The molecular regulation of organelle transport in mammalian retinal pigment epithelial cells
Summary
Retinal pigment epithelial cells contain large numbers of melanosomes that can enter the apical processes extending between the outer segments of the overlying photoreceptors. Every day the distal portion of the photoreceptor outer segment is shed and phagocytosed by the retinal pigment epithelial cell. The phagosome is then transported into the cell body and the contents degraded by lysosomal enzymes. This review focuses on recent progress made in the identification of molecules that regulate the transport of melanosomes into the apical processes and the transport of phagosomes into the cell body. Myosin VIIa is a key player in both processes and, at least in the case of melanosome movement, myosin VIIa is recruited to the melanosome via the GTPase, Rab27a. The possible role played by defects in the transport of melanosomes and phagosomes in the development of retinal degenerative diseases is discussed.
Retinal pigment epithelial (RPE) cells are a pigmented monolayer of cells that form part of the blood/retina barrier. Their apical surface forms numerous long processes that partially envelop the outer segments of the photoreceptors. This close interaction between the RPE and photoreceptors is critical for the maintenance of visual function. It facilitates (i) the transfer of retinal between photoreceptors and RPE which is required for the visual cycle of retinal and the maintenance of photoreceptor excitability; and (ii) the phagocytosis by RPE cells of the daily shed tips of the outer segments of photoreceptors, a process essential for photoreceptor survival. The basolateral surface of the RPE lies on Bruch's membrane, which separates the RPE from the choriocapillaris, a layer of fenestrated capillaries. The RPE transports nutrients from the blood to the retina and transports ions, water and waste products from the sub-retinal space to the blood. This review will focus on two aspects of RPE cell biology; the regulation of melanosome transport and phagosome transport.
Engulfment of the distal tip of the photoreceptor outer segment is regulated by light and circadian rhythm and requires alphavbeta5 integrins, CD36 and the Mer tyrosine kinase (reviewed in Strauss, 2005). Less is known about the molecular regulation of the subsequent processing of the phagosome which must move into the cell body to fuse with lysosomes before degradation of the phagosome content (see Figure 1) (Bosch et al., 1993; Herman and Steinberg, 1982a,b). However, it is becoming clear that defects in the processing of the products of photoreceptor phagocytosis are likely to contribute to the pathogenesis of many retinal degenerative diseases.
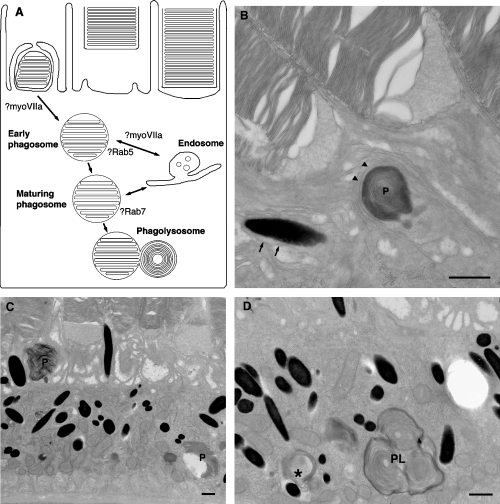
Phagocytosis of rod outer segments in RPE cells. (A) A possible model for phagosome maturation and fusion with the lysosome, indicating where myoVIIa and Rabs might act. (B) A phagosome (P) where the engulfment by apical processes (arrowheads) is almost complete. Arrows indicate the distension of the membrane of the apical process around the melanosome. (C) Phagosomes both newly engulfed and deep in the cell body. (D) A putative phagolysosome (PL) and a possible product of melanosome–phagosome fusion (asterisk). Bar = 0.5μm.
Another organelle within the RPE whose motility is regulated by a light and/or circadian rhythm is the melanosome. Melanin within melanosomes of the RPE plays an important role in the development of the neural retina. Albino animals show abnormal patterns of cell proliferation during development and at maturity have reduced numbers of photoreceptors and abnormal chiasmatic pathways (Jeffery, 1997). Melanin within melanosomes of RPE cells also absorbs light scatter and is likely to aid in protection against photo-oxidation. Melanosomes within the RPE of fish and amphibians exhibit a dramatic redistribution from the cell body to the apical processes upon light onset, which is reversed in the dark (Burnside and Laties, 1979). Until recently the melanosomes of mammalian RPE cells were thought not to move but have now been shown to undergo a modest redistribution into the apical processes of murine RPE cells after light onset (Futter et al., 2004) and to exhibit motility in isolated primary murine RPE cells (Gibbs et al., 2004). The regulation of melanosome motility has been extensively studied in melanocytes of the skin where melanosomes move to the cell periphery along microtubules and are trapped in the cell periphery by interaction with the cortical actin cytoskeleton, a process which is essential for their transfer to neighbouring keratinocytes (Wu et al., 1998). Interaction of the melanosome with the actin cytoskeleton is mediated by a tripartite complex of the small Ras-like GTPase, Rab27a, melanophilin and myosin Va, where Rab27a binds to the melanosome, myosinVa to the actin cytoskeleton, and melanophilin acts as a linker (Wu et al., 2001, 2002; Fukuda et al., 2002; Hume et al., 2001, 2002; Provance et al., 2002; Strom et al., 2002).
Recent studies on the molecular regulation of melanosome movement in RPE cells have revealed both parallels and differences with skin melanocytes. In addition, apparently some of the same molecular machinery that regulates melanosome movement may also regulate phagosome movement. That some of these molecular regulators are defective in human diseases that exhibit retinal degenerations suggests that a fuller understanding of organelle motility in RPE cells may shed light on the pathogenesis of these diseases.
Myosin VIIa – a regulator of melanosome and phagosome distribution in RPE cells
Myosin VIIa was the first molecular regulator of melanosome distribution to be identified in mammalian RPE cells. The shaker-1 mouse is deficient in myosin VIIa (Gibson et al., 1995; Mburu et al., 1997), a plus-end-directed motor (Inoue and Ikebe, 2003) that within the retina has been localized to the cilium of the photoreceptors, to the apical region of RPE cells (Wolfrum et al., 1998), and to melanosomes within RPE cells (El-Amraoui et al., 2002; Gibbs et al., 2004). Three functions of myosin VIIa have been identified within the retina: (i) the regulation of the distribution of melanosomes within the apical processes of RPE cells (Liu et al., 1998); (ii) the transport of opsin from the inner to the outer segment of the photoreceptors (Liu et al., 1999); and (iii) the transport of phagosomes from the apical region to the cell body in RPE cells (Gibbs et al., 2003).
Within the RPE of shaker-1 mice melanosomes are found exclusively in the cell body and are excluded from the apical processes, indicating that myosin VIIa is required for movement of melanosomes into, or retention of melanosomes within, the apical processes (Liu et al., 1998). In RPE cells, F-actin is found just beneath the apical plasma membrane, in the circumferential actin filaments extending from the adherens junctions, and within the apical microvilli, and is largely absent from the cell body (Figure 2). In the absence of myosin VIIa melanosomes reside exclusively in the microtubule rich cell body, and so myosin VIIa may be required for transfer from microtubules to the actin rich apical region and for transport through it. Microtubules are completely absent from the apical processes (Figure 2) (Burnside and Laties, 1979; Futter et al., 2004), and so the actin cytoskeleton is likely to be involved in the subsequent movement up the apical processes. Whether myosin VIIa or other motor proteins are required for this process has yet to be shown. The narrow diameter of the apical process, compared with the diameter of the melanosome, and the distortion of the plasma membrane of the apical process that occurs as the melanosome moves up it (see example in Figure 1) suggests that force is required for this movement.
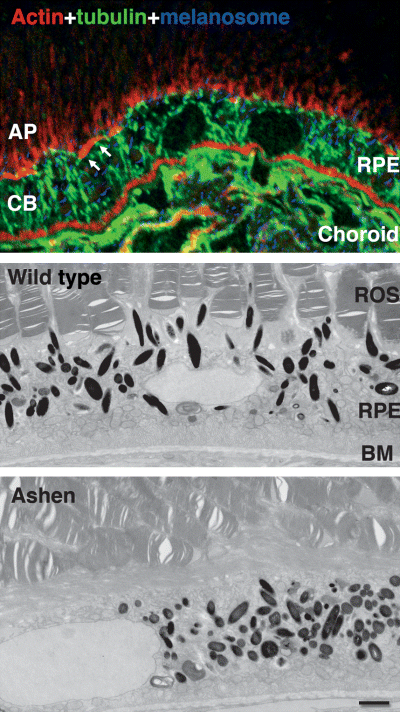
Transfer of melanosomes from the microtubule rich cell body to the actin rich apical processes. Confocal microscopy of sections through wild-type retina shows a meshwork of microtubules (green) in the cell body of RPE cells but microtubules are absent from the apical processes. Phalloidin staining (red) shows F-actin associated with the infoldings of the basal plasma membrane, with the circumferential actin ring extending from the adherens junctions (visible in 0.5 μm sections as short stretches, indicated by arrows) and within the apical processes. Melanosomes (artificially coloured blue) are in the microtubule rich cell body but are also found associated with the actin-rich apical processes. Transmission EM shows that in wild type mice the melanosomes are oriented parallel to the longitudinal axis of the RPE cell and enter the apical processes that extend between the rod outer segments (ROS). BM, basement membrane. In the ashen mouse, the melanosomes are excluded from the apical processes. Bar = 2.0μm. Reproduced from Futter et al. (2004).
Thus, myosin VIIa plays a key role in regulating melanosome distribution in RPE cells which may be equivalent to the role of myosin Va in skin melanocytes. Although myosin Va is present on melanosomes in RPE cells the distribution of melanosomes within RPE cells of the dilute mouse (lacking myosin Va) is normal (Gibbs et al., 2004).
The shaker-1 mice also exhibit a defect in the uptake and processing of phagocytosed rod outer segments (Gibbs et al., 2003). It is not clear whether the reduced phagocytosis is due to a defect in shedding of outer segments or in the uptake process. However, there is a very clear inhibition in the subsequent movement of the phagosome from the apical region to the cell body and in the degradation of the phagosome content (Gibbs et al., 2003). This at first appears somewhat paradoxical as myosin VIIa is required for the transport of melanosomes in a basal to apical direction and of phagosomes in the opposite direction. Possibly the role of myosin VIIa in phagosome transport is indirect and may be required for efficient interaction of the phagosome with the endocytic pathway, as has been proposed for myosin Va (Al-Haddad et al., 2001).
In macrophages myosin V colocalises with the fully internalized phagosome (Swanson et al., 1999) and in macrophages of dilute lethal mice (lacking myosin Va) phagosomes show altered motility. Their accumulation in the perinuclear region of the cell is accelerated because short-range movements to the cell periphery are prevented (Al-Haddad et al., 2001). These authors proposed that the short-range movements to the cell periphery are necessary for interaction of the phagosome with the endocytic pathway. Phagosome maturation, at least in macrophages where it has been studied extensively, involves interaction first with the early endosome and then with late endosomes and lysosomes (Desjardins et al., 1994). These interactions require sequentially Rab5 and then Rab7. Rab5 recruits the phosphatidylinositol-3-kinase, Vps34, the activity of which recruits EEA1 (Fratti et al., 2001; Vieira et al., 2003). EEA1 is a phosphatidylinositol-3P-binding protein localized to early endosomes that acts as a docking factor required for early endosome–endosome fusion (Christoforidis et al., 1999). EEA1 recruitment to the phagosome is required for subsequent phagosome maturation, whilst Rab7 is required for fusion with lysosomes and degradation of phagosome content (Harrison et al., 2003). Although phagosome maturation has been less extensively studied in RPE cells phagosomes containing latex beads stain for Rab5 at early time points and markers of the lysosome at later time points (Hoppe et al., 2004). A crucial question is where within RPE cells do phagosomes acquire the markers of phagosome maturation, such as Rab 5, EEA1 and Rab 7 (see Figure 1), and what is the effect of loss of myosin Va and myosin VIIa on their acquisition by the phagosome?
Inhibitors of both the microtubule and actin cytoskeletons inhibit phagosome maturation (Desjardins et al., 1994) and phagosome-lysosome fusion (Funato et al., 1997). Rab5 regulates early endosome association with and movement along microtubules (Nielsen et al., 1999). If myosin VIIa is required for association of the phagosome with early endosomes, and/or the acquisition of Rab5, then loss of myosin VIIa could indirectly affect microtubule-dependent transport into the cell body and subsequent lysosome fusion.
Rab27a and its effectors in the regulation of melanosome distribution in RPE cells
Studies using cells from the ashen mouse, which lacks Rab27a, have shown that in melanocytes of the skin this GTPase binds to the melanosome and mediates interaction with the actin cytoskeleton via myosin Va (Wu et al., 2001, 2002; Hume et al., 2001). Rab27a also binds to the melanosome in RPE cells and in the RPE of the ashen mouse the melanosomes remain in the cell body (Figure 2), failing to move beyond the level of the adherens junctions and thus have a phenotype with respect to melanosome distribution indistinguishable from the RPE of the shaker mouse (Futter et al., 2004; Gibbs et al., 2004).
Further studies have shown that Rab27a is a marker of mature secretory organelles and may play an important role in regulated exocytosis from both conventional secretory cells (such as endocrine and exocrine glands) and haematopoietic cells (Tolmachova et al., 2003). Rab27a appears to be important for actin-based motility of both melanosomes and secretory granules via the recruitment of unconventional myosin motors. The interaction with unconventional myosins is indirect, via linker myosin and Rab-interacting proteins. One such protein acting in skin melanocytes is melanophilin, which is required for the peripheral capture of melanosomes (Fukuda et al., 2002; Provance et al., 2002; Strom et al., 2002). Another rab effector, MyRIP, has been implicated in RPE melanosome motility because of its expression in RPE cells and its ability to bind both Rab27a and myosin VIIa (El-Amraoui et al., 2002). Based on these observations, it was proposed that a tripartite complex of Rab27a-MyRIP-myosin VIIa regulates melanosome distribution in RPE cells, in a manner analogous to the Rab27a–melanophilin–myosin Va complex that operates in melanocytes (El-Amraoui et al., 2002). However, in the absence of a MyRIP knockout mouse no direct evidence for a role for MyRIP in the maintenance of melanosome distribution in RPE cells has been obtained. Furthermore, MyRIP has been shown in vitro to bind myosin Va as well as myosin VIIa, and to bind actin directly (Fukuda and Kuroda, 2002). MyRIP also has a role in secretory granule exocytosis that may be independent of myosin V or VIIa (Desnos et al., 2003; Waselle et al., 2003). It has been reported that MyRIP/myosin VIIa can rescue the normal peripheral distribution of melanosomes in melanocytes lacking melanophilin (Kuroda and Fukuda, 2005), indicating that in an intact cell MyRIP can bind myosin VIIa and act as a linker between Rab27a on melanosomes and the actin cytoskeleton. MyRIP, therefore, is a likely candidate for playing a role in maintaining melanosome distribution in RPE cells but at which step remains to be shown.
Rab27a is able to recruit at least 11 different effector proteins (Fukuda, 2005) which fall into four groups: (i) Slps (Slp1 through 5), for ‘synaptotagmin-like proteins’ which contain two C2 domains (Ca2+ and phospholipid-binding domains); (ii) melanophilin and MyRIP which bind Rab27a and myosins but lack C2 domains (also referred to as Slacs for ‘Slps lacking C2 domains’); (iii) Rabphilin and Noc2 which also contain C2 domains and bind promiscuously to both Rab3 and Rab27 proteins; and (iv) Munc13–4, a putative regulator of SNARE complexes. A recent study showed that, in addition to melanophilin, a second Rab27a effector, Slp2-a, is bound to the melanosome and regulates melanosome distribution in melanocytes (Kuroda and Fukuda, 2004). Therefore, possibly in RPE cells more than one Rab27a effector protein regulates the distribution of melanosomes, and multiple Rab27a effectors are expressed in the RPE (Gibbs et al., 2004). The fact that melanosomes are unable to access the actin-rich apical region within RPE cells in both ashen and shaker mice suggests that Rab27a–MyRIP (probably)–myosin VIIa regulates transfer of melanosomes from the microtubule-rich cell body to the apical actin cytoskeleton, although other Rab27a effectors could be involved in subsequent transport steps.
A recent study has indicated that another Rab protein, Rab8, is also involved in actin-dependent movement of melanosomes in melanocytes, but is unlikely to operate through myosin V (Chabrillat et al., 2005). Rab8 may directly regulate transient interactions with the actin cytoskeleton. Rab27a and Rab8 can localize to the same melanosome but how the activities of the two Rab proteins is co-ordinated is as yet unclear. A role for Rab8 in regulating melanosome distribution in RPE cells has not been reported.
Whether Rab27a, like myosin VIIa, plays a role in uptake of photoreceptor outer segments or in their subsequent intracellular transport and degradation has yet to be determined. As described above for myosin VIIa, Rab27a could play an indirect role in phagosome processing by either regulating interactions with endosomes or even by regulating interactions with melanosomes. A link between phagosomes and melanosomes has been suggested by the frequently observed fusion profiles between melanosomes and phagosomes (see Figure 1 for a possible example). Indeed following phagocytosis of gold labelled outer segments which have been injected into the subretinal space, gold particles are found within melanosomes within the RPE (Schraermeyer et al., 1999). Melanosomes are lysosome-related organelles and contain some degradative enzymes. Whether they play any role in the degradation of the phagosome content, the degradation products of which could be used for generation of more melanin pigment, is not clear. It is interesting to note, however, that mature melanosomes within melanocytes are not accessible to endocytic probes (Raposo et al., 2001), suggesting a potential functional specialisation of melanosomes within RPE cells.
The potential roles of defects in organelle transport within RPE cells in the pathology of eye diseases
Defects in the degradation of phagocytosed outer segments
The shaker-1 mouse, deficient in myosin VIIa, is the mouse model for the human disease, Usher syndrome 1B (Weil et al., 1995). Patients with this disease suffer from progressive retinal degeneration, are profoundly deaf and exhibit vestibular defects (Petit, 2001). The finding that RPE cells of shaker-1 mice have a defect in both the initial uptake of outer segments and in their subsequent transport raises the possibility that this could cause the retinal degeneration observed in Usher syndrome 1B.
A major defect in phagocytosis of outer segments would be expected to promote photoreceptor degeneration, as has been observed in the RCS rat (Bok and Hall, 1971; Dowling and Sidman, 1962; Herron et al., 1969) which lacks functional mer tyrosine kinase (D'Cruz et al., 2000), in the mer knockout mouse (Duncan et al., 2003) and in the β5 knockout mouse (Nandrot et al., 2004). A defect in the processing of phagocytosed photoreceptor outer segments might be expected to lead to the progressive accumulation of toxic degradation products of photoreceptor outer segments within the RPE. The accumulation of lipofuscin within RPE cells that occurs with age, and is increased in patients suffering from age-related macular disease (AMD), is believed to originate from incompletely digested outer segments (Kennedy et al., 1995). A severe inhibition of degradation of photoreceptor outer segments in transgenic mice expressing a mutant form of cathepsin D, the most critical enzyme for the digestion of rhodopsin, leads to accumulation of lipofuscin and basal deposits that have some, but not all, the characteristics of the basal deposits (Drusen) observed in AMD (Rakoczy et al., 2002). The shaker-1 mice exhibit some electro-retinographic abnormalities, namely a reduction in the wave response to light (Libby and Steel, 2001), but do not show any retinal degeneration. The accumulation of lipofuscin and basal deposits have not been described in the shaker-1 mice, but studies on aging mice have not been reported. They do, however, exhibit hearing and vestibular defects. The reason for this difference between the effects of loss of function of myosin VIIa between mice and humans is not clear. If the retinal degeneration seen in Usher Syndrome 1B is caused by a reduction in the ability of the RPE to take up or degrade outer segments, it may be that the mice simply do not live long enough to exhibit these effects. Each RPE cell is exposed to approximately 40 photoreceptors and as there is a little or no proliferation of adult RPE cells a single human RPE cell can phagocytose an enormous number of photoreceptor discs during a 70 year life span. Even a minor defect in the processing of the phagocytosed discs could lead to a considerable accumulation of undigested material within the RPE over this time scale, but possibly not during the more limited life time of a mouse.
Defects in melanosome movement in RPE cells
The dilute, ashen and leaden mice are models of Griscelli syndrome types 1, 2 and 3, respectively (Menasche et al., 2000, 2003; Pastural et al., 1997). All three types are characterized by pigment dilution but mutations in myosin V also lead to a severe neurological impairment (Pastural et al., 1997), mutations in rab27a lead to a haemophagocytic syndrome (Menasche et al., 2000), whilst the disease caused by mutations in melanophilin is restricted to hypopigmentation (Menasche et al., 2003). Patients do not suffer retinal degeneration but this could be due to their early death. The ashen mice also do not exhibit retinal degeneration (Futter et al., 2004) but, as suggested above for the shaker-1 mouse, the comparatively short life of the mouse may prevent this. Several potential roles for melanosome movement in protection from retinal degeneration can be envisaged. It has been proposed that the presence of melanosomes within the apical processes might reduce disc shedding and their phagocytosis by shielding the photoreceptor outer segments from light (Sarangarajan and Apte, 2005). The rate of disc shedding has not been measured in the ashen or shaker-1 mice. Phagocytosis, at least in the shaker-1 mouse, is reduced but this could be due to a direct role for myosin VIIa in phagocytosis. Phagocytosis itself produces peroxides and having melanin granules surrounding the newly formed phagosome may rapidly absorb peroxides and prevent their damaging effects.
Rab27a has been implicated in retinal degeneration through studies of X-linked choroideraemia (CHM) (Seabra et al., 2002), which is caused by mutations in the Rab Escort protein, REP1 (Cremers et al., 1990; Merry et al., 1992). The disease affects not only the RPE, but also the adjacent photoreceptors and the choroid and affected males exhibit slow retinal degeneration and are blind by middle age (Heckenlively and Bird, 1988; McCulloch, 1988). Rab escort proteins are required for Rab prenylation, which is an absolute requirement for Rab function (Seabra and Wasmeier, 2004). Many Rabs are prenylated normally in X-linked CHM patients through the action of REP2 (Cremers et al., 1994; Seabra, 1996). However, Rab27a appears to be a poor substrate for REP2 and lympoblasts from CHM patients have an excess of unprenylated Rab27a (Seabra et al., 1995). This observation, together with the high expression of Rab27a in the RPE and the choroid, lead to the suggestion that defects in the function of Rab27a might be responsible for CHM. As other tissues where Rab27a has important functions are comparatively unaffected in CHM it now appears that at least some Rab27a must be prenylated in these patients. Indeed, in recently developed conditional mouse models of choroideremia in which the degeneration of photoreceptors and RPE cells has been shown to arise independently, different subsets of Rabs show defective prenylation in the two cell types (Tolmachova et al., 2006).
Conclusion
This review serves to highlight as much what we do not know as what we do know about the molecular regulation of melanosome movement and phagosome maturation within RPE cells. This is partly because immortalized RPE cell lines and isolated RPE cells in culture do not readily maintain their pigmentation or their extensive apical processes and, although they can phagocytose rod outer segments, aspects of their in vivo regulation by interaction with photoreceptors and Bruch's membrane, are lost. Recently, mouse models have allowed the delineation of the roles of proteins like myosin VIIa and Rab27a in phagocytosis and melanosome motility. Differences between mice and humans in terms not only of their retinal architecture, but also of their longevity, has so far made the roles of defects in these processes in human retinal degenerative diseases difficult to determine. In addition, elucidation of the role of other molecular components regulating organelle transport within the RPE is limited by the lack of suitable mouse models. The development of systems to manipulate primary RPE cells in culture, under conditions where they more closely reproduce their in vivo phenotype, should allow further progress in our understanding of the processes regulating organelle transport in a cell that plays a central role in maintaining the neural retina and hence vision.
Acknowledgements
The author would like to thank Miguel Seabra and Steve Moss for critical reading of this review. Work in the author's laboratory is supported by Cancer Research UK, Wellcome Trust, BBSRC, Fight for Sight and the Special Trustees of Moorfields Eye Hospital.