Studies of pigment transfer between Xenopus laevis melanophores and fibroblasts in vitro and in vivo1
The first and second authors have contributed equally.
Summary
Frog melanophores rapidly change colour by dispersion or aggregation of melanosomes. A long-term colour change exists where melanosomes are released from melanophores and transferred to surrounding skin cells. No in vitro model for pigment transfer exists for lower vertebrates. Frog melanophores of different morphology exist both in epidermis where keratinocytes are present and in dermis where fibroblasts dominate. We have examined whether release and transfer of melanosomes can be studied in a melanophore-fibroblast co-culture, as no frog keratinocyte cell line exists. Xenopus laevis melanophores are normally cultured in conditioned medium from fibroblasts and fibroblast-derived factors may be important for melanophore morphology. Melanin was exocytosed as membrane-enclosed melanosomes in a process that was upregulated by α-melanocyte-stimulating hormone (α-MSH), and melanosomes where taken up by fibroblasts. Melanosome membrane-proteins seemed to be of importance, as the cluster-like uptake pattern of pigment granules was distinct from that of latex beads. In vivo results confirmed the ability of dermal fibroblasts to engulf melanosomes. Our results show that cultured frog melanophores can not only be used for studies of rapid colour change, but also as a model system for long-term colour changes and for studies of factors that affect pigmentation.
Introduction
Melanophores from fish and amphibians are cells specialized for rapid, synchronous and bi-directional transport of pigment organelles. The black or brown pigment melanin is found in membrane-enclosed melanosomes, which co-ordinately disperse or aggregate after appropriate external stimuli and allow rapid colour changes in response to variations in the environment. When the melanosomes aggregate to the cell centre, skin lightening occurs, whereas dispersal of pigment throughout the cell results in skin darkening. This is known as physiological colour change. Environmental information is processed in the central nervous system (CNS) and transmitted to the melanophores. Both hormonal and neuronal regulations result in appropriate chromatic reactions allowing quick colour changes that are used for camouflage and communication between individuals (for reviews see Fujii, 2000, Sugden et al., 2004).
In Xenopus laevis and Rana pipiens, melanophores are found both in epidermis and in dermis, in contrast to the restricted localization of melanocytes to the epidermis in mammals. Several differences exist between epidermal and dermal melanophores. They are smaller and more oval in epidermis where they are located among keratinocytes. In dermis, the melanophores are larger and have long dendritic processes, and they are surrounded with connective tissue where fibroblasts are common. It is the melanophores in the dermis that are usually associated with the rapid physiological colour change (Zuasti et al., 1998).
Details of melanophore colour change have recently been successfully studied in melanophore cell cultures and pigment transport is highly dependent on both microtubules and microfilaments (Nascimento et al., 2003; Nilsson Sköld et al., 2002). The most common experimental system is Xenopus laevis melanophores derived from immortalized cellular lines (Daniolos et al., 1990). Recently, we have become interested in not only the physiological colour change, but also the release and transfer of melanosomes from melanophores, an important part of the long-term morphological colour change of frogs. Melanosomes are observed in keratinocytes in frog skin, suggesting an active transfer of pigment (Hadley and Quevedo, 1967; Zuasti et al., 1998). To learn more about these processes it is necessary to work with cell cultures. However, no keratinocyte Xenopus cell lines are available today. We decided, therefore, to explore whether we could co-culture Xenopus melanophores with fibroblasts, based on the knowledge that conditioned Xenopus fibroblast medium with so far unknown fibroblasts-derived factors is necessary for successful culturing of Xenopus melanophores. We were also interested to determine whether fibroblasts could affect melanophore morphology to explain the morphological differences in dermis and epidermis.
The mechanism of transfer of melanosomes into other cells is not yet fully understood, but four processes have been postulated for the mechanism of export of pigment based on experiments in mammals: (1) cytophagocytosis; (2) release of melanosomes into the intracellular space, followed by endocytosis of melanosomes by recipient keratinocytes; (3) transfer of melanosomes through a communication pore between the pigment cell and the keratinocyte; and (4) direct inoculation of melanosomes to keratinocytes (Jimbow and Sugiyama, 1998). Several differences exist between mammalian melanocytes and frog and fish melanophores both regarding regulation and intracellular transport of pigment. No in vitro studies of transfer have been performed with melanophores, making it interesting to determine whether similar transfer mechanisms exist, or if the mechanisms have changed in correspondence with the loss of the ability of rapid colour changes in mammalian melanocytes.
Our results show that release and transfer of melanosomes can be studied and also stimulated by α-melanocyte-stimulating hormone (α-MSH) in co-cultures of frog melanophores and fibroblasts, making this cell culture even more useful. The fibroblasts induced dendritic morphological changes of the melanophores, indicating that fibroblasts-derived factors could be of importance for the dendritic morphology of melanophores in frog dermis. We found that melanosomes could be taken up by the fibroblasts. We found evidence for exocytosis/endocytosis and cytophagocytosis, but no direct evidence for inoculation or transfer through communication pores. Melanin was transferred as membrane-surrounded melanosomes and membrane proteins were most probably of importance, as the specific uptake pattern of melanosomes in clusters of fibroblasts was distinct from a more general phagocytosis of latex beads. In addition, the results in vitro were supported by in vivo studies, which confirmed presence of extracellular pigment in dermis and uptake of melanosomes by fibroblasts.
Results
Fibroblasts affect the cellular morphology of melanophores
Xenopus laevis melanophores derived from immortalized cellular lines can be grown in culture as long as medium conditioned by corresponding fibroblasts is added. When the melanophores were cultured together with fibroblasts, the melanophores initially appeared to change their shape and the position of their pigment mass in response to the distribution of fibroblasts. The melanophores became curved and tended to spread in the direction of groups of fibroblasts (Figure 1A,B). Some melanophores also protruded dendritic extensions between fibroblasts and over the surface of individual cells (Figure 1C,D). In areas where melanophores had spread over the plastic before they were surrounded by fibroblasts, the pigment cells had a somewhat rounded morphology (Figure 1E). The boundaries were even and the cells had very few protrusions. In contrast, when the melanophores competed with fibroblasts for space during division, the cells were much more dendritic with long and slender protrusions (Figure 1F).
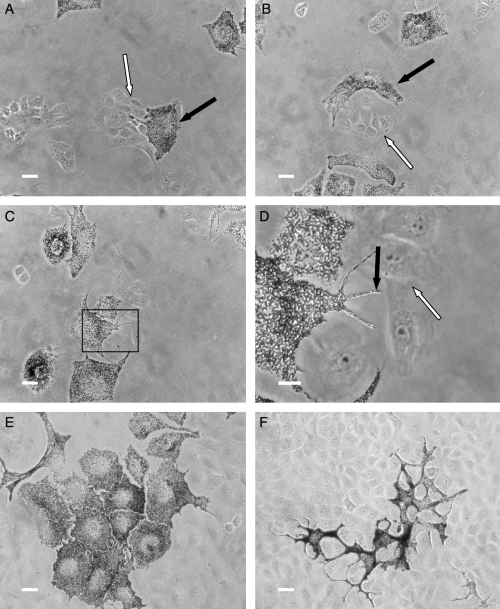
Early co-cultures of Xenopus laevis fibroblasts and melanophores. The presence of fibroblasts (white arrows) altered the cellular morphology of pigment cells (black arrows). (A) Melanophores tended to position their dendrites and pigment mass in the direction of fibroblasts. (B) A melanophore that has become curved around a group of fibroblasts. (C) In some cases melanophores protruded dendrites towards a cluster of fibroblasts. (D) Magnification of the box in (C). (E) Melanophores that spread over the plastic before contact with fibroblasts had a rounded morphology and few protrusions. (F) Melanophores that competed with fibroblasts for space became much more dendritic. Scale bar = 10 μm.
With these experimental settings, the cultures lasted approximately 6–7 d. When the cultures became confluent, melanophores tended to aggregate their pigment and detach from the culture plate.
Melanophores transfer pigment to fibroblasts in co-culture
Within 16 h of co-culturing, transfer of pigment from melanophores to fibroblasts was observed. Transfer took place in fibroblasts in close proximity of pigment cells (Figure 2A, B) as well as in fibroblasts without any apparent melanophores in the vicinity (Figure 2C,D). This indicates that cell–cell contact is not required for transfer. At first transfer was limited to distinct groups of fibroblasts. The melanosomes were evenly distributed in the fibroblast cytoplasm (Figure 2B), and the amount of transfer of melanosomes increased over time.
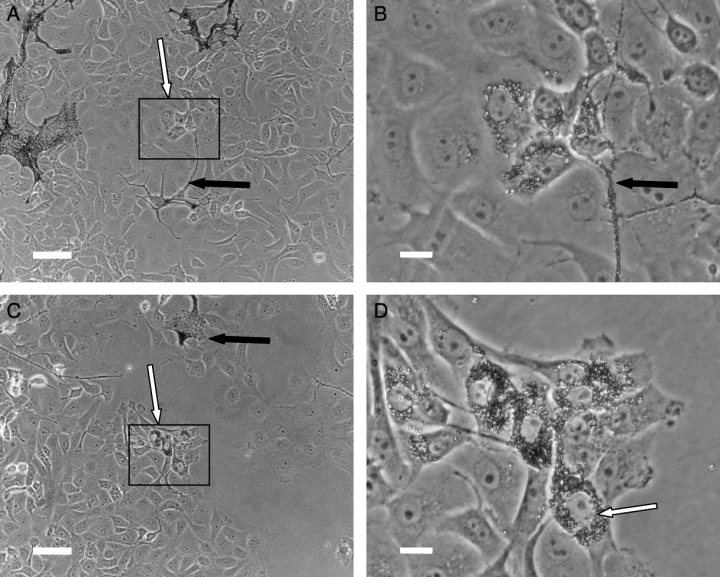
Co-cultures of Xenopus laevis fibroblasts and melanophores. Melanosomes were transferred to fibroblasts situated at a distance from melanophores (A,B) as well as to cells that were located close to melanophores (C,D). The transfer was restricted to discrete groups of fibroblasts. Scale bar (A,C) = 50 μm, (B,D) = 10 μm.
To conclude that cell material is actually transferred between the two cell types, and to exclude that the pigment is synthesized inside the fibroblasts after contact with melanophores, we used two different cell trackers. 5-Chloromethylfluorescein diacetate (CMFDA) is a tracking dye that can pass freely through cell membranes, but once inside the cell it is transformed into a cell-impermeant reaction product. CMFDA is colourless and non-fluorescent until cytosolic esterases cleave off the acetates, revealing a strongly fluorescent product that stains the cytoplasm in a relatively uniform manner. Since the fluorescent CMFDA is unable to diffuse across the membrane, any dye present in previously unlabelled cells must be the result of active transfer of cell material, in this case melanosomes or cell fractions containing melanosomes. Transfer of pigment occurred in co-cultures with melanophores pre-stained with CMFDA (Figure 3A, B). The subsequent staining of fibroblasts was granular and co-localized with transferred pigment, which confirms transfer of melanosomes from melanophores to fibroblasts in culture. We can also confirm that the pigment is not produced by the fibroblasts themselves.
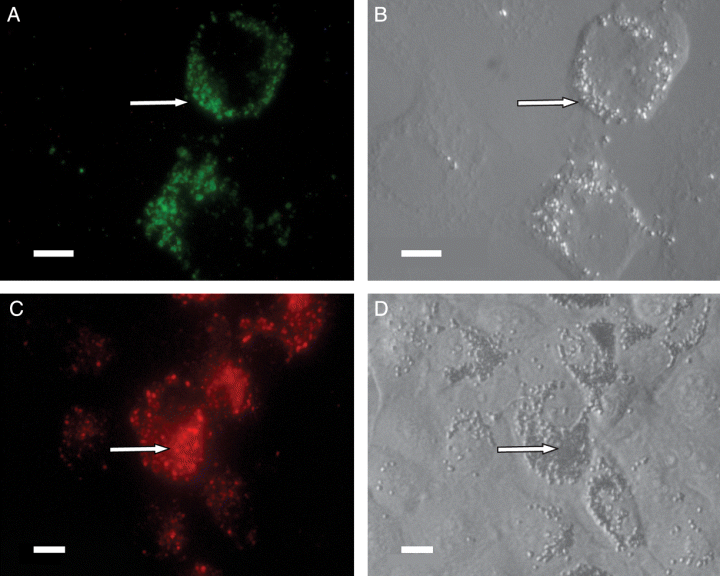
Melanophores labelled with CMFDA (green fluorescence) or DiI (red fluorescence) were co-cultured with fibroblasts. Subsequent fluorescence in fibroblasts (A and C) co-localized with uptake of melanosomes (B and D), confirming transfer of cell material from melanophores to fibroblasts in membrane-bound vesicles. Scale bar = 50 μm in all panels.
To further investigate whether the pigment is transferred as free melanin or in membrane-bound vesicles, we used 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI), which is a lipophilic dye that incorporates in the plasma membrane and various intracellular membranes. We observed transfer of DiI to the fibroblasts, and the punctuate staining co-localized with transferred melanosomes (Figure 3C,D). As DiI is trapped in membranes, this experiment indicates that melanin is transported between cells in membrane-enclosed vesicles or with cell–cell interactions.
Effects of α-MSH on pigment distribution, transfer and phagocytic capacity of fibroblasts
Dispersion of melanosomes to the dendritic tips is a pre-requisite for transfer of pigment in human melanocytes. α-MSH induces pigment dispersion in a dose-dependent manner in Xenopus melanophores (Figure 4A). There was no difference in dispersion rate between the three concentrations used after 24 or 48 h (not shown). α-MSH is also a major physiological signal that modulates skin pigmentation. To determine whether α-MSH influences transfer and uptake of pigment, we incubated co-cultures with different concentrations of α-MSH. The highest concentration of α-MSH (120 nM) significantly increased the transfer of melanosomes in co-cultures but incubation with the lower concentrations of α-MSH did not significantly affect the melanosome transfer (Figure 4B). In order to investigate whether α-MSH affects the general phagocytic capability of fibroblasts, we incubated fibroblast monocultures with fluorescent beads and different concentrations of α-MSH. α-MSH did not significantly increase the uptake of beads by fibroblasts (Figure 4C), suggesting that it is melanosome release rather than the efficiency of melanosome uptake that is affected by α-MSH in co-cultures.
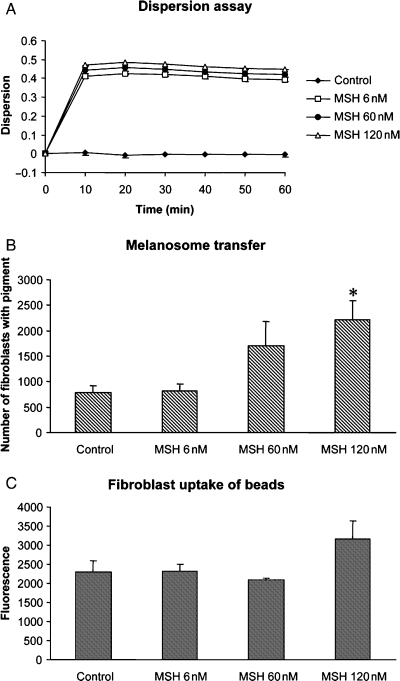
Effects of α-MSH on pigment distribution, transfer and phagocytic capacity of fibroblasts. (A) Microplate assay showing kinetic responses of dispersion in melanophores. Concentration-dependent dispersion was induced by α-MSH. Absorbance data was measured every 10th minute for 90 min. Dispersion was calculated as 1–10Ai-Af and displayed as means ± SEM (n = 3). (B) Transfer of pigment to fibroblasts after 48 h of co-culturing as quantified by counting the number of fibroblasts with pigment in 100 grids per plate in six plates per α-MSH concentration. α-MSH (120 nM) significantly increased the transfer of melanosomes in co-cultures (*P < 0.05). Data are presented as means ± standard error (SE). C: α-MSH did not affect the capacity of fibroblasts to phagocyte fluorescent beads (n = 6). Data are presented as means ± standard error (SE).
Melanosomes are released and transferred in several different ways
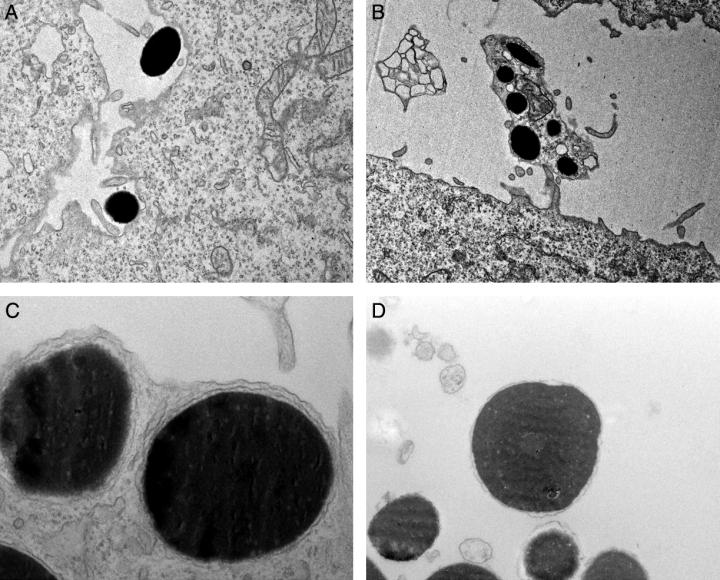
To study transfer in more detail, we used electron microscopy. We could distinguish fibroblasts from melanophores by the larger size and dendritic shape of the pigment cells. In addition, the cytoplasm of melanophores is scattered with melanosomes. Using electron microscopy, we found melanosomes inside fibroblasts throughout the co-cultures, often in neighbouring cells (Figure 5A). Transfer was found close to melanophores, but also in fibroblasts located without melanophores in the vicinity, confirming the results from the light microscopy. The melanosomes did not seem to be organized, but were scattered throughout the fibroblast cytoplasm. In many cases, melanosomes seemed to be taken up individually, surrounded by a double membrane (Figure 5B). Some melanosomes were taken up in groups (Figure 5C,D). A double membrane surrounded each pigment granule in the cluster, but they were also enclosed in a common outer double membrane.

Co-cultures of melanophores and fibroblasts were examined by electron microscopy. (A) Uptake of melanosomes by two neighbouring fibroblasts. The pigment granules are spread in the fibroblast cytoplasm. 5000×. (B) A melanosome that has been taken up by a fibroblast. A double membrane surrounding the pigment is clearly visible. 40 000×. (C and D) Clusters of membrane-enclosed melanosomes were found inside fibroblasts. A common double membrane surrounds the cluster. (C) 8000×. (D) 40 000×.
Released melanosomes were also observed outside cells (Figure 6A). They were sometimes located in invaginations of the plasma membrane of fibroblasts, and in at least one occasion it appeared as if a fibroblast had begun to take up the released melanosome. What seemed to be small pieces of melanophores were also found in extracellular areas (Figure 6B). These melanophore portions may account for the intracellular clusters of pigment granules in fibroblasts. However, we cannot exclude that these melanophore pieces are in fact parts of a dendritic arm of a melanophore located at a distance from the fibroblast, and that this phenomenon is due to sectioning. In addition, it is a possibility that the individual melanosomes observed in the extracellular space are due to break or leakage of a former common outer membrane surrounding groups of pigmented granules.
Release and uptake of melanosomes. (A) Single melanosomes were found in extracellular areas. Note the fibroblast protrusions that encircle the lower melanosome. 8000× (B) Groups of melanosomes were also found outside fibroblasts. 8000×. (C) Melanosomes are located very close to the plasma membrane in the dendritic arms of melanophores. 40 000×. (D) Released melanin is surrounded by a double membrane. 25 000×.
In melanophores, the melanosomes are surrounded by a double membrane and located very close to the plasma membrane in the dendritic arms (Figure 6C). In order to study whether a membrane surrounds released melanosomes, we collected cell medium from melanophore monocultures and isolated melanosomes that had been released. The isolated melanosomes were sectioned and observed with electron microscopy, and we found that the pigment is membrane-enclosed when released from melanophores in culture (Figure 6D). This is consistent with the results of the DiI experiments, which also imply that the released and transferred pigment is surrounded by a membrane.
In vivo study
By using conventional light-microscopy and staining methods we confirmed that melanophores are present in both epidermis and dermis in skin from X. laevis (Figure 7A). Interestingly, we found what could be free or transferred melanosomes in the dermis (Figure 7B,C). To further characterize these melanosomes, we used electron microscopy methods. Free melanosomes were often found in close proximity of unpigmented and extremely flat cells in the dermis (Figure 8A,B), and the extracellular melanosomes had outer membranes (Figure 8B). We could also detect transferred melanosomes in fibroblast-like cells (Figure 8C).
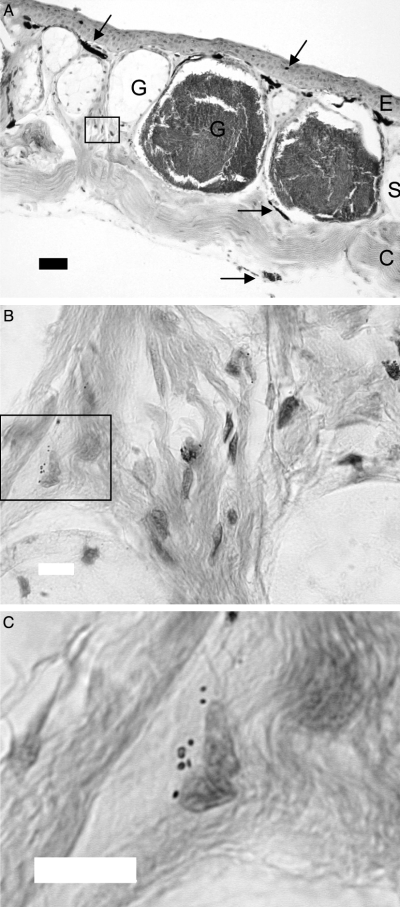
(A) Cross-section of Xenopus laevis dorsal skin stained with haematoxylin and eosin. E, epidermal layer; G, glands; S, stratum spongiosum; C, stratum compactum; black arrows, melanophores. Scale bar = 50 μm. (B) Magnification of the box in (A). Melanosomes are present in the stratum spongiosum of the dermis. Scale bar = 10 μm (C) Magnification of the box in (B). Melanosomes located near the nucleus of a cell. Scale bar = 10 μm.
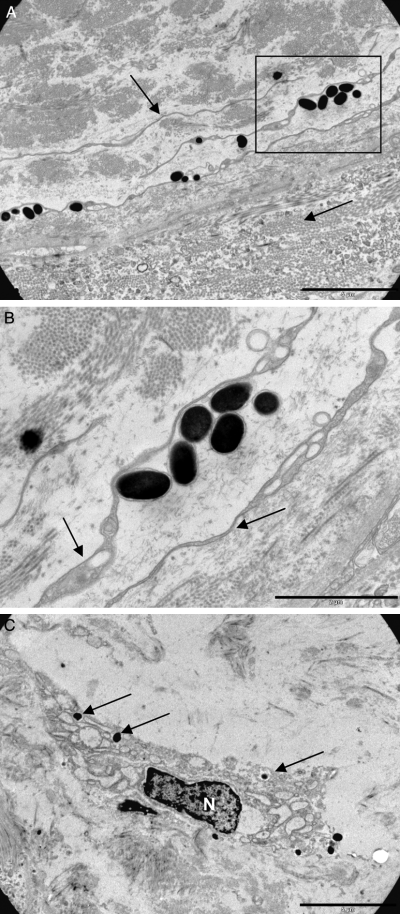
(A) Free melanosomes close to the border between spongiosum and compactum. The cells are associated but only rarely incorporated into the very flat adjacent cells. Scale bar = 5 μm. (B) The free melanosomes have intact outer membranes. Scale bar = 2 μm. (C) A dermal cell with extensive ER and Golgi apparatus similar to a fibroblast with incorporated melanosomes. N, nucleus. Scale bar = 5 μm.
Discussion
In the present study, we have shown that melanosomes are released and transferred in a co-culture of X. laevis melanophores and fibroblasts. It is the first study in an in vitro system from lower vertebrates and the results show that both the highly specialized bi-directionally transporting frog melanophores and mammalian melanocytes are capable of melanosome exocytosis in spite of their differences. Furthermore, we found that melanosomes can be released by melanophores in vivo and taken up by dermal fibroblasts.
Multiple factors are known to affect human and mouse pigmentation but much less is known for frogs and fish. However, conditioned medium from Xenopus fibroblasts is necessary to grow the melanophores, indicating that fibroblast-derived factors are involved. The presence of fibroblasts in the culture was also found to have a morphological effect on the melanophores. In the vicinity of fibroblasts melanophores became curved, seemed to spread towards the fibroblasts and extended dendritic protrusions. The results indicate that fibroblast-derived factors could be of relevance for the dendritic morphology of melanophores in frog dermis where fibroblasts are present, in contrast to the smaller melanophores in the epidermis where keratinocytes dominate. Mammalian keratinocytes are well known to affect mammalian epidermal melanocytes suggesting paracrine links between the different cell types (Hirobe, 1992; Imokawa et al., 1998). In mammals, melanocyte proliferation, pigment production, adhesion and migration are controlled by cytokine inflammatory mediators, growth factors and matrix components synthesized not only by keratinocytes, but also mast cells and fibroblasts, which all elaborate the basal membrane of the epidermis, where the melanocytes reside (Norris et al., 1998). In a fibroblast-melanocyte co-culture model, fibroblast extracellular matrix (ECM) was found to have an increasing effect on melanocyte cell size (Buffey et al., 1994). Fibroblast ECM was also found to increase melanocyte tyrosinase activity. On the contrary, others have suggested that fibroblasts play a suppressive role in melanocyte pigmentation (Hedley et al., 2002). Further studies are needed to explore more details of the effects of different cell–cell signalling.
Melanosomes were surprisingly found to be taken up by the fibroblasts in cell culture. The melanosomes spread throughout the fibroblasts in contrast to the selective and predominating translocation to the apical pole of human keratinocytes (Boissy, 2003). The melanosomes were predominately transferred to distinct groups of fibroblasts. This suggests a paracrine fibroblast signalling, even if we cannot exclude the presence of discrete subpopulations of fibroblasts with a high phagocytotic activity as shown by McCulloch and Knowles (1993). The uptake was melanosome-specific and melanophore-independent; there was no difference in uptake pattern to groups of fibroblasts in the absence or presence of melanophores. Melanosomes contain many transmembrane proteins, and one or several of these may be involved.
Human dermis normally contains no melanocytes or melanin. However, in inflammatory skin disorders melanins can be transferred abnormally to the dermis in a process called pigmentary incontinence or dermal melanosis (Masu and Seiji, 1983). Melanocytes can also be abnormally located in the dermis in a condition called dermal melanocytosis (Kuniyuki, 1997). These cells synthesize melanin, but so far no studies have been performed which report transfer to keratinocytes or dermal cells. For a review of these diseases, see Ortonne and Nordlund (1998). In a condition known as cutaneous amyloidosis, fibroblasts have been reported to engulf melanosomes (Ishii et al., 1984). The authors also suggested that melanophage-like cells that have previously been known to take up melanosomes are in fact fibroblasts. When we studied sections of the dorsal skin of X. laevis, we found melanosomes in the extracellular space of dermis as well as in the cytoplasm of fibroblasts. Extracellular melanosomes were surrounded by a membrane in vivo, which supports the results in our in vitro model. Free pigment was found in areas distant from any observed melanophores, suggesting that exocytosis is the main mechanism of transfer in this model. However, other mechanisms may be present in the epidermis and this will be a subject for future studies. The low presence of released pigment and transfer in vivo compared with in vitro may be explained by a lower rate of exocytosis in the dermis than in cell culture. In addition, barriers in the extracellular space in the dermis are probably capable of restricting access between cells and free pigment. These barriers do not exist in the cell culture. Based on the present results it seems to be an evolutionary difference between humans and frogs; in humans uptake in fibroblasts is connected to a pathological condition whereas in frog is a rare but naturally occurring phenomenon.
The molecular mechanism of transfer of melanosomes into surrounding cells is not well characterized, but as mentioned in the introduction several possible mechanisms have been proposed. Based on our DiI results together with EM pictures we found evidence for exocytosis of membrane-bound vesicles followed by endocytosis. Spontaneously released melanosomes were commonly found in the culture medium. Melanosomes were taken up distantly from melanophores and added isolated melanosomes were also readily taken up by cultured fibroblasts. Using electron microscopy, we observed both single melanosomes and groups of pigment granules surrounded by a common membrane in fibroblasts. It is not clear if the melanosomes are taken up individually before grouping in lysosomal structures, or if melanosomes taken up in groups become separated after dissipation of the common membrane. Cytophagocytosis might, therefore, be a plausible mechanism, even if the evidence is not conclusive. The same issue has been debated in papers about melanosome uptake in keratinocytes. Based upon observations of clustered melanosomes inside keratinocytes, Okazaki et al. (1976) concluded that engulfment of melanosome-filled processes had occurred, but Virador et al. (2001) observed phagosomal structures filled with melanosomes inside keratinocytes after uptake of individual melanosomes from purified melanosome preparations.
We found no evidence for direct inoculation of melanosomes to skin cells or transfer of melanosomes through a communication pore, but since uptake of pigment is observed at a distance from melanophores as well as in monocultures of fibroblasts incubated with isolated melanosomes without any pigment cells present, these mechanisms cannot be exclusive. We, therefore, suggest that exocytosis/endocytosis and cytophagocytosis are the main mechanisms in our system.
α-MSH is well known to increase pigmentation, even if the exact regulatory pathway is not known. Here α-MSH increased the melanosome transfer most probably as a result of a stimulated melanosome exocytosis or by an augmented release of protrusions filled with melanosomes. One possible explanation for an increased transfer is an increased dispersion of melanosomes to the cell periphery, but there was no significant difference in dispersion rate between the different concentrations of α-MSH, not even after 48 h. α-MSH did not have an effect on the general phagocytotic ability of fibroblasts, as the uptake of fluorescent latex beads was unaffected. This effect differs from what is known for α-MSH regulation of pigment transfer between mammalian keratinocytes/melanocytes where α-MSH increases pigment transfer from murine melanocytes as well as uptake in keratinocytes in a co-culture (Lei et al., 2002; Virador et al., 1999, 2001). In mammals, α-MSH binds to the MC-1R receptor, which is present on both keratinocytes (Chakraborty et al., 1995; Jiang et al., 1996) and fibroblasts (Böhm et al., 2004).
SNARE proteins have been proposed to have an important role in docking and exocytosis of melanosomes in melanocytes (Scott and Zhao, 2001), and could be cellular targets for α-MSH signalling. With this new cell co-culture we have a very promising in vitro system for detailed molecular studies on the mechanism for exocytosis of melanosomes from melanophores and preliminary results show that several SNARE proteins are present. We will focus on establishing a new Xenopus keratinocyte cell line for the co-cultures before performing further mechanistic studies. Even if co-culturing with a keratinocyte cell line is a more physiological relevant system, the present system can be used to make interesting comparisons between the effects of keratinocyte- and fibroblast-derived factors on melanophores. This is especially relevant in frog skin with melanophores in both epidermis and dermis. It will further give us a possibility to explore evolutionary differences between melanophores from lower vertebrates and melanocytes from mammalians.
Materials and methods
Chemicals
The cell trackers Vybrant® DiI cell-labelling solution and CMFDA were bought from Molecular Probes (Eugene, OR, USA). Epoxy resin, α-MSH and melatonin were from Sigma (St Louis, MO, USA). Stock solutions of α-MSH and melatonin were stored at −20°C and diluted to the experimental concentrations in fresh medium just before use. Fluorescent beads (FluoresbriteTM carboxylate microspheres 0.5 μm) were obtained from Polysciences Inc. (Warrington, PA, USA).
Cell culture
Xenopus laevis fibroblasts and melanophores were generously provided by Michael Lerner, Arena Pharmaceuticals (San Diego, CA, USA). The fibroblasts were cultured at 27°C in 0.7-x Leibovitz L-15 medium, which was diluted with sterile water and supplemented with 20% foetal bovine serum, 2 mM l-glutamine, 100 IU/ml penicillin, and 100 μg/ml streptomycin. L-15 medium that had been conditioned by fibroblasts for 3–4 d was collected, filtrated and used in the melanophore culture. For co-culturing, a cell suspension with 20 000 melanophores and 60 000 fibroblasts was used in a 60 mm culture dish. The medium in the co-cultures contained one part conditioned medium and two parts 0.7-x Leibovitz L-15 medium. All cell culture reagents were obtained from Life Technologies (Renfrewshire, UK) and the cell culture plastics were from Sarstedt (Nümbrecht, Germany).
Cell tracker experiments
In experiments with CMFDA, melanophores in suspension were centrifuged for 5 min at 700 rpm and the supernatant was removed. The pelleted melanophores were resuspended to a concentration of 106 cells/ml, and stained in serum free L-15 medium containing 2.5 μM CMFDA for 30 min. The cells were then re-pelleted by centrifugation and resuspended in normal L-15 medium. After 30 min of incubation the cells were centrifuged again, resuspended in medium and co-cultured with fibroblasts on sterile coverslips for 48 h. The coverslips with the cells were fixed in −20°C methanol for 6 min and washed three times with PBS before mounting in DABCO mixed with glycerol. The transfer of CMFDA from melanophores to fibroblasts was observed using fluorescence microscopy.
The DiI experiments were performed similarly; 5 μl DiI per ml medium in melanophore suspension was used instead of CMFDA. After 20 min of incubation the cells were centrifuged and resuspended in normal medium. This was repeated twice before the cells were co-cultured with fibroblasts on coverslips. The cells, in this experiment, were not fixed prior to fluorescence microscopy.
Effects of α-MSH
For dispersion assays, melanophores were seeded at a concentration of 40 000 cells per well in a flat bottom 96-well culture plate and cultured for 3 d. The cells were then pre-aggregated with melatonin at 10 nM for 1 h. After that α-MSH was added to final concentrations of 6, 60 and 120 nM. Control cells were treated with serum free medium only. Absorbance was measured at 650 nm directly after addition of α-MSH and then every 10 min until 90 min, using a SPECTRAmax 190 microplate reader and the SOFTmax PRO version 3:1:2 software (Molecular Devices, Sunny Wale, CA, USA). We also measured absorbance after 24 and 48 h. Dispersion was calculated as 1–10Ai−Af. Ai is the initial absorbance and Af is the absorbance at the specified time-point. Data from three independent dispersion assays, each performed in eight wells per treatment, were analysed.
Co-cultures were seeded in plastic tissue culture plates with grids and allowed to rest for 48 h. Subsequently, they were incubated with co-culture medium containing 6, 60 or 120 nM α-MSH. Controls were incubated in normal co-culture medium. After another 48 h, the co-cultures were fixed with ice-cold methanol, rinsed with PBS, and the number of fibroblasts that had taken up pigment was counted in 100 grids per plate in six plates per MSH concentration.
In order to study whether α-MSH affects uptake of fluorescent beads in fibroblasts, the cells were seeded in plastic culture plates, allowed to rest for 24 h and then incubated with α-MSH (6, 60 or 120 nM) or control medium over night. Thereafter beads were added and cells were incubated for another 24 h. The cells were washed three times with PBS to remove beads that had not been internalized, and their cytoplasmic contents were extracted with buffer as described previously (Virador et al., 2001). Fluorescence of the supernatants was measured in a VICTORTM 1420 Multilabel Counter from Wallac Sverige AB (Upplands Väsby, Sweden).
In vivo studies
Xenopus laevis frogs were kept at the Department of Zoology, University of Göteborg, Sweden. The animals were anaesthetized with MS222 and sacrificed by decapitation. The dorsal skin was used for microscopy preparations (ethical permit 242–2005). Skin for light microscopy was fixed in 4% paraformaldehyde in 0,7 × PBS and embedded in Paraplast+. Preparations were cryosectioned (5 μm) and stained with haematoxylin and eosin.
Electron microscopy
Melanophores and fibroblasts were seeded together in 24-well plates. After 96 h of incubation they were fixed with 2.5% glutaraldehyde for 2 h followed by a 2 h incubation with 1% OsO4 and 1% K4Fe(CN)6, and 0.5% uranylacetate in dH2O for 1 h. This was followed by dehydration in 70% ethanol for 2 × 5 min, 85% EtOH for 1 × 5 min, 95% EtOH for 1 × 5 min, 99,5% EtOH for 4 × 5 min, epoxy resin/EtoH 1 + 2 for 30 min and epoxy resin/EtOH 2 + 1 for 30 min. Thereafter, the cells were embedded in epoxy resin and polymerized at 40°C for 15 h and at 60°C for 48 h. Ultra-thin sections (60–70 nm) were contrasted with uranylacetate and lead citrate and examined in a Leo 912AB Omega electron microscope (Carl Zeiss SMT, Oberkochen, Germany). Isolated melanosomes were obtained by centrifugation of medium used in melanophore culture at 15 000 g for 5 min. The granules were then fixed and sectioned as described.
Skin for electron microscopy was fixed in Karnovsky's fix over-night and then rinsed with 0.15 M sodium cacodylate buffer. Post-fixation was made in 1% OsO4 with 1% K4Fe(CN)6. For contrast-enhancement, the tissues were washed three times in dH2O and incubated in 0.5% uranylacetate in dH2O for 1 h. Dehydration was made in a series of ethanol baths (70%, 85% and 95% 5 min each and 99.5% 4 × 5 min) and propylene oxide 3 × 15 min followed by propylene oxide/Epon (1 + 1) for 90 min in room temperature and then Epon overnight in 4°C. This was followed by embedding in Epon and polymerization at 40°C in 15 h and at 60°C in 48 h.
Statistical analyses
Data were tested for homogeneity of variances using the Levene's test (>0.05). The data were analysed using non-parametric Kruskal–Wallis followed by two-tailed Mann–Whitney U-tests. Data are presented as means ± standard error (SE) and the significance level is P < 0.05. The statistical analyses were performed using SPSS 12.0.1 software from SPSS Sweden AB (Sundbyberg, Sweden).
Acknowledgements
We want to thank Prof. Bengt R. Johansson, Dr Ulf Nannmark, Yvonne Josefsson and Gunnel Bokhede for excellent assistance with the electron microscopy. We are also indebted to Elisabeth Norström for outstanding help with the cell cultures and Monika Sundqvist for help with the frogs. This work was financially supported by Stiftelsen Längmanska Kulturfonden, Helge Ax:son Johnsons Stiftelse and Stiftelsen Lars Hiertas Minne.