Patterning skin by planar cell polarity: the multi-talented hair designer
Abstract
Abstract: In mammals, the skin can form complex global and local patterns to meet diverse functional requirements in different parts of the body. To date, the fundamental principles that underlie skin patterning remain poorly understood because of the involvement of multiple interacting processes. Genes involved in the planar cell polarity (PCP) signalling pathway, which is capable of polarizing cells within the planar plane of an epithelium, can control the orientation and differentiation of hair follicles, underlining their involvement in skin pattern formation. Here, we summarize recent progress that has been made to understand the PCP signalling pathway and its function in mammalian skin, including its role in hair follicle morphogenesis, ciliogenesis and wound healing. We argue that dissecting PCP signalling in the context of hair follicle formation might reveal many as-yet-undiscovered functions for PCP in the development, homeostasis and regeneration of skin.
Introduction
As with almost all epithelial tissue types, the epidermis is polarized in strictly regulated manner and this polarization is essential for its appearance and function. Recent studies on planar cell polarity (PCP, the polarized organization of cells within the epithelial planar plane) have revealed that PCP signalling (otherwise known as non-canonical Wnt signalling) can control global and regional patterning events in the epithelium through the propagation and restriction of polarization signals along the surface of the epithelium. In mammalian skin, genes involved in the PCP signalling pathway control the orientation and differentiation of hair follicles, which makes hair follicles an ideal model to investigate PCP signalling in relation to skin patterning. Current evidence suggests that PCP might regulate the formation of hair follicles through interactions with other signalling pathways and by controlling the orientated division, migration and adhesion of cells, processes that are common to many invertebrate and vertebrate epithelia. Thus, understanding PCP signalling in the context of hair follicle formation has important implications for epithelial biology and regenerative medicine.
Hair follicles are an ideal model to understand the formation of skin patterns in mammals
The integument is the interface between an organism and the environment (1). For animals to survive well in the environment, the skin evolves diverse structural and functional features in unique anatomical locations in the body (2). As a result, visible patterns emerge on the skin.
How do the patterns on the skin form? Pattern formation involves a set of fundamental biological processes that disrupt the homogeneity of the cells and lead to the emergence of new structures or arrangements of cells (3). Skin patterning occurs at a number of hierarchical levels, from the polarized distribution of organelles within a single cell, to the highly sophisticated global patterning of multicellular appendages, such as hair follicles.
The hair follicles, which determine almost all properties of the hair, are unique to mammals. Because hair pattern is one of the most prominent features that result from patterning of the skin (Fig. 1), the investigation of events that are associated with hair follicle patterning will assist in our understanding of how mammalian skin becomes patterned.

Regional specificity of the skin in mice, dogs, monkeys and humans. Note that the soles of the feet of these mammals and the faces of monkeys and humans are devoid of terminal hairs. In the hairy regions, hairs generally point in the same direction along the axes of the body or orientate to form a whorl.
Substantial progress has been made in our understanding of the site-specific positioning signals for hair follicles (4–7). Reaction–diffusion (8–10) and activation–inhibition (11) signalling models have been postulated. Recent studies demonstrate that a number of genes involved in the PCP signalling pathway also participate in hair follicle development (Table 1). These PCP genes control the orientation or the differentiation of developing hair follicles, underscoring the importance of the PCP signalling pathway in hair follicle patterning. Conversely, the extensive involvements of PCP genes in hair follicle development also make the hair follicles an ideal model to dissect the PCP signalling pathway and answer biological questions that might be applicable to other model systems.
| Genes | Role in PCP signalling | Functions in hair follicles | References |
|---|---|---|---|
| Fzd6 | Core PCP component | Orientation | (50,59) |
| Vangl2 | Core PCP component | Orientation | (51) |
| Celsr1 | Core PCP component | Orientation | (51,57) |
| Invs | Core PCP component | Orientation | (58) |
| Rac1 | Ubiquitous PCP effector | Differentiation, integrity, and cycling | (74) |
| Cdc42 | Ubiquitous PCP effector | Differentiation | (75) |
| Fuz | Tissue-specific PCP effector | Differentiation | (65) |
- PCP, planar cell polarity.
Planar cell polarity is an evolutionarily conserved signalling mechanism
The formation and maintenance of appropriate polarities are essential for a variety of cellular functions, such as determination of the division plane, directional transfer of molecules, maintenance of cell shape and directional migration (12–16). The two types of polarities that have been extensively studied are the apical–basal polarity (15–18) and PCP (19–21). This viewpoint article focuses on the PCP pathway and its role in hair follicle formation.
Planar cell polarity, which is also called tissue polarity, refers to the polarized organization of cells within the epithelial planar plane; a concept that is primarily based on studies on the Drosophila wing epithelium and the eye (22,23). PCP signalling mechanisms are conserved strongly through evolution, such that molecular genetic interactions that are remarkably similar to those in Drosophila can be observed in vertebrate animals (19,21,24–29).
In mammals, the two groups of genes that have been shown to be involved in PCP signalling are the core PCP genes and the tissue-specific PCP effector genes. Core PCP genes (Fzd3, Fzd6, Vangl1, Vangl2, Celsr1, Dvl2, Dvl3, Prickle1 and Invs) are essential for convergent extension (30–38) – a process that involves medial–lateral intercalation of cells and the subsequent lengthening and narrowing of tissue. Convergent extension is important during neural tube closure (39), formation of the inner ear cochlea (40), kidney development (41) and eyelid closure (29). Tissue-specific PCP effector genes (Fuz, Intu and Frzb) are essential for directional cell movement (42,43) and the formation of the primary cilium or non-motile cilium (42,44–46) – a cellular protrusion that exists in most mammalian cell types and is essential for several signalling pathways (47,48).
The establishment of PCP is reflected by the polarized accumulation of PCP proteins at opposing lateral cell membranes within a sheet of epithelial cells. The asymmetric localization of various PCP proteins functions as a unique signalling mechanism. However, the polarized localization of PCP proteins cannot regulate gene transcription directly. PCP signalling requires downstream effector proteins to control the morphology and biological behaviour of polarized cells (19,29,49). The genetic hierarchy of the PCP signalling pathway has been postulated (Fig. 2), but its spatial and temporal specificity, antagonizing or diffusion properties, dose–effect relationships, impact on neighbouring cells and structures, and effects on cellular functions are not clear. Recent studies support the notion that the skin and hair follicles are ideal models with which to address these questions.

Known components of the planar cell polarity (PCP) signalling pathway and related cellular functions. Examples of core (Fzd) and Fat/Dachsous (Fat) PCP components, PCP effectors and their involvement in the cytoskeleton and cellular functions in vertebrate animals are shown. Note that these cellular functions are often tissue- and cell-type specific.
Planar cell polarity genes control hair follicle orientation and differentiation
Hair follicles are precisely patterned across the entire surface of the body, in a manner that correlates with the body axes (5). In mammals, hair follicles often point caudally on the dorsal skin and distally on the leg, and form symmetric whorls on the chest and tufts along the midline of the flank (Fig. 1), features determined early during hair follicle morphogenesis. Recent studies demonstrate that core PCP genes control hair follicle orientation and PCP effector genes control hair follicle differentiation and cycling (Table 1 and Fig. 3).

Involvement of different components of the planar cell polarity (PCP) signalling pathway during hair follicle morphogenesis. The illustrations represent hair follicles at different developmental stages as described by Schneider et al. (105). While the induction of hair germ formation requires canonical Wnt (β-catenin) signalling, core PCP genes are required for the formation of hair follicle orientation, and the tissue-specific PCP effector gene is required for follicular keratinocyte differentiation.
Core planar cell polarity genes control hair follicle orientation
Proteins encoded by homologues of three Drosophila core PCP genes –Fzd6, Vangl2 and Celsr1– are polarized in epidermal keratinocytes before the orientation of hair follicles is established (50–52). The expression of core PCP genes has been disrupted in genetically engineered mutant mice (Fzd6−/− and Celsr1−/−) or in those with naturally occurring mutations [Looptail (Lp) (53,54), Crash (Crsh) (36) or Inversin (Inv) (55,56). This disruption of core PCP gene expression disturbs hair follicle orientation (50,51,57,58). Specifically, hair follicles fail to orientate caudally in the dorsal skin (50,51,57,58) and distally on the hind legs and feet (50,57), and fail to maintain invariable whorls on the upper chest (50). These defects can be traced back to the early developmental stages of hair follicles (50,51,57). Furthermore, the polarization of PCP proteins is interdependent: disrupting one core PCP gene can affect the subcellular localization of the others (51). These studies have demonstrated that core PCP genes are required genetically for the correct orientation of hair follicles and have confirmed the need for the establishment of PCP during hair follicle development.
The orientation of hair follicles coordinates with the body axes. It also responds readily to polarization cues from neighbouring follicles. Although hair follicles in PCP mutants fail to orientate globally, they can coordinate and form local patterns such as whorls and ridges (50,57) and reorientate readily (59). In chimeric skin formed from wild-type and Fzd6−/− or Lp/Lp mutant keratinocytes, the orientation of wild-type hair follicles is disrupted by neighbouring follicles formed by mutant cells (50,51). It is thought that PCP is crucial for the propagation of polarization cues that coordinate the global patterning of hair follicles and, in addition, that a secondary regulatory event exists beyond PCP (59).
The global determinants that create the initial polarity signal that specifies the expression and polarization of core PCP proteins are unclear (60). It remains to be determined whether signals formed by the Fat/Dachsous complex, which has conserved functions in the regulation of core PCP proteins (21,27,61), is capable of specifying global hair orientation. To understand complex PCP signalling in the epidermis, the geometric localization and interdependent profiles of core PCP proteins have to be determined precisely. This task might be a challenge, because multiple isoforms of the PCP genes might be expressed in the skin and act independently or redundantly. To understand the redundant or non-overlapping roles of different isoforms of PCP genes completely, it might be necessary to generate double or triple knockout mouse models, such as for the Fzd3 and Fzd6 (57), Dvl1, Dvl2 and Dvl3 (30), Celsr2 and Celsr3 (33), and Vangl1 and Vangl2 genes (62). In addition, given that the functions of PCP genes are pleiotropic and that severe developmental defects are often associated with their disruption, epidermal-specific knockout mouse models and novel in vitro assay systems might be required (63,64).
Tissue-specific planar cell polarity effector genes control hair follicle differentiation
Homologues of three Drosophila tissue-specific PCP effector genes (fuzzy, inturned and fritz) exist in mammals (Fuz, Intu, and Frzb) (19). Fuz is expressed in both epidermal and dermal cells (65). In mutant mice that lack Fuz, the morphogenesis of hair follicles is markedly impaired as a result of failure of hair follicle differentiation (65). The differentiation of hair follicles requires a complex of reciprocal interactions between epidermal keratinocytes and dermal papilla cells and the activation of a number of signalling pathways, such as Hh (66). Indeed, the phenotype for hair follicle differentiation observed in Fuz−/− mutants is associated with attenuation of the Hh signalling pathway (65) and resembles that observed in Hh mutant mice (67–69).
During the characterization of Fuz−/− mutants, we determined that formation of the primary cilium was disrupted in epidermal and follicular keratinocytes and dermal fibroblasts in these mutants (65). Grafting Fuz−/− keratinocytes with wild-type fibroblasts or Fuz−/− fibroblasts with wild-type keratinocytes resulted in similar hair follicle differentiation defects, demonstrating that the formation of primary cilia is cell autonomous, whereas epidermal–dermal crosstalk requires the formation of primary cilia in both follicular keratinocytes and dermal papilla cells. The study also revealed the presence of interactions between the PCP and Hh signalling pathways in the skin, which converge at the primary cilia. Molecular mechanisms by which tissue-specific PCP effectors control ciliogenesis have been documented recently in Xenopus skin (39,42,44), but how these PCP effectors control ciliogenesis in mammalian skin remains to be determined.
Disruption of Fuz does not affect the orientation of hair follicles or the polarized distribution of core PCP proteins in basal keratinocytes (65). This suggests that Fuz functions downstream of core PCP genes, a correlation that is consistent with findings in Drosophila (70,71). However, whether and how tissue-specific PCP effectors mediate polarization signals generated by core PCP proteins remains unclear in mammals. Several recent studies have suggested that tissue-specific PCP effectors mediate PCP signals through regulation of the microtubule and actin cytoskeletal networks, in a tissue-specific and cilia-dependent or cilia-independent manner (39,42,44,72).
Planar cell polarity effectors are involved in hair follicle maintenance and cycling
Rac1 and Cdc42 are Rho family small GTPases (73) that act as Frizzled/PCP effectors of the PCP signalling pathway (19). Tissue-specific disruption of these genes in the epidermis affects the differentiation and postnatal cycling of hair follicles (74,75), which suggests they have a role in hair follicle maintenance and the activation of keratinocyte stem cells during cycling. In addition, Cdc42 is capable of modulating the canonical Wnt signalling pathway by regulating the degradation of β-catenin in keratinocytes (75). Because Rac1 and Cdc42 serve as points of integration of many signalling pathways, it is important to determine whether the hair follicle phenotypes that are associated with these genes are controlled primarily by PCP signalling.
Planar cell polarity interacts with Hh signalling pathways in the primary cilium
Primary cilia were considered previously to be vestigial organelles. However, their biological importance has been determined recently (47,48) and linked to PCP signalling in mammalian tissues (76–78). For example, many PCP proteins (Vangl2, Dvl, Intu and Fuz) are localized at the base of cilia (79,80). Many genes that function in the PCP signalling pathway are also involved in the formation and positioning of the primary cilia (80) with Invs regarded as both a PCP and ciliary gene (38,58,81). Furthermore, the disruption of PCP and ciliary genes often results in overlapping phenotypes in mammals, for example during neural tube closure (39,82).
In mammalian skin, primary cilia are found in keratinocytes and fibroblasts (Fig. 4) (65,83,84). The disruption of cilia in either cell type can result in the arrest of hair follicle morphogenesis, primarily due to disrupted Hh signalling (65,83). In adults, primary cilia can also mediate the development of basal cell carcinoma through the regulation of Hh signalling (85). Interestingly, tissue-specific disruption of two ciliogenic genes, Ift88 and Kif3a, in the epidermis disrupted primary cilia formation and Hh signalling with minimum impact on hair follicle morphogenesis (86). Instead, disruption of these ciliogenic genes results in abnormal epidermal differentiation and reduction in label-retaining cells in the bulge area (86). A recent study has further demonstrated that primary cilia regulate epidermal differentiation through Notch signalling (87).
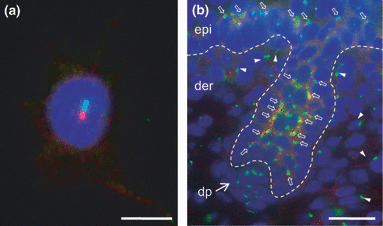
Immunofluorescence labelling of primary cilia in mouse epidermal keratinocytes and dermal fibroblasts. (a) Primary cilium (green) and basal body (red) in a cultured primary keratinocyte. (b) Primary cilia in epidermal and follicular keratinocytes (open arrows), dermal fibroblasts (arrowheads), and dermal papilla cells (arrow). Dotted line marks the epidermal–dermal junction. Primary cilia are labelled with an ADP-ribosylation factor-like 13B (Arl13b) antibody, the basal body is labelled with an gamma-tubulin antibody, and the nuclei (blue) are stained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bar, 10 μm in (a) and 20 μm in (b).
In contrast, mutants that lack the tissue-specific PCP effector gene Fuz display phenotypes for hair follicle differentiation that are consistent with those of Hh mutants (65). These findings demonstrate that the functions of primary cilia in the skin are just start to be understood. Disruption of primary cilia through different genetic pathways may generate cilia (and Hh)-dependent and cilia-independent phenotypes, which may be dependent on the developmental status of the skin and hair follicles. Thus, dissecting the mechanisms through which PCP and other ciliogenic genes control ciliogenesis in the context of hair follicle development, hair cycling and epidermal homeostasis is an important approach to understand the interplay between PCP and primary cilia.
Planar cell polarity is involved in skin wound healing
The establishment and maintenance of PCP is important for skin development and for the maintenance of epidermal integrity and its repair (88). In mice, PCP genes (Vangl2 and Celsr1) interact genetically with Grhl3 (89), a transcription factor that is essential for formation of the skin barrier and wound healing (90). Compound heterozygous Vangl2+/−; Grhl3+/− and Celsr1+/−; Grhl3+/− mutants show impaired skin healing (89). At the molecular level, PCP signalling is required for actin polymerization and the polarization of subcellular organelles at the leading edge (89), which are integral processes in epidermal keratinocytes in response to wounding. A recent study has demonstrated that the Fuz gene also plays a crucial role in the directional migration of keratinocytes in vitro (43). Taken together, these studies suggest that the PCP signalling pathway is involved extensively in skin repair. Furthermore, given that PCP genes are also expressed in the mesenchyme and can regulate angiogenesis (91), further studies are required to define the roles of these genes at different stages of wound healing, such as stem cell activation, reepithelialization, angiogenesis and wound bed remodelling.
Open questions and translational medicine perspectives for planar cell polarity signalling
We are only beginning to understand the functions of PCP signalling in mammalian skin. The involvement of PCP genes during the development and maintenance of hair follicles indicates that PCP signalling is an important pathway for the regulation of the fundamental cellular functions of epidermal keratinocytes. These functions may include determination of the division plane, formation of intercellular junctions, maintenance of cell shape and directional cell migration. The evidence for the involvement of PCP signalling in a few of these processes is summarized in Table S1.
Beyond what we know now about the function of PCP signalling on keratinocytes, molecular genetic evidence of PCP signalling suggest that there might be more extensive involvement of PCP signalling in skin biology. For example, it was recently identified that PCP genes regulate vesicle trafficking, membrane fusion and stability (44,92,93), directional cell movement (94,95), processes required during keratinocyte–melanocyte melanosome transfer (96–98). Thus, it is becoming intriguing to investigate the potential functions of PCP signalling in melanocyte biology (99).
As more mutations in PCP genes are associated with human disorders, such as those identified in VANGL1 (31,100) and VANGL2 (101), FUZ (43), FRZB (42), and PRICKLE1 (102,103) and PRICKLE2 (102,104) genes, a complex association of various mutations and disease symptoms has appeared. As predicted, some of these PCP mutations are lethal and hence can only be identified in stillborn babies, whereas others are associated with a wide spectrum of neural tube defects and ciliopathies phenotypes in patients. The association of PCP mutations with dermatological conditions will certainly advance our understanding of the PCP signalling pathway in the skin.
Furthermore, the PCP signalling network is comprised of a number of kinases and small GTPases that can be targeted by drugs and small molecules. Understanding the PCP signalling pathway has important future implications for translational medicine.
Conclusion
The importance of PCP signalling in mammalian skin is only beginning to emerge. Understanding PCP in the context of the morphogenesis and maintenance of hair follicles will advance our understanding of skin pattern formation and lead to new insights into the behaviour of keratinocytes and other cell types in the skin. This group of genes are involved in processes such as cell division, differentiation, migration and interaction with neighbouring cells and structures – all of which are essential in forming and reforming of skin patterns and maintenance of skin functions.
Acknowledgements
We thank Dr. Ralf Paus for his encouragement and inspiration, Erika Guevara for editorial assistance, and Aaron Huebner for pictures. JC is supported by a Research Career Development Award from the Dermatology Foundation and a grant from NIH/NIAMS (AR061485). CMC is supported by grants from NIH/NIAMS (AR042177, AR060306 and AR047364). JC prepared the figures, collected information and wrote the manuscript. CMC contributed images and wrote the manuscript.
Conflict of interests
The authors have declared no conflicting interests.




