Inhibitory effect of piperlonguminine on melanin production in melanoma B16 cell line by downregulation of tyrosinase expression
Summary
Tyrosinase is a key enzyme for melanin biosynthesis, and hyperpigmentation disorders are associated with abnormal accumulation of melanin pigments, which can be improved by treatment with depigmenting agents. In the present study, piperlonguminine from Piper longum was discovered to inhibit melanin production in melanoma B16 cells stimulated with α-melanocyte stimulating hormone (α-MSH), 3-isobutyl-1-methylxanthine or protoporphyrin IX, where the compound exhibited stronger depigmenting efficacy than kojic acid. However, piperlonguminine did not affect 1-oleoyl-2-acetyl-sn-glycerol-induced melanogenesis and did not affect protein kinase C-mediated melanin production. Surprisingly, piperlonguminine did not inhibit the catalytic activity of cell-free tyrosinase from melanoma B16 cells but rather suppressed tyrosinase mRNA expression. This effect was attributed to the inhibitory action of piperlonguminine on α-MSH-induced signaling through cAMP to the cAMP responsive element binding protein that in turn regulates the expression of the microphthalmia-associated transcription factor, a key activator of the tyrosinase promoter. This study demonstrates that piperlonguminine is an efficient depigmenting agent with a novel mechanism of action.
Introduction
In humans, skin pigmentation results from the synthesis and distribution of melanin. This process, called as melanogenesis, is a complex phenomenon occurring in melanocytes after differentiation of the non-pigmented precursors, melanoblasts (Slominski et al., 2004). The biochemical pathway for melanin biosynthesis begins through two distinct reactions of the conversion of tyrosine to dopaquinone catalyzed by tyrosinase (the albino locus) with tyrosine hydroxylase activity and 3,4-dihydroxyphenylalanine (dopa) oxidase activity (Ferguson and Kidson, 1997; Hearing and Tsukamoto, 1991). In the absence of thiol substances, dopaquinone is spontaneously converted to dopachrome. Tyrosinase related protein (TRP)-2 (the slaty locus) isomerizes dopachrome to 5,6-dihydroxyindole-2-carboxylic acid (Tsukamoto et al., 1992). TRP-1 (the brown locus) has been shown in mice to oxidize 5,6-dihydroxyindole-2-carboxylic acid to indole-5,6-quinone-2-carboxylic acid, while human TRP-1 seems to be devoid of this catalytic activity (Boissy et al., 1998). Tyrosinase in mice can oxidize 5,6-hydroxyindole in addition to tyrosine and dopa, but not 5,6-dihydroxyindole-2-carboxylic acid (Miranda et al., 1985). However, human tyrosinase is able to utilize 5,6-dihydroxyindole-2-carboxylic acid as well as tyrosine, l-dopa and 5,6-hydroxyindole as the substrates (Miranda et al., 1985). In mammals, two major types of melanins are produced, the black/brown eumelanins and the yellow/red pheomelanins (Jimbow et al., 1992). Tyrosinase is absolutely required for both eumelanin and pheomelanin production. TRP-1 and -2 are more crucial for eumelanin biosynthesis.
Melanogenesis is regulated by ultraviolet (UV) light that can act directly on melanocytes or indirectly through release of keratinocyte-derived factors (Gilchrest et al., 1996). In cultured melanocytes, UV light can increase directly tyrosinase activity and expression (Abdel-Naser et al., 2003). Direct melanogenesis effects of UV light on melanocytes might involve the modification of membrane phospholipids that can activate phospholipase C, thereby releasing diacylglycerol (DAG), which in turn activates protein kinase C (PKC; Saito et al., 2002). Melanogenesis is also attributable to an indirect effect of UV light on melanocyte activation through a paracrine action of keratinocyte-secreted factors (Duval et al., 2001). As the paracrine factors, nitric oxide (NO) activates cGMP-mediated melanogenesis pathway in melanocytes (Romero-Graillet et al., 1996), while α-melanocyte stimulating hormone (α-MSH), adrenocorticotropic hormone and prostaglandin E2 activate the cAMP-mediated pathway (Im et al., 1998; Kadekaro et al., 2003). However, several cytokines are also released from keratinocytes in response to UV light and they inhibit melanogenesis (Morelli and Norris, 1993). Therefore, the balance of keratinocyte-secreted factors plays an important role in the fine tuning of melanocyte activation, thereby controlling skin pigmentation.
However, disturbance in the amount and distribution of melanin pigments might ultimately provide clues to several disorders. Albinism is a genetic abnormality caused by deficiency in melanin synthesis, which manifests as hypopigmentation of the skin, hair and eyes (Oetting, 1999). Hypopigmentation in the skin is associated with sensitivity to UV radiation and predisposition to skin cancer (Broekmans et al., 2003; Takeuchi et al., 2004). However, abnormal accumulation of melanin pigments is responsible for hyperpigmentations including melasma, freckle and senile lentigines (Cayce et al., 2004), which show satisfactory improvement by treatment of depigmenting agents such as arbutin, hydroquinone and kojic acid-targeting tyrosinase activity (Briganti et al., 2003).
In our attempt to discover depigmenting agents from medicinal plants, oxyresveratrol from Morus alba was identified with depigmenting efficacy and its mechanism of the action was previously reported as reversible inhibition of tyrosinase activity (Kim et al., 2002b; Shin et al., 1998). In the present study, piperlonguminine (Figure 1) from Piper longum is discovered to inhibit melanin production in melanoma B16 cells stimulated with α-MSH, 3-isobutyl-1-methylxanthine (IBMX) or protoporphyrin IX. As a mechanism of the depigmenting action shown by piperlonguminine, downregulation of tyrosinase expression at the transcription level has been demonstrated.
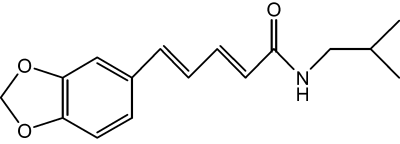
Chemical structure of piperlonguminine.
Results
Effect of piperlonguminine on α-MSH or IBMX-induced melanogenesis
Piperlonguminine showed inhibitory effects on melanogenesis in melanoma B16 cell line stimulated with α-MSH or IBMX, elevators of intracellular cAMP (Im et al., 1998). Melanoma B16 cells, in resting state, released a basal amount 56 μg/ml of melanin during incubation for 72 h (Figure 2A). Upon exposure to α-MSH alone, the cells produced a pronounced amount 127 μg/ml of melanin for the same period (Figure 2A). Piperlonguminine showed dose-dependent inhibitory effects with 85% inhibition at 25 μM, 62% at 13 μM and 36% at 6 μM, showing an IC50 value of 9.6 μM on the α-MSH-induced melanin production in melanoma B16 cells (Figure 2A, B). As a positive control, kojic acid also inhibited α-MSH-induced melanogenesis in a dose-dependent manner with an IC50 value of 44.6 μM (Figure 2B). Furthermore, piperlonguminine inhibited melanin production in IBMX-stimulated melanoma B16 cells in a dose-dependent manner, corresponding to 104% inhibition at 25 μM, 71% at 13 μM and 34% at 6 μM with an IC50 value of 9.2 μM (Figure 2C, D). Kojic acid also showed dose-dependent inhibitory effect with an IC50 value of 83.5 μM on the IBMX-induced melanogenesis (Figure 2D).
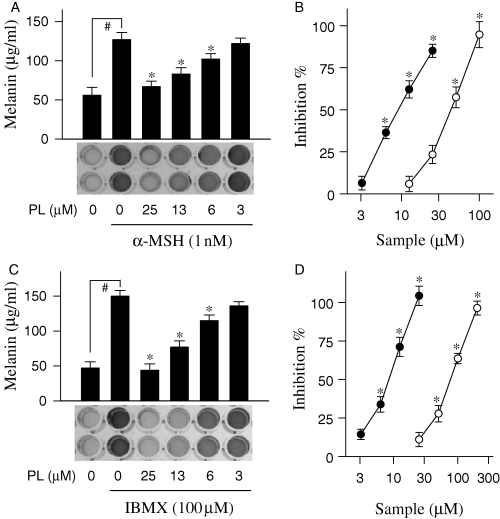
Inhibition of α-melanocyte stimulating hormone (α-MSH) or IBMX-induced melanogenesis by piperlonguminine (PL). Melanoma B16 cells were treated with piperlonguminine and stimulated with either α-MSH (A) or 3-isobutyl-1-methylxanthine (IBMX; C). Amounts of melanin were determined with cell-free culture media. Effects of piperlonguminine (solid circle) and kojic acid (open circle) on α-MSH- (B) or IBMX-induced melanogenesis (D) are also represented as inhibition (%). Values are mean ± SE from five separate experiments. #P < 0.01 versus media alone-treated group. *P < 0.01 versus α-MSH or IBMX alone-treated group.
Effect of piperlonguminine on protoporphyrin IX or OAG-induced melanogenesis
Protoporphyrin IX is an activator of soluble guanylate cyclase, which can induce melanogenesis (Ignarro et al., 1984). Melanoma B16 cells in resting state released 53 μg/ml of melanin during incubation for 72 h, but markedly increased melanin production, up to 109 μg/ml, upon exposure to protoporphyrin IX alone for the same period (Figure 3A). Piperlonguminine showed dose-dependent inhibitory effects with 103% inhibition at 25 μM, 69% at 13 μM and 34% at 6 μM, showing an IC50 value of 9.1 μM on melanin production in protoporphyrin IX-stimulated melanoma B16 cells (Figure 3A, B). As a positive control, kojic acid also showed an IC50 value of 43.2 μM on protoporphyrin IX-induced melanogenesis (Figure 3B). 1-Oleoyl-2-acetyl-sn-glycerol (OAG) is an activator of PKC, which can induce melanogenesis (Park et al., 1999). Upon exposure to OAG alone, melanoma B16 cells markedly increased melanin production over the basal level (Figure 3C). Piperlonguminine did not show any significant inhibitory effects on melanin production in OAG-stimulated melanoma B16 cells (Figure 3C, D). However, kojic acid as a positive control showed dose-dependent inhibitory effect with an IC50 value of 34.6 μM on OAG-induced melanogenesis (Figure 3D).
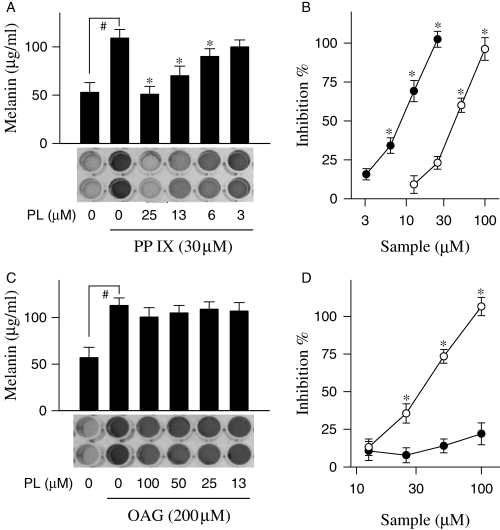
Inhibition of protoporphyrin IX (PP IX) or 1-Oleoyl-2-acetyl-sn-glycerol (OAG)-induced melanogenesis by piperlonguminine (PL). Melanoma B16 cells were treated with piperlonguminine and stimulated with either protoporphyrin IX (A) or OAG (C). Melanin contents were determined with cell-free culture media. Effects of piperlonguminine (solid circle) and kojic acid (open circle) on protoporphyrin IX- (B) or OAG-induced melanogenesis (D) are also represented as inhibition (%). Values are mean ± SE from five separate experiments. #P < 0.01 versus media alone-treated group. *P < 0.01 versus protoporphyrin IX or OAG alone-treated group.
Effect of piperlonguminine on proliferation of melanoma B16 cells
Proliferation of melanoma B16 cells was measured using trypan blue exclusion. The melanoma B16 cells in resting state were rapidly proliferated at 2–3 days after seeding on culture plates. Upon exposure to α-MSH alone, growth rate of melanoma B16 cells was significantly retarded but viability of the cells was not affected because of insignificant numbers of trypan blue-stained cells (Figure 4A). Piperlonguminine did not show any significant cytotoxic effects to α-MSH-stimulated melanoma B16 cells during incubation for 3 day (Figure 4B), indicating that inhibitory effect of piperlonguminine on melanin production was not attributable to its non-specific cell toxicity.
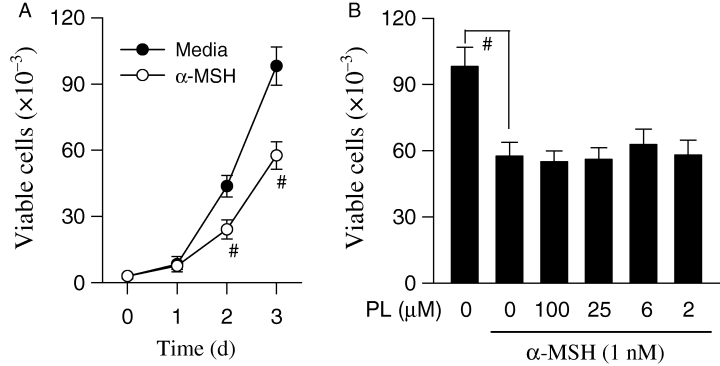
Cytotoxic effect of piperlonguminine (PL). Melanoma B16 cells were treated with either media (solid circle) or α-melanocyte stimulating hormone (α-MSH) alone (open circle) for indicated times (A), and with α-MSH plus piperlonguminine for 72 h (B). Numbers of the cells were counted by trypan blue exclusion. Values are mean ± SE from three separate experiments. #P < 0.01 versus media alone-treated group.
Effect of piperlonguminine on tyrosinase zymogram
To investigate the mechanism of depigmenting action shown by piperlonguminine, l-dopa oxidation zymography of tyrosinase on acrylamide gel was carried out. Melanoma B16 cells were treated with either α-MSH alone or α-MSH plus piperlonguminine, and their lysates were resolved on the gel by electrophoresis. Tyrosinase intensity on the gel was very low in the resting melanoma B16 cells, but markedly increased by stimulation of the cells with α-MSH alone (Figure 5A). Piperlonguminine inhibited α-MSH-enhanced tyrosinase intensity on the gel in a dose-dependent manner, corresponding to 92% inhibition at 25 μM, 88% at 13 μM and 35% at 6 μM with an IC50 value of 8.1 μM (Figure 5A). Upon exposure to OAG alone, the B16 cells also increased tyrosinase intensity on the gel (Figure 5B). Piperlonguminine, even at 100 μM, did not show significant inhibitory effect on OAG-enhanced tyrosinase intensity (Figure 5B).
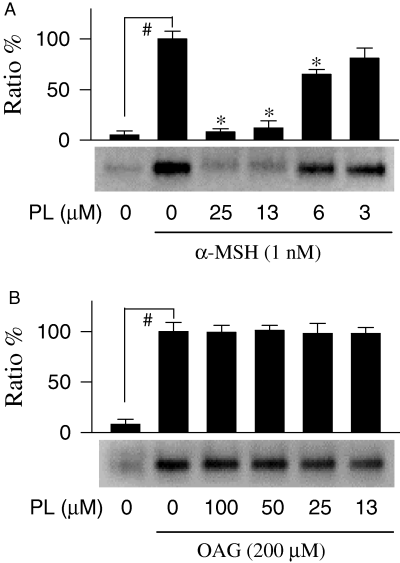
Effect of piperlonguminine (PL) on tyrosinase zymogram. Melanoma B16 cells were treated with piperlonguminine and stimulated with either α-melanocyte stimulating hormone (α-MSH; A) or OAG (B), and lysates of the cells were resolved on SDS-acrylamide gel by electrophoresis. The gels were then soaked with sodium phosphate buffer containing l-dopa. One of similar results is represented and relative ratio (%) is also shown. Values are mean ± SE from three separate experiments. #P < 0.01 versus media alone-treated group. *P < 0.01 versus α-MSH alone-treated group.
Effect of piperlonguminine on cell-free tyrosinase activity
We next determined whether piperlonguminine can affect catalytic activity of cell-free tyrosinase. Lysates of melanoma B16 cells stimulated with α-MSH alone were used as enzyme sources of the cell-free tyrosinase, and l-dopa oxidation velocity was measured as the catalytic activity. Piperlonguminine did not show significant inhibitory effects on l-dopa oxidation activity of the cell-free tyrosinase (Figure 6). However, kojic acid as a positive control inhibited catalytic activity of the cell-free tyrosinase in a dose-dependent manner with an IC50 value of 38.1 μM (Figure 6).

Effect of piperlonguminine (PL) on cell-free tyrosinase activity. Melanoma B16 cells were stimulated with α-melanocyte stimulating hormone (α-MSH) alone, and lysates of the cells were used as enzyme sources. Effects of piperlonguminine (solid circle) and kojic acid (open circle) on l-dopa oxidation velocity of the cell-free tyrosinase are represented as inhibition (%). Values are mean ± SE from five separate experiments. *P < 0.01 versus enzyme source alone-treated group.
Effect of piperlonguminine on tyrosinase expression
To investigate whether piperlonguminine can influence tyrosinase synthesis, Western immunoblot analysis was carried out with lysates of α-MSH-stimulated melanoma B16 cells. The B16 cells produced basal levels of tyrosinase with mature and immature forms (Figure 7A). Upon exposure to α-MSH alone, melanoma B16 cells markedly increased amounts of both mature and immature forms of tyrosinase (Figure 7A). Piperlonguminine attenuated α-MSH-induced tyrosinase synthesis in a dose-dependent manner (Figure 7A). To further investigate whether inhibitory effect of piperlonguminine on tyrosinase expression was influenced at the transcription level, a promoter activity was analyzed using melanoma B16 cells transfected transiently with pTyro construct encoding murine tyrosinase promoter (−2236/+59) fused to luciferase gene as the reporter (Bertolotto et al., 1996). Upon exposure to α-MSH alone, the transfected cells increased luciferase expression up to 19-fold over the basal level (Figure 7B), indicating cellular tyrosinase promoter is transcriptionally functional. Piperlonguminine inhibited α-MSH-induced luciferase expression in a dose-dependent manner, corresponding to 83% inhibition at 25 μM, 61% at 13 μM and 33% at 6 μM with an IC50 value of 10.1 μM (Figure 7B).
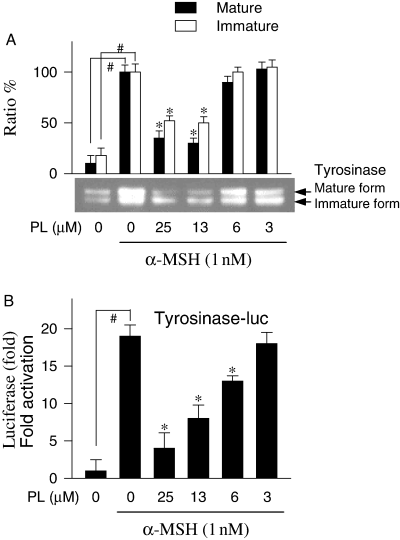
Inhibition of α-melanocyte stimulating hormone (α-MSH)-induced tyrosinase expression by piperlonguminine (PL). Melanoma B16 cells were treated with piperlonguminine and stimulated with α-MSH. Lysates of the cells were subjected to Western blot analysis with anti-tyrosinase antibody. One of similar results is represented and relative ratio (%) is also shown, where mature and immature forms of tyrosinase are indicated by an arrow (A). The B16 cells transfected transiently with both pTyro reporter construct and pSV-β-galactosidase control vector were treated with α-MSH plus piperlonguminine. Luciferase and β-galactosidase activities were measured with lysates of the cells. Luciferase expression as a reporter of tyrosinase promoter activity is represented as relative fold, where luciferase activity was normalized to β-galactosidase activity (B). Values are mean ± SE from three separate experiments. #P < 0.01 versus media alone-treated group. *P < 0.01 versus α-MSH alone-treated group.
Effect of piperlonguminine on MITF production and CREB phosphorylation
Once phosphorylated, cAMP responsive element binding protein (CREB) can upregulate microphthalmia-associated transcription factor (MITF) that subsequently binds to tyrosinase promoter for transcriptional activation (Bertolotto et al., 1998; Ganss et al., 1994). To investigate whether piperlonguminine can affect MITF production and CREB phosphorylation, Western immunoblots were carried out with lysates of α-MSH-stimulated melanoma B16 cells. The B16 cells produced basal levels of MITF and markedly increased MITF production upon exposure to α-MSH alone (Figure 8A). Piperlonguminine decreased α-MSH-induced MITF production in a dose-dependent manner (Figure 8A). Phosphorylated CREB was hardly detectable in resting B16 cells, but markedly increased upon exposure to α-MSH alone (Figure 8B). Piperlonguminine decreased α-MSH-induced CREB phosphorylation in a dose-dependent manner (Figure 8B).
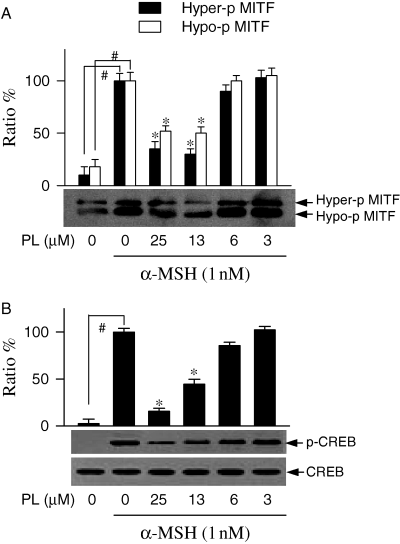
Inhibition of microphthalmia-associated transcription factor (MITF) production and cAMP responsive element binding protein phosphorylation by piperlonguminine (PL). Melanoma B16 cells were treated with piperlonguminine for 2 h and stimulated with α-melanocyte stimulating hormone (α-MSH) for 3 h. Lysates of the cells were subjected to Western blot analysis with anti-MITF antibody. One of similar results is represented and relative ratio % is also shown, where hyper-phosphorylated (Hyper-p) and hypo-phosphorylated (Hypo-p) forms of MITF are indicated by an arrow (A). The cell lysates were also subjected to Western blot analysis with anti-CREB antibody or anti-phospho-CREB antibody. One of similar results is represented and relative ratio (%) is also shown, where phosphorylated CREB (p-CREB) signal was normalized to total CREB signal (B). Values are mean ± SE from three separate experiments. #P < 0.01 versus media alone-treated group. *P < 0.01 versus α-MSH alone-treated group.
Discussion
In the present study, piperlonguminine was discovered to inhibit melanin production in α-MSH- or IBMX-stimulated melanoma B16 cell line (Figure 2). Further, the compound inhibited protoporphyrin IX-induced melanogenesis (Figure 3A, B). However, piperlonguminine did not affect OAG-induced production (Figure 3C, D). These results indicate that piperlonguminine could inhibit cAMP- or cGMP-mediated melanin production but did not affect PKC-mediated melanin production.
Tyrosinase has been evidenced to catalyze the rate-limiting step of melanin biosynthesis, and is the primary target of arbutin or kojic acid used as cosmetic and medical materials with depigmenting efficacy (Hearing and Tsukamoto, 1991; Kahn, 1995; Maeda and Fukuda, 1996). To investigate a mechanism of the depigmenting efficacy shown by piperlonguminine, l-dopa oxidation zymography of tyrosinase was carried out with lysates of α-MSH-stimulated melanoma B16 cells. Piperlonguminine inhibited α-MSH-enhanced tyrosinase intensity on the gel, but did not affect OAG-enhanced tyrosinase intensity (Figure 5). These results are well-correlated to effects of piperlonguminine on α-MSH- or OAG-induced melanin production, respectively, shown in 2, 3. We next determined whether piperlonguminine can affect catalytic activity of cell-free tyrosinase from melanoma B16 cells. Piperlonguminine did not inhibit l-dopa oxidation activity of the cell-free tyrosinase (Figure 6). To facilitate our understanding of the depigmenting efficacy of piperlonguminine targeting tyrosinase, Western immunoblot analysis and promoter activity assay were carried out. Mature and immature forms of tyrosinase were identified in the Western immunoblot analysis (Figure 7A), where mature form of the enzyme was only detectable in the l-dopa oxidation zymogram (Figure 5A). Piperlonguminine attenuated α-MSH-induced tyrosinase synthesis at the protein level, but also luciferase expression as a reporter of tyrosinase promoter activity, in parallel (Figure 7). Therefore, depigmenting efficacy of piperlonguminine in melanoma B16 cells was attributable to its downregulatory mechanism of tyrosinase expression at the transcription level.
cAMP has been evidenced to play a key messenger in the regulation of skin pigmentation (Busca and Ballotti, 2000). α-MSH can increase intracellular cAMP content by activation of adenylate cyclase after binding to melanocortin 1 receptor (Mas et al., 2003). IBMX also increases intracellular cAMP content by inhibiting cAMP phosphodiesterase (Huai et al., 2004). cAMP can activate PKA that is able to phosphorylate CREB known as a transcription factor (Sassone-Corsi, 1998). Once phosphorylated, CREB can upregulate MITF that binds to M-box and E-box motifs in tyrosinase promoter for transcriptional upregulation of the key enzyme in melanin production (Bertolotto et al., 1998; Ganss et al., 1994). Piperlonguminine decreased α-MSH-induced MITF production but also CREB phosphorylation (Figure 8). Therefore, primary target of piperlonguminine on α-MSH-induced melanin production would be upstream PKA-dependent CREB phosphorylation.
Protoporphyrin IX is an endogenous activator of soluble guanylate cyclate, elevating intracellular cGMP content (Ignarro et al., 1984). cGMP can elicit its effects through different pathways. cGMP was reported to inhibit cAMP phosphodiesterase, leading to an increase of cAMP content (Brechler et al., 1992). In addition, cGMP activates PKG, which can phosphorylate activator protein 1, another transcription factor for upregulation of tyrosinase expression (Englaro et al., 1995). OAG can activate PKC that is able to phosphorylate tyrosinase at Ser-505 and -509 residues, resulting in an increase of melanin production (Park et al., 1999). In this study, piperlonguminine inhibited not only cAMP- or cGMP-mediated melanin production in melanoma B16 cells (2, 3), but also suppressed tyrosinase expression at the transcription level (Figure 7). However, piperlonguminine did not inhibit PKC-mediated melanin production (Figure 3C, D).
Recently, C2-ceramide was reported to decrease melanin production in human melanocytes and its primary target was elucidated as a prolonged activation of extracellular signal-associated protein kinase (ERK; Kim et al., 2002a). The C2-ceramide could downregulate tyrosinase expression at the transcription level via an increase of MITF degradation, where ERK-dependent MITF phosphorylation at Ser-73 residue is marked for ubiquitination followed by proteasome-mediated degradation (Kim et al., 2002a; Xu et al., 2000). This study demonstrated that piperlonguminine could downregulate α-MSH-induced tyrosinase expression at the transcription level via a decrease of CREB phosphorylation, which is a rare mechanism for depigmenting efficacy. Several depigmenting agents have been also reported with diverse mechanisms of interference on melanin synthesis and deposition; arbutin and kojic acid targeting for tyrosinase activity (Kahn, 1995; Maeda and Fukuda, 1996), α-linolenic acid for tyrosinase degradation (Ando et al., 1998), RWJ-50353 for protease-activated receptor 2-mediated melanosome transfer (Seiberg et al., 2000), and calcium D-pantetheine-S-sulfonate for tyrosinase glycosylation (Franchi et al., 2000).
Taken together, piperlonguminine from Piper longum inhibited cAMP- or cGMP-mediated melanin production in melanoma B16 cells with five to ninefold stronger efficacy than kojic acid. As a mechanism of the depigmenting action, piperlonguminine could downregulate tyrosinase expression at the transcription level, but did not affect catalytic activity of cell-free tyrosinase. These results indicate that piperlonguminine is a useful inhibitor of melanogenesis in melanoma B16 cells and suggest that it might have beneficial effects in the treatment of hyperpigmentation disorders.
Methods
Materials
Fetal bovine serum (FBS), Dulbecco's modified Eagle's medium and other media supplements were purchased from Invitrogen (Carlsbad, NM, USA). Antibodies against tyrosinase, MITF, CREB or phospho-CREB were obtained from Santa Cruz Biotech (Santa Cruz, CA, USA). Piperlonguminine (purity, >98%) was isolated from Piper longum as described in our previous work (Lee et al., 2004). The other chemicals including α-MSH, IBMX, protoporphyrin IX and OAG were otherwise obtained from Sigma-Aldrich (St. Louis, MO, USA).
Cell culture
Melanoma B16 cells (CRL 6323) were obtained from ATCC (Manassas, VA, USA), cultured in DMEM (13.4 mg/ml Dulbecco's modified Eagle's medium, 10 mM HEPES, 143 U/ml benzylpenicillin potassium, 100 μg/ml streptomycin sulfate, 24 mM NaHCO3, pH 7.1) containing 10% FBS, and incubated at 37 °C under 5% CO2 atmosphere. The cells were detached from culture dishes using 0.025% trypsin and 0.5 mM ethylenediaminetetra acetic acid (EDTA) in PBS.
Melanin quantification
Melanoma B16 cells were seeded at a density of 2.5 × 103 cells/well of 96-well culture plates and incubated at 37 °C under 5% CO2 atmosphere for 24 h. The cells were then treated with piperlonguminine followed by each stimulant of α-MSH (1 nM), IBMX (100 μM), protoporphyrin IX (30 μM) or OAG (200 μM) for 72 h. Amounts of melanin in the cell-free culture media were spectrophotometrically measured at 405 nm.
Measurement of cell proliferation
Melanoma B16 cells were seeded at a density of 2.5 × 103 cells/well of 96-well culture plates and then treated with either α-MSH (1 nM) alone or α-MSH plus various concentrations of piperlonguminine for indicated times. After detaching from culture plates, numbers of the cells were counted by a method of trypan blue exclusion.
Tyrosinase zymography
Melanoma B16 cells were treated with piperlonguminine and stimulated with α-MSH (1 nM) or OAG (200 μM) for 72 h. Protein concentration of the cell lysates was determined using an assay kit (Bio-Rad, Hercules, CA, USA) with bovine serum albumin (BSA) as a standard. Equal amounts of the cell lysates were mixed with Laemmli sample buffer without β-mercaptoethanol, boiling was avoided and then resolved on sodium-dodecyl-sulfate (SDS)-acrylamide gel by electrophoresis. The gels were then equilibrated in 100 mM sodium phosphate buffer (pH 6.8) and then soaked with 5 mM l-dopa until colorimetric detection of tyrosinase.
Measurement of cell-free tyrosinase activity
Lysates of melanoma B16 cells treated with α-MSH alone were used as enzyme sources of cell-free tyrosinase. l-dopa oxidation activity of tyrosinase was spectrophotometrically determined as described previously (Kim et al., 2002b). Briefly, 40 μl of 25 mM l-dopa, 80 μl of 100 mM sodium phosphate buffer (pH 6.8), and 40 μl of the same buffer with or without test sample were added into a 96-well microplate, and then 40 μl of the tyrosinase (125 U/ml) was mixed. Initial rate of dopachrome formation from the reaction mixture was measured as the increase of absorbance at 492 nm/min. One unit of tyrosinase activity was defined as the conversion of 1 nmol l-dopa to dopachrome/min.
Western blot analysis
Melanoma B16 cells were pretreated with piperlonguminine for 2 h and stimulated with α-MSH for 3 h (MITF, CREB, phospho-CREB) or for 48 h (tyrosinase). The cells were then lysed in a buffer (50 mM Tris-HCl, 1% Triton X-100, 0.5% NP-40, 0.1% SDS, 1 mM phenylmethanesulfonyl fluoride (PMSF), 1 mM EDTA, pH 7.4). Equal amounts of the cell lysates were resolved on SDS-acrylamide gel by electrophoresis, and then transferred to PVDF membranes (Millipore, Bedford, MA, USA). The membranes were blocked with 5% non-fat dry milk in TBST buffer (25 mM Tris-HCl, 150 mM NaCl, 0.05% Tween 20, pH 7.5) for 1 h and subsequently incubated overnight at room temperature with anti-tyrosinase antibody (1:200 dilution), anti-MITF antibody (1:500 dilution), anti-CREB antibody (1:250 dilution) or anti-phospho-CREB antibody (1:250 dilution). After washing with TBST buffer, the blots were incubated with horseradish-conjugated anti-goat IgG antibody (1:2500 dilution) for 2 h at room temperature. After washing with TBST buffer, the blots were reacted with ECL reagent (Amersham-Pharmacia, San Francisco, CA, USA) and exposed to X-ray film.
Measurement of tyrosinase promoter activity
Both pTyro reporter construct (Bertolotto et al., 1996) and pSV-β-galactosidase control vector (Promega, Madison, WI, USA) were transiently transfected to melanoma B16 cells using LipofectAMINE (Invitrogen, Carlsbad, NM, USA). The transfected cells were treated with α-MSH plus piperlonguminine for 48 h. Lysates of the cells were subjected to luciferase assay using Luciferase Reporter Assay System (Promega) and to β-galactosidase assay using β-galactosidase Enzyme Assay System (Promega).
Statistical analysis
Results are expressed as mean ± SE. Data were analyzed using the Dunnett's test. Values of P < 0.01 were considered significant.
Acknowledgements
We thank Dr R. Ballotti (INSERUM, France) for his kind supply of pTyro reporter construct. This work was financially supported by a grant (KRF-2003-002-E00179) from Korea Research Foundation.