) was performed for 10 min. No antigen demasking was necessary for α-SMA detection. Both antibodies were applied overnight at 4°C in a humidified chamber. After washing in PBS for 3 × 5 min, sections were incubated with Alexa Fluor® 568 goat anti-mouse IgG for 1 hr at RT.
For negative controls, the primary antibodies were replaced by the isotype-matched control IgG. Cell nuclei were counterstained with 0.5 μg/mL bisbenzimide in PBS (Hoechst 33342; Sigma, Munich, Germany). After completing the immunofluorescence staining, slides were rinsed with distilled water, dried and mounted in H-1000 Vectashield medium (Vector Laboratories Inc., Burlingame, CA, USA).
Microscopy and image collection
Sections were viewed in an Axioplan 2 microscope (Carl Zeiss, Jena, Germany). For each tissue section, monochrome images of four representative fields were recorded from each channel with an AxioCam HRc2 Color CCD digital camera at 10× and 40× magnification respectively. To minimize background signals, the exposure times for the MT1 (and other antigens) antibody staining were evaluated for a certain magnification and kept constant between the samples. Background subtraction was uniformly carried out with the Axiovision 4.6 software (Carl Zeiss Vision GmbH, Aalen, Germany).
Quantification of MT1 and nestin immunostaining
All carcinoma and corresponding noncarcinoma sections showing distinct MT1 staining where a clear localization of MT1 could be recognized were further analyzed for the number of cells with MT1 and nestin immunostaining. Of each tissue section, four representative images (40× magnification) were analyzed using the Tissue-Quest® 2.2 software (TissueGnostics GmbH, Vienna, Austria). Single cells were identified by their nuclei (blue bisbenzimide staining). This identification mask was then used to measure gray values in the two corresponding channels (MT1 and nestin) of each object in all images [30].
For the quantification of MT1- and nestin-containing cells, their fluorescence intensity signals were plotted against the bisbenzimide signal, as a parameter for the cell number, in scattergrams. Each scattergram represents average values calculated from analysis of all four images of one entire tissue section. Based on the degree of fluorescence intensity in the negative controls (control IgG), appropriate threshold values for positive immunofluorescence staining were defined. Because isotype controls were always less than 10%, the threshold for considering the samples positive for MT1 or nestin staining was set at 10%. MT1 staining levels were considered to be different between cancer and noncancer tissues, when the difference was ≥10%.
Statistical analysis
All calculations were performed with the SPSS 14.0 statistical software package (SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± standard deviation, and were compared with the two-sided paired or unpaired t-test, if normally distributed. Otherwise, the two-sided Mann–Whitney U-test was used. Differences between two groups of categorical variables were tested with the two-sided Fisher’s exact test. A value of P ≤ 0.05 was considered statistically significant.
Results
Overall, in paired samples from 37/42 patients green staining for MT1 immunoreactivity was observed in the Ca and adjacent NCa specimens (Fig. 1). In NCa sections, MT1 immunoreactivity is located in myoepithelial and luminal epithelial cells of the milk ducts. Occasionally, green MT1-stained stromal cells are visible of normal tissue (Fig. 1A,B). The inset in Fig. 1A further shows that MT1 immunoreactivity is mainly located in the cell membrane of ductal epithelial cells in this specimen. This was only seen in 15/42 NCa specimens, while in 10/42 of NCa specimens, in addition to cell membranes, cytosolic compartments were also stained for MT1. In the remaining 15/42 MT1-positive samples, the subcellular localization of MT1 was diffuse and not clearly attributable to the membrane or the cytosol. An analogous staining pattern for MT1 was also observed in immunohistochemistry experiments (Fig. 1C).
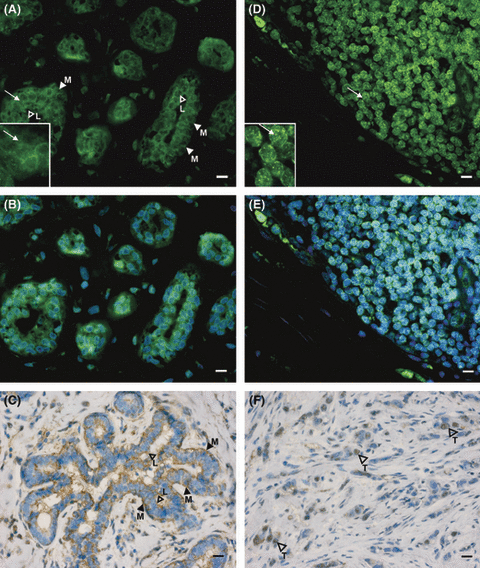
Expression of MT1 in noncancerous and cancerous breast tissue. Paraffin-embedded sections from breast tissue were stained for MT1 by immunofluorescence (A,B,D,E) and immunohistochemistry (C,F). MT1 staining of epithelial cells in milk ducts is seen in noncancerous tissue (A–C). In milk ducts, outer myoepithelial cells (M) and inner luminal cells (L) show green MT1 fluorescence (A,B) and brown staining of MT1 with DAB (C). As shown in the inset (arrow), MT1 staining is mainly in the membrane. MT1 staining of carcinoma cells (T, arrowheads in image F) of invasive ductal tumors of G2 (F; patient 12) and G3 (D,E; patient 17). The area shown at higher magnification in the inset is marked by an arrow. Green spots in carcinoma cells suggest MT1 localization in cytoplasmic compartments. Note that the images (B) and (E) correspond to (A) and (D), but are additionally counterstained with the DNA intercalating dye bisbenzimide. In (C) and (F), nuclei were counterstained with hematoxylin. Inset bars represent 10 μm. Number of samples correspond to that in Table 1 and Fig. 3 (panel B).
In contrast to the more frequent membrane, MT1 localization in the NCa specimens, MT1 immunolocalization in the cytosol was seen in the majority of all Ca specimens (21/42), while preferential cell membrane staining was seen in 10/42 Ca samples. In the remaining eight samples, the localization of MT1 was not clearly attributable to either group. However, comparison of the subcellular localization of MT1 in Ca and paired NCa sections did not reveal significant differences between the two groups (P = 0.74). MT1 immunofluorescence staining patterns of Ca sections from a highly undifferentiated (G3) invasive ductal tumor are shown in Fig. 1D,E. Fig. 1F represents an immunohistochemical staining for MT1 in a better differentiated ductal carcinoma (G2). As demonstrated in the inset of Fig. 1D, MT1 immunoreactivity is spotted in cytoplasmic compartments in ductal epithelial cells in this Ca specimen.
MT1 immunoreactivity in cell membranes and in cytoplasmic compartments, or both, was further verified by confocal immunofluorescence laser scanning microscopy (data not shown). As nestin was previously described to be expressed in breast tumors in myoepithelial and cancer cells with stem cell properties [23, 24], we investigated nestin immunolocalization with respect to that of MT1 to: (i) confirm myoepithelial localization of MT1 and (ii) identify cancer cell subpopulations positive for nestin and MT1.
In the immunofluorescence analysis, overall positive cytoplasmic nestin staining was observed in 38/42 of NCa, where it was located in myoepithelial cells in milk ducts and lobules. From 31 nestin-positive Ca specimens, in nine nestin was seen only in myoepithelial cells of milk ducts, in another nine in myoepithelial cells and infiltrating cells and in the remaining 13 in infiltrating tumor cells only. In the latter, nestin was observed in tumor cell clusters and vascular-like structures (Fig 2A and B). Double staining for MT1 and nestin is shown in Fig. 3A(a–c) in NCa and Ca sections from a patient with an invasive ductal carcinoma (G2). In parts of this tumor, intact milk ducts are still present. MT1–nestin coexpression is found in myoepithelial cells of the milk ducts in the NCa and Ca sections (Fig. 3Aa,b), while in image c, overlap of MT1 and nestin staining is found in only a small fraction of tumor cells.
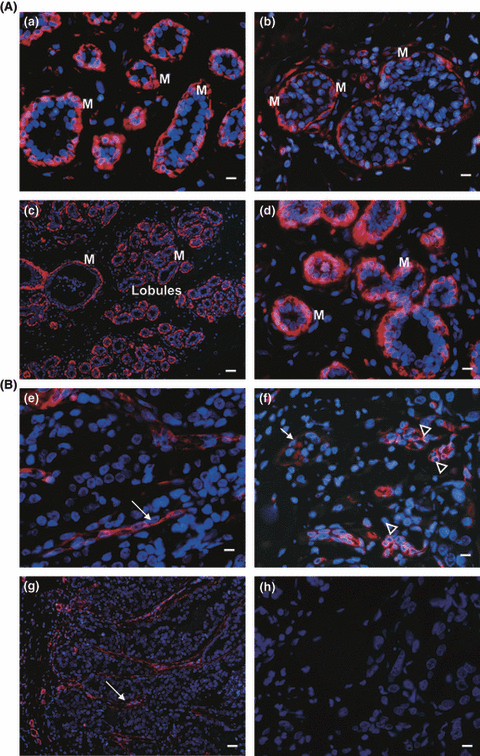
Immunofluorescence staining of nestin, α-SMA and CD10 in malignant and nonmalignant breast tissue. (A): Red nestin staining of milk ducts in malignant (b) and nonmalignant (a) tissue. Image (b) shows the intraductal component of an invasive ductal carcinoma, G2, from patient 3. (c) shows red α-SMA and (d) red CD10 staining of milk ducts and lobules in nonmalignant tissue. (B) These serial sections show an apocrine tumor (G2), obtained from patient 2. Red nestin staining in the invasive part of the tumor, without milk duct structures (e,f). Note, that long stretched structures of nestin staining (e, indicated by arrows) resemble the pattern of α-SMA staining in picture (g). In (f), nestin-positive cells are forming clusters (indicated by arrowheads), which are negative for the myoepithelial marker CD10 (h). The arrow indicates nestin expression in endothelial cells of a blood vessel. (g) shows red α-SMA staining in the invasive part of the tumor, without milk duct structures. (h) No staining for CD10 is observed in this tumor. Cell nuclei were detected by staining of the DNA with bisbenzimide (blue). Inset bars represent 10 μm for nestin (a,b,e,f) and CD10 (d,h), and 40 μm for α-SMA (c,g) staining pictures.
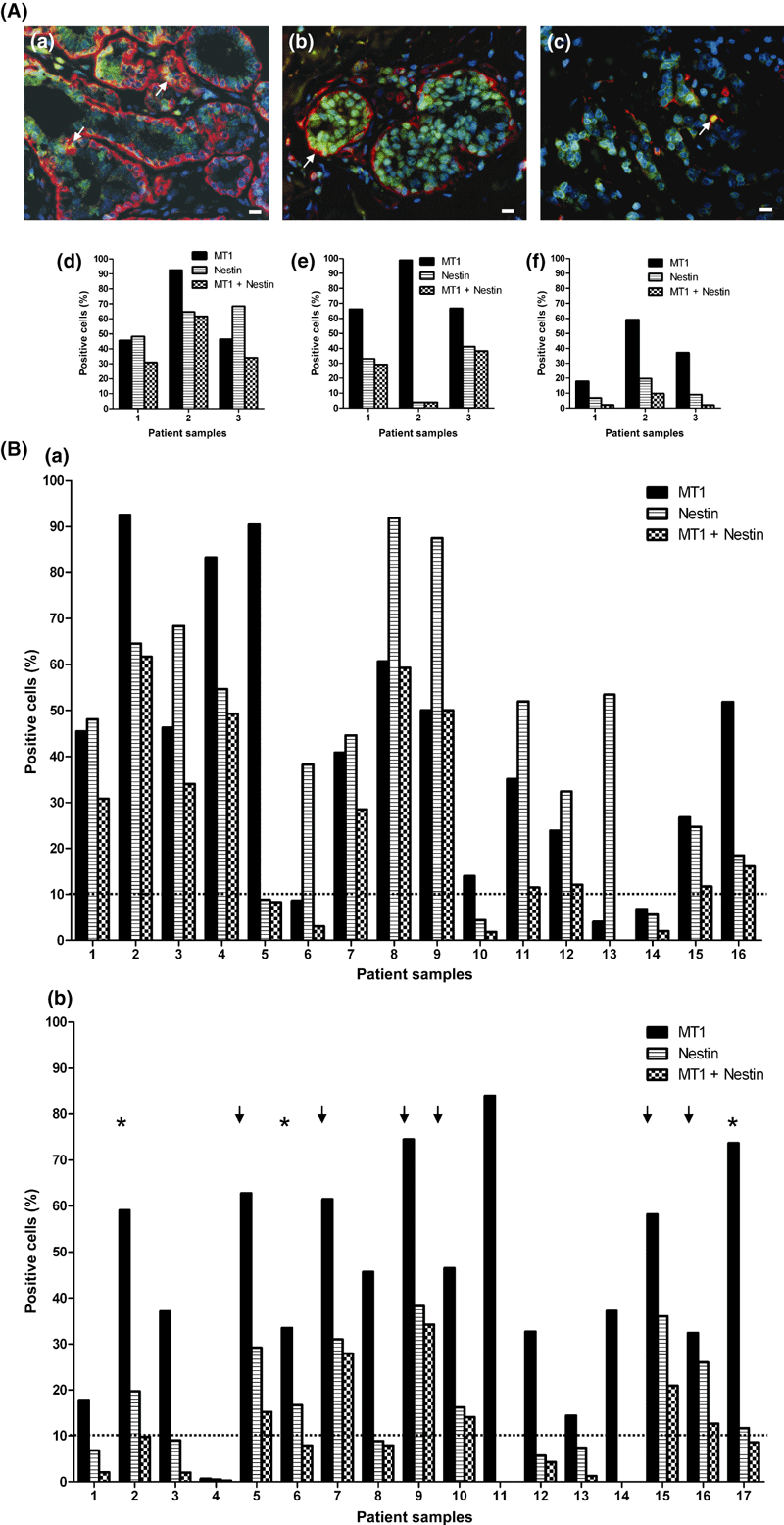
MT1- (green) and nestin (red)-co-staining in the noncancerous (a) and cancerous parts (b,c) from breast cancer specimens and its quantitative evaluation. (A) (a–c) Coexpression is seen in milk ducts in the noncancerous (a) and cancerous (b) parts and in cells in the invasive component (c) from patient 3 (see B) with a G2 invasive ductal tumor (c). In the merged picture of green MT1 and red nestin staining, orange to yellow staining (arrows) indicates overlay of MT1 and nestin fluorescence. Inset bars: 10 μm. (d–f) Quantitative evaluation of MT1-, nestin- and MT1–nestin-coexpressing cells. Data were obtained from Tissue-Quest® 2.2 image analysis of specimens from patient 3 (shown above), and from two additional patients. Noncancerous (d) and cancerous (e) milk ducts and invasive component (f). Note, that only patient 2 had >10% of MT1 and nestin double-positive cells in the invasive part of the tumor respectively. (B): Number of MT1-, nestin- and MT1–nestin-coexpressing cells in specimens from noncancer (a) and invasive cancer (b) sections from breast tumors from 17 patients. The percentage of MT1- (black bars), nestin- (shaded bars) and MT1–nestin (checkered bars) coexpressing cells was calculated from data in the scattergrams, where cells in the upper right quadrants were considered MT1 positive. In panel (b), the stars and the arrows indicate patients with high numbers of MT1- and nestin-expressing cells, showing <10% (stars) and >10% (arrows) of MT1–nestin-coexpressing cells respectively. *For patient 17, NCa tissue was not analyzable.
To estimate the percentage of cells in the Ca and NCa samples, we applied quantitative Tissue Quest analysis. For this analysis, paired Ca and NCa samples were chosen in which MT1 membranous and/or cytoplasmic staining was pronounced in a uniform pattern in the whole specimen. This was the case for 17 patients, but for patient 17, only the Ca tissue was available. Clinical data of these 17 patients are given in Table 1. We found that: i) 8/16 tumors had more MT1-positive cells (>10%) in the Ca than in adjacent NCa section; ii) in 6/16 tumors, the number of MT1-positive cells was higher (>10%) in the NCa than in corresponding Ca section; and that iii) the remaining two patients showed no difference (<10%) between the NCa and Ca sections in the amount of cells positive for MT1.
| Patient no. | Agea (yr) | Stage | Histology | Grade | ER | PR | HER-2 |
|---|---|---|---|---|---|---|---|
| 1 | 63 | I | Invasive ductal | 1 | + | + | − |
| 2 | 47 | I | Apocrine | 2 | − | − | + |
| 3 | 55 | I | Invasive ductal | 2 | − | − | + |
| 4 | 68 | IIA | Invasive ductal | 2 | + | − | − |
| 5 | 53 | IIA | Invasive ductal | 3 | − | − | − |
| 6 | 42 | IIB | Invasive ductal | 2 | − | + | + |
| 7 | 68 | IIA | Invasive ductal | 2 | + | + | − |
| 8 | 75 | IIA | Invasive ductal | 3 | − | − | + |
| 9 | 29 | IIA | Invasive ductal | 3 | + | + | + |
| 10 | 71 | IIIA | Invasive ductal | 2 | + | + | + |
| 11 | 66 | IIA | Invasive ductal | 2 | + | − | − |
| 12 | 49 | IIA | Invasive ductal | 2 | − | + | + |
| 13 | 57 | IIA | Medullary | 3 | − | − | + |
| 14 | 53 | IIA | Invasive ductal | 3 | − | − | + |
| 15 | 64 | IIB | invasive lobular | 2 | + | + | − |
| 16 | 44 | IIIC | Invasive lobular | 3 | − | − | + |
| 17 | 44 | I | Invasive ductal | 3 | − | − | + |
- Patient numbers correspond to Fig. 3B.
- aAge at primary diagnosis.
In Fig. 4A, images (a–c) and the corresponding scatter plots (d–f) are given for the quantification of MT1-expressing cells in NCa and surrounding invasive Ca specimens from a representative patient. In Fig. 4B, data on the analysis of MT1-expressing cells from invasive Ca and corresponding NCa sections from 16 patients (Table 1) are depicted.
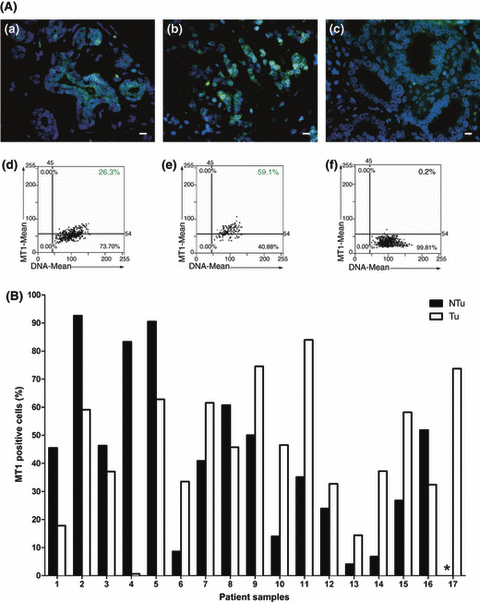
Quantitative evaluation of MT1 levels in cancerous and adjacent noncancerous breast specimens. (A) Immunofluorescence staining of MT1 (green) in nonmalignant (a) and malignant tissue (b) from patient 15 with invasive lobular breast carcinoma (G2). Nuclei (blue) were counterstained with bisbenzimide and were used for cell recognition (DNA mean) in the image analysis. Their number is given in the dot blots (d–f) which correspond to the immunofluorescence pictures (a–c). Cells were analyzed for DNA content (x) and MT1 fluorescence (y). The upper right quadrant in the dot blot shows the number of MT1 stained cells in one specimen. Detection thresholds are indicated by black lines, which have been set after image analysis of the negative control (noncancerous breast tissue, c,f), in which unspecific control IgG were used instead of the MT1 antibody. Inset bars represent 10 μm. (B) Number of MT1-expressing cells in specimens from noncancer (black bars) and invasive tumor (gray bars) sections from breast tumors from 17 patients. The percentage of MT1-expressing cells was calculated from data in the scattergrams, where cells in the upper right quadrants were considered MT1 positive. *For patient 17, NCa tissue was not analyzable.
We found no significant relationship between the amount of MT1-expressing cells in the invasive Ca and ER, PR or HER2 status, the menopausal status and the tumor differentiation grade. Moreover, quantification of cells expressing nestin was carried out in the Ca and NCa specimens from the 17 patients (Table 1). Nestin was found in 13/16 NCa specimens, and in 9/17 Ca specimens. Remarkably, these nine patients also had high MT1 staining levels (panel b in Fig. 3B). Therefore, in the next step we investigated whether MT1 might be expressed together with nestin in the same cell.
MT1–nestin double-positive myoepithelial cells were found in 11/16 NCa samples (Fig. 3B image a). As shown in Fig. 3B(b), remarkable MT1–nestin coexpression was found in 6/17 Ca sections lacking intact milk ducts. Representative images of MT1-, nestin- and MT1–nestin overlay staining in invasive parts of Ca from two patients with and without MT1–nestin coexpression are shown in Fig. 5A and B respectively.
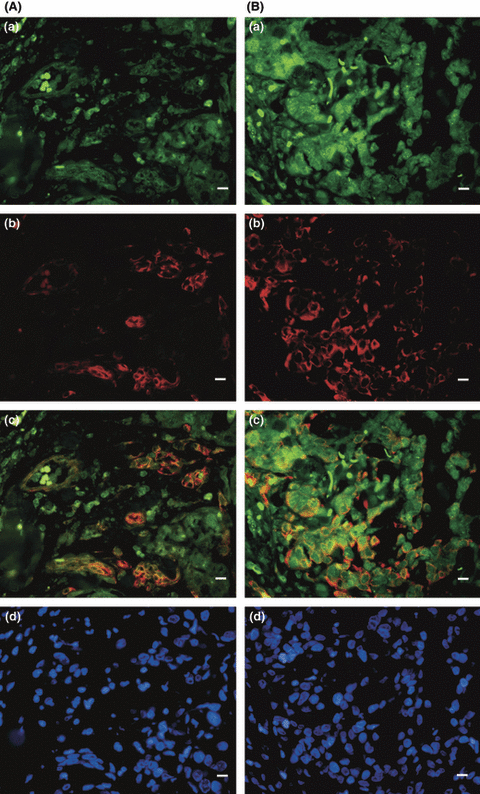
MT1 and nestin coexpression in invasive tumors containing >10% MT1- and nestin-expressing, CD10-negative cells. Immunofluorescence staining for MT1 (a), nestin (b) and the merged pictures (c) are shown in specimens from a G2 apocrine carcinoma (patient 2, A), and from an invasive ductal G3 carcinoma (patient 5, B). MT1 expression in nestin-positive/CD10-negative cells is seen in <10% in Ca sections from patient 2 (A) and >10% in patient 5 (B). In (d), blue stained cell nuclei in the apocrine (A) and the invasive ductal (B) carcinoma are shown. Inset bars: 10 μm.
In Table 2 we show that MT1 and nestin co-localization is found in six patients being in a more advanced tumor stage (IIA–IIIC). By contrast, patients without remarkable MT1–nestin colocalization were at a low tumor stage (2/3 in stage I). However, in this explorative study, differences in tumor stage between the two groups did not reach significance (P = 0.083). In addition, interestingly, 4/6 patients with MT1–nestin coexpression had ER and PR double-positive tumors (two with ER+/PR+/HER2+; and two with ER+/PR+/HER2−), whereas 2/3 patients without remarkable coexpression showed HER2-like phenotype (ER−/PR−/HER2+).
| MT1–nestin coexpression | ||
|---|---|---|
| Absent (n = 3)Patient no. 2, 6, 17 | Present (n = 6)Patient no. 5, 7, 9, 10, 15, 16 | |
| Tumor grade | ||
| G1 | 0 | 0 |
| G2 | 2 | 3 |
| G3 | 1 | 3 |
| Stage | ||
| I | 2 | 0 |
| II | 1 | 4 |
| III | 0 | 2 |
| Risk of relapsea | ||
| Low | 0 | 0 |
| Intermediate | 3 | 4 |
| High | 0 | 2 |
| Relapse | ||
| No | 1 | 5 |
| Localb | 2 | 0 |
| Distantc | 0 | 1 |
| Death | ||
| Alive | 3 | 5 |
| Dead | 0 | 1 |
| Age, mean ± SD (yr) | 44.6 ± 2.5 | 54.7 ± 15.9 |
- aRisk categories were defined as described in the Materials and methods section.
- bLocal relapse within 49 months after primary diagnosis.
- cDistant metastases in the lung, brain and bone within 8 months after primary diagnosis. This patient (16, Table 1, Fig. 3B) died from disease 11 months after primary diagnosis.
Discussion
In this study, we showed that MT1 and nestin immunoreactivity was detectable in a great majority of Ca and adjacent NCa specimens from 42 patients with breast cancer. MT1 staining was detected in luminal and myoepithelial cells in milk ducts of NCa and also in differentiated tumor areas, giving a considerable overlap with the nestin staining. In invasive tumor parts, MT1 was present in single tumor cells and cell clusters invading the stroma as well as in perivascular cells, which were also positive for nestin. The striking colocalization of MT1 with the intermediate filament protein nestin raises the question about the role of MT1 in nestin-expressing myoepithelial and also in tumor cells in breast tissue.
Mammary gland structure constantly changes in size, shape and function from the time of puberty to menopause, and it is well known that myoepithelial cells play a key role in these processes [31]. Moreover, the loss of or change in myoepithelial cell function is a key step in the transition from the in situ (i.e. tumor limited to the inside of the milk duct) to invasive breast cancer, as, under normal or even premalignant conditions, the myoepithelial cells are forming a structural barrier between the luminal epithelial cells and the surrounding stroma, thus preventing cell invasion [31, 32]. Additionally, myoepithelial cells have been shown to inhibit growth and invasion of breast cancer cells through secretion of paracrine factors, like extracellular matrix proteins and protease inhibitors [31]. These considerations support our findings that MT1 is expressed in some, but not all, nestin-positive myoepithelial cells in NCa and Ca milk ducts. Moreover, the presence of MT1 in luminal, nestin-negative cells, might be important, as the majority of breast cancers are developing from these cells.
We found MT1–nestin coexpression also in carcinoma cells in the invasive Ca parts. Both, luminal and myoepithelial cells are thought to be derived from a common precursor/stem cell, and, interestingly, in a recent experiment it was shown that neural stem cells can settle in a new microenvironment and can adopt the function of similarly endowed mammary progenitors [33]. This could also apply for human breast tumors. Therefore, it is interesting to mention that the coexpression of MT and nestin had recently been reported in a mouse neural stem cell line [26].
Cancer development and progression is characterized by dynamic changes in the expression and function of protein kinases which regulate mitogenesis, motility, invasion, cell survival and angiogenesis [34]. Various alterations in the expression or function of protein kinases or their associated signaling pathways can lead to malignant transformation of cells in the breast [35]. For example, overexpression of several receptor tyrosine kinases and deregulation of the phosphoinositide-3-kinase (PI3K) pathway were found to contribute to the development of breast cancer [36, 37]. This consideration is important, as MT1 activation by melatonin was found to regulate a number of different signaling pathways involving the second messengers cAMP, diacylglycerol, inositol triphosphate (IP3), arachidonic acid and intracellular Ca2+ ([Ca2+]i) [38, 39]. However, if melatonin was used for MT1 activation in experimental studies, rather an inhibitory effect on pathways, like the PI3K pathway, was observed, as shown by a recent study in MCF-7 breast cancer cells, where MT1 activation by melatonin, via coupling to G(αq), inhibited phospholipid hydrolysis and the induction of IP3 production [6]. Moreover, an enhancement of the retinoid-induced RARα transcriptional activity is achieved via G(αq) coupling.
Other oncostatic properties of melatonin most likely to be conferred via binding to MT1 are related to its interaction with estrogens and the ER [40]. For example, via epigenetic processes, melatonin downregulates the expression of genes responsible for the local synthesis or activation of estrogens, e.g. aromatase. Melatonin further inhibits telomerase activity and cyclin D1 expression induced by estrogens, thereby modulating the cell cycle [5]. Although these mechanisms could contribute to potential beneficial effects of melatonin on tumor progression, as shown in animal models [10, 14], they are related to the estrogen responsiveness of the cells, i.e. requiring a functional ER. Therefore, the high levels of MT1 found in our study also in ER-negative Ca specimens, are difficult to explain, but tumor suppressive effects of melatonin via MT1 in hormone-independent tumors have also been described in other studies, like in hormone refractory prostate cancer [41]. These findings suggest additional hormone-independent functions of MT1.
Both abundant and aberrant expressions of nestin were previously observed under malignant transformation [42]. In a recent study, nestin upregulation in infiltrating breast cancer cells with stem cell properties was found to be associated with the highly aggressive breast cancer subtypes [23, 24]. Moreover, in our study, high levels of nestin were present in tumor cells invading the stroma. Moreover, it has been shown that nestin-expressing progenitor cells in newly tumor-initiating populations might possess the capacity to differentiate not only into epithelial but also into endothelial cells which would be relevant for tumor growth and vascularization [25, 43]. Like in other tumors, a high rate of neovascularization is considered as poor prognostic factor in breast cancer [44].
Remarkably, higher numbers of MT1–nestin-coexpressing tumor cells invading the tissue stroma were related to a higher stage of disease, as (i) all six patients were at stages >I and as (ii) in this subgroup of six patients both stage III patients of the 17 quantified patients were included. Moreover, two patients had a high risk of relapse, if parameters like tumor size, presence of tumor cells in local lymph nodes, tumor differentiation grade, HER2 status and peritumoral vascular invasion (but not hormone receptors) were taken into consideration according to the St Gallen consensus 2005 [28]. Indeed, one patient died from distant metastases in the lung, brain and bone within 1 yr after primary diagnosis. By contrast, specimens without notable MT1–nestin coexpression in the invasive tumor derived from 3 patients being in a lower stage (<III) tumors, where two-thirds of the patients were still in stage I. However, these tumors were of the ER- and PR-negative and HER2-positive phenotype, which might explain that these patients had already an intermediate risk of relapse despite being in a low stage of disease. Despite these interesting findings, it should be mentioned that the sample size in our study was relatively low, so that further studies with a higher sample number are necessary to confirm our results.
In summary, this study revealed that MT1 is coexpressed with nestin in NCa and Ca in a considerable number of cells. High levels of MT1 in the Ca when compared with NCa specimens and the co-localization of MT1 and nestin in breast cancer cells invading the stroma were associated with a more advanced tumor stage and worse prognosis in a subset of patients. This warrants further studies on MT1-mediated signaling pathways in nestin-expressing breast cancer cells in order to elucidate the role and function of MT1 in the progression of breast cancer.
Acknowledgments
We thank Helga Stracker, Susanne Wein and Prisca Pondorfer for excellent technical assistance. This study was supported by P 2312 (Med. Wiss. Fonds des Bürgermeisters der Stadt Wien) (M.K.), Hochschuljubiläumsstiftung P 10331 (T.T.) and a grant from the Jubiläumsfonds der Österreichischen Nationalbank 12600 (W.J.).
Disclosure/conflict of interest
The authors have no conflict of interests.




