IL-17 downregulates filaggrin and affects keratinocyte expression of genes associated with cellular adhesion
Abstract
Abstract: Atopic eczema and psoriasis are common skin diseases. While it is well established that the pathogenesis of these diseases varies, both are characterized by impairment in epidermal barrier function and abnormal IL-17 expression in the skin and peripheral blood. Recent findings indicated that filaggrin is essential during barrier formation and its insufficiency underlies the pathogenesis of atopic eczema. Filaggrin downregulation has also been reported in psoriasis. It is clear that Th1/Th2 bias influences expression of the protein, but an analysis of the effects of interleukin-17 (IL-17) on the expression of the protein and profilaggrin-processing enzymes has not yet been reported. In addition, the effect of the cytokine on components of functional epidermal barrier, tight junctions and adhesion/desmosomal proteins, has not been elucidated. Keratinocytes were exposed to interleukin-17A, and microarray analysis was performed. Filaggrin protein level was assessed by western blot. We have observed a significant decrease in profilaggrin mRNA level in interleukin-17A-exposed cultures (P = 0.008). Expression of processing enzymes was also altered, indicating an indirect effect of the cytokine on filaggrin production/degradation. Moreover, expression of many genes involved in cellular adhesion was also decreased. A significant downregulation of filaggrin at the protein level was detected by western blot in immortal and primary keratinocytes. Gene ontology analysis indicated changes in keratinization, epidermal differentiation and formation of the cornified envelope. We conclude that IL-17A downregulates the expression of filaggrin and genes important for cellular adhesion which could affect epidermal barrier formation. This effect potentially contributes to barrier dysfunction and could become a possible therapeutic target.
Introduction
Functional epidermis is indispensible to protect the organism from infections, allergens, radiation and other threats of the external environment. While primary role of keratinocytes is to build a continuous multilayered tissue that provides a mechanical barrier, these cells, together with other cellular populations, participate in the formation of actively secreting organ that maintains interactions with immune cells and provides antimicrobial protection. Therefore, maintaining the integrity of epidermis is essential for supporting both interactions with external environment and the homeostasis of the organism. Defective epidermal barrier is a prominent feature of common inflammatory skin diseases, atopic eczema (AE) and psoriasis.
In recent years, the importance of a late keratinocyte protein, filaggrin, in the formation of functional epidermis has been especially highlighted by studies of the pathogenesis of AE (1–3). Filaggrin is expressed at the final stages of keratinocyte differentiation and is involved in the aggregation of a scaffold-like cornified envelope (4), which initiates programmed cell death through keratinization. This process is essential for the formation of functional epidermal barrier. In addition to this, the filaggrin molecule itself further enhances barrier function of the skin by provision of important constituents of natural moisturizing factor (NMF) that retain water and maintain low pH of the upper epidermal layers (5).
Many null mutations of the filaggrin gene have been identified to date (6,7), together representing a highly increased risk of AE development (8). The importance of these findings is further confirmed by the correlation of enhanced barrier dysfunction, measured by an assessment of transepithelial water loss (9,10) and enhanced penetration of both lipophilic and hydrophilic substances (11–17) in individuals with AE. In the view of these findings, the presently accepted model of AE inflammation points at the impairment of the skin barrier as a factor of critical primary importance. According to this, barrier dysfunction would lead to an increase in antigen penetration and priming of specific T cells (18). In line with this, sensitization to environmental antigens is frequently observed in atopic patients (19). The model of transepithelial antigenic exposure is further supported by a correlation between presence of filaggrin mutation and frequencies of dust mite antigen-specific T cells and clinically observed severity of the disease (20). In cohort studies, the presence of filaggrin mutations seems to correlate well with other manifestations of the allergic march as well as allergy to certain foods (8,21), which further supports a notion of a relationship between environmental allergen exposure and systemic inflammation in atopy.
While studies reported the lack of correlation between filaggrin mutation and psoriasis, decreased filaggrin expression has been detected in psoriatic skin, especially in acute plaques, suggesting that genetic predisposition may not be required. This observation can be explained by the existence of non-genetic factors that can affect barrier function by decreasing filaggrin content in the skin independently from the genetic background. Specifically, IL-4, IL-13 and, very recently, TNF-α were reported to reduce the amount of the protein in keratinocytes (22,23). Th2 bias is a hallmark of atopic disease and can be also observed in other manifestations of the allergic march (24–27), while TNF-α involvement in psoriasis has been well recognized. In addition to altering filaggrin expression, all these cytokines also seem to affect additional components of an effective epidermal barrier namely the tight junctions (28), which may contribute to the increase in the barrier permeability. Interestingly, an aberrant tight junction formation has been reported in both psoriasis (29–35) and eczema (36).
Constitutive expression of IL-17 receptor in keratinocytes has been previously reported (37). IL-17 seems to be a cytokine important in both diseases, with serum and skin levels increased in patients with both acute psoriasis and AE compared to healthy controls (38–43). In addition, while one group has reported a relatively lower upregulation of Th17-related genes in AE compared with psoriasis (43,44), the Th17 cells are found to accumulate at early stages of skin inflammation in both dermatoses and therefore likely to be critical for the direction of evolving immune response (39,45).
In view of those findings, it is surprising that the detailed study on the effect of IL-17 on filaggrin and components involved in cellular adhesion (tight junctions, adhesion molecules, etc.) has not been conducted to date. Therefore, we have sought to investigate whether IL-17 could also affect the expression of filaggrin and genes associated with cellular adhesion in the epidermis.
Methods
The work has been approved by the Oxfordshire Research Ethics Committee.
Cells
HaCaT cells were cultured in DMEM medium (Sigma Aldrich, St.Louis, MO, USA) supplemented with 10% fetal calf serum, l-glutamine and penicillin/streptomycin. Normal human epidermal keratinocytes were purchased from Lonza (Verviers, Belgium) or obtained from normal donors through ethics approval 09/H0606/71. Briefly, skin samples were incubated in dispase overnight, and the epidermis was peeled off. Cultures were obtained after digestion in trypsin and subsequent passing of cell suspension through a 70-μm nylon strainer (BD) and PBS washes. Primary cells were maintained in KGM Gold keratinocyte medium (Lonza) at 0.06 mm calcium; cells that were used for the experiments were of early passage (up to passage five). To stimulate differentiation and filaggrin expression, calcium concentration was increased to 1.5 mm in cultures of primary cells for up to five days before using the cells in the experiments. IL-17A was added at the concentration of 20 or 50 ng/ml for 24 h.
Microarrays
HaCaT cells (a gift from Dr N. Fusenig) were cultured in DMEM medium, supplemented with 10% fetal calf serum, l-glutamine and penicillin/streptomycin (D10 medium). IL-17A was added to the cultures at a concentration of 200 ng/ml when cells reached 80–90% confluence. The cells were lysed after 12 h of incubation, and RNA was extracted with RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The microarray acquisition was performed by Service XS (Holland, Leiden) on Illumina BeadArray platform. The comparisons were made between control samples and IL-17A-exposed cultures (the experiment was set up in triplicates). Expression of filaggrin transcript was assessed based on the hybridization to the Illumina probe ILMN1720695 (GGAGACATGGCAGCTATGGTAGTGCAGATTATGATTATGGTGAATCCGGG). The data have been submitted to GEO (GSE27533).
Western blot
Cells were lysed with RIPA buffer (Cell Signalling Technology, Danvers, MA, USA), supplemented with protease inhibitors (Complete Protease Inhibitor Cocktail, Roche, Mannheim, Germany) after 24 h. The lysates were fractionated by SDS-PAGE on either 7% TA or 12% BT gels (Invitrogen, Carlsbad, CA, USA). Western blot assays were carried out on PVDF membrane, using anti-filaggrin (AKH-1, Santa Cruz, Santa Cruz, CA, USA) and anti-GAPDH antibodies (6C5; Bioo Scientific, Austin, TX, USA).
Immunohistochemistry
Cells were grown in chamberslides (BD) in the media as described earlier, until 50–60% confluent. IL-17A was added at 50 ng/ml for 24-h incubation, and the staining was conducted using anti-E-cadherin, anti-γ-catenin, anti-ZO-1 or isotype control rabbit and mouse antibodies (Epitomics, Abcam, Imgenex, Invitrogen and BD, respectively). The antigens were visualized using Dako Envision DAB polymer IHC system.
Data analysis
Microarray data were normalized using lumi (46) analysed with LIMMA (47). To further identify the processes, cellular components and molecular functions that showed significant differential expression by the IL-17A treatment, GOrilla (Gene Ontology enRIchment anaLysis and visuaLizAtion tool) (48) analysis was also performed on 1000 of significantly changed genes ranked according to their P values.
Results
IL-17 downregulates filaggrin at the mRNA level and affects the expression of profilaggrin- and filaggrin-processing enzymes
To investigate the effect of IL-17A on keratinocytes, a microarray study was performed. We could detect genes that were differentially expressed in the control versus IL-17A-exposed HaCaT cultures. We have observed a downregulation of profilaggrin mRNA expression after as short as 12-h stimulation with the cytokine (Fig. 1a). We could not detect any changes in the expression of filaggrin 2 and proteins of the filaggrin-like family (repetin, hornerin, cornulin and trichohyalin) at that time point (data not shown). Changes in the expression levels of other proteins that are characteristic of keratinocyte differentiation (loricrin, involucrin and transglutaminases) were also not detected in our array (data not shown).
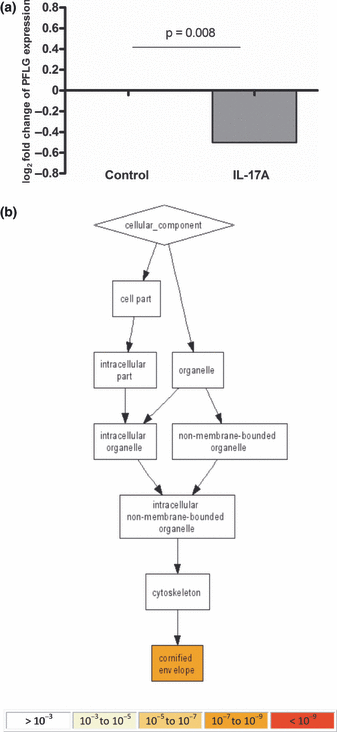
Interleukin-17 downregulates the expression of profilaggrin mRNA and affects epidermal cell differentiation and formation of cornified envelope. HaCaT cells were cultured in D10 medium at 1.5 mm calcium and exposed to IL-17A (200 ng/ml) for 12 h. Cells were lysed, total mRNA was extracted and microarray analysis was carried out using Illumina HT12 platform. (a) Expression of profilaggrin mRNA in IL-17A-stimulated cultures versus control is shown as log2 fold change. (b) Gene ontology analysis was performed using the data available from microarray study. About 1000 of significantly altered genes were ranked according to their P value and analysed using Gorilla gene ontology tool. The enrichment of gene ontology terms is shown for cellular compartments.
However, we have identified a number of genes encoding proteins thought to be important in profilaggrin- and filaggrin-processing pathways that were affected by IL-17A (Table 1). Specifically, we found that cathepsin D was most pronouncedly decreased. In addition, kallikrein-related peptidase 5, as well as two transcripts of calpain small subunit 1 (essential for the formation of calpain I), was also downregulated. In addition, we have observed the upregulation of two other genes potentially important in profilaggrin processing, namely cathepsin L1, transcript variant 1, and cathepsin B, transcript variant 1. These changes, however, were less pronounced. As cathepsins are also critically involved in processing filaggrin into amino acids (49), we believe that these alterations in gene expression could affect the generation of natural moisturizing factor.
| Gene | Symbol | Log2 fold change | Adj. P value |
|---|---|---|---|
| Homo sapiens cathepsin D | CTSD | −1.21 | 0.0045 |
| Homo sapiens calpain, small subunit 1, transcription variant 2 Homo sapiens kallikrein-related peptidase 5 | CAPNS1 | −0.81 | 0.0097 |
| KLK5 | −0.79 | 0.0019 | |
| Homo sapiens calpain, small subunit 1, transcription variant 1 | CAPNS1 | −0.79 | 0.0241 |
| Homo sapiens cathepsin L1, transcription variant 1 | CTSL1 | 0.73 | 0.0032 |
| Homo sapiens cathepsin B, transcription variant 1 | CTSB | 0.57 | 0.0416 |
These data suggest that IL-17A downregulates filaggrin expression at mRNA level directly and indirectly, by affecting profilaggrin mRNA expression, production of functional filaggrin monomers and their degradation at the level of enzymatic processing.
IL-17 downregulates the expression of proteins involved in keratinocyte adhesion
Apart from filaggrin, other components are required for the formation and maintenance of a functional mechanical barrier by epidermal keratinocytes (50). To understand the effect of IL-17 treatment on cellular adhesion, we have adapted a non-hypothesis-driven approach. The microarray study showed that many relevant genes are significantly downregulated (Table 2). Specifically, the downregulation of 16 transcripts, including various integrins, plakoglobins, plakophilins, cadherins (including E-cadherin), claudin 7 and ZO-2 proteins, was observed at the mRNA level (Table 2). There was a trend towards a reduced transcription of ZO-1, the protein critical to the tight junction formation, under IL-17 stimulation; however, the change in mRNA expression did not cross the threshold level of log2FC = 0.5 (data not shown).
| Gene | Symbol | Log2fold change | Adj. P |
|---|---|---|---|
| Homo sapiens tight junction–associated protein 1 | TJAP1 | 0.70 | 0.0031 |
| Homo sapiens gap junction protein, beta 5 | GJB5 | 0.56 | 0.0046 |
| Homo sapiens claudin 12 | CLDN12 | 0.52 | 0.0034 |
| Homo sapiens integrin, beta 4, transcription variant 2 | ITGB4 | −1.70 | 0.0095 |
| Homo sapiens integrin, beta 4, transcription variant 3 | ITGB4 | −1.63 | 0.0204 |
| Homo sapiens junction plakoglobin, transcription variant 1 | JUP | −1.38 | 0.0014 |
| Homo sapiens plakophilin 3 | PKP3 | −1.14 | 0.0062 |
| Homo sapiens junction plakoglobin, transcription variant 2 | JUP | −1.06 | 0.0054 |
| Homo sapiens desmoplakin, transcription variant 2 | DSP | −0.98 | 0.0084 |
| Homo sapiens cadherin 3, type 1, P-cadherin (placental) | CDH3 | −0.96 | 0.0376 |
| Homo sapiens periplakin | PPL | −0.90 | 0.0022 |
| Homo sapiens adhesion-regulating molecule 1, transcription variant 2 | ADRM1 | −0.82 | 0.0035 |
| Homo sapiens integrin alpha FG-GAP repeat containing 3 | ITFG3 | −0.78 | 0.0074 |
| Homo sapiens cadherin, EGF LAG seven-pass G-type receptor 2 | CELSR2 | −0.67 | 0.0041 |
| Homo sapiens integrin, beta 5 | ITGB5 | −0.66 | 0.0048 |
| Homo sapiens integrin, alpha 2 | ITGA2 | −0.65 | 0.0317 |
| Homo sapiens cadherin 1, type 1, E-cadherin (epithelial) | CDH1 | −0.61 | 0.0315 |
| Homo sapiens tight junction protein 2 (zona occludens 2), transcription variant 2 | TJP2 | −0.57 | 0.0032 |
| Homo sapiens claudin 7 | CLDN7 | −0.52 | 0.0394 |
To further confirm the results obtained in our microarray study, we have carried out the immunohistochemistry staining on keratinocytes growing in culture chamberslides, using E-cadherin and γ-catenin as our target proteins. We also carried out additional staining with anti-ZO-1 antibody, as we hypothesized that the changes could be observed at the protein level. We could see a decrease in the staining of cells that have been subjected to IL-17 stimulation in the case of all three junction and adhesion molecules (Fig. S2).
Taken together, these results show that IL-17 downregulates expression of the vast majority of genes encoding various components of functional epidermal barrier, including tight junction proteins and adhesion molecules, and that this decrease can be observed at the protein level.
IL-17 affects the expression of genes involved in the formation of functional epidermal barrier
Using the results obtained from microarray experiments, we have also performed a gene ontology enrichment analysis using an online tool (GOrilla Gene Ontology enRIchment anaLysis and visuaLizAtion tool) (48). This enabled for the identification of biological processes changed as a result of the stimulation. The analysis was performed on the 1000 genes which ranked highest according to the adjusted P value as reported by LIMMA. GOrilla analysis indicated that processes such as keratinization, epithelial/epidermal and keratinocyte differentiation were differentially regulated by IL-17A at mRNA level (Fig. S1A and Table S1). In particular, early keratinocyte differentiation markers, including small proline-rich proteins (SPRRs), were induced in the IL-17A-exposed cultures, reflecting the cessation of terminal differentiation. Interestingly, such a pattern of increased SPRR expression along with filaggrin downregulation has been identified in psoriasis (51). This further validates our microarray data, as IL-17 is observed at high levels in the disease. In addition, the inflammatory response, locomotory and chemotactic behaviour as well as response to external stimulus and wounding were also processes that were enriched in IL-17A-exposed cultures.
Furthermore, GOrilla analysis of cellular components that were especially altered in the cytokine-stimulated cultures pointed at the formation of cornified envelope as the main structure regulated by IL-17A at the mRNA expression level (Fig. 1b and Table S2). This further supports our hypothesis that the cytokine is potentially an important modulator of epidermal barrier. In respect to changes in molecular function, chemokine receptor binding/chemokine activity was also highlighted (see Figure S1B and Table S3).
In addition to this, we have also compared the expression of S100 family proteins under the stimulation with IL-17, as these proteins are important indicators of epidermal stress, with levels increased in many skin inflammatory states. In agreement with this, we found that main epidermal S100 proteins are indeed induced following IL-17 stimulation (Table S4), suggesting their function in keratinocyte stress and recovery.
In summary, these results indicate that stimulation of keratinocytes with IL-17A alters many cellular processes that have a potential to affect barrier function of the skin, with a special influence on epidermal differentiation, formation of cornified envelope, response to inflammation and stress as well as chemotaxis.
IL-17 downregulates the generation of filaggrin monomers in HaCaT cell line and primary keratinocytes
Following the results of the microarray analysis, we determined the expression of filaggrin at the protein level by a western blot assay. HaCaT cells were stimulated with IL-17A at low or high dose. The expression was assessed against the expression of a housekeeping gene (GAPDH). We have observed that exposure to IL-17A decreased expression of the 37-kDa filaggrin monomer band using AKH-1 antibody (52) in a dose-dependent manner compared to the control samples (Fig. 2a).
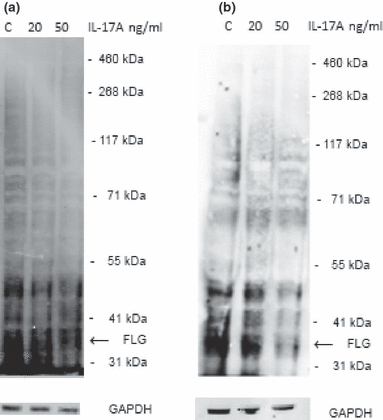
Interleukin-17 downregulates expression of filaggrin monomer in keratinocytes. (a) HaCaT cells were grown in D10 medium and exposed to increasing concentrations of IL-17A. The cells were harvested by trypsinization and lysed after 24, and western blot assay was performed. Figure shows a representative of three blots; (b) Normal human epidermal keratinocytes were maintained in KGM Gold medium at 0.06 mm of calcium and exposed to increased calcium concentration (1.5 mm) for up to 5 days before the experimental set-up. The cells were stimulated with 20 or 50 ng/ml of IL17A for 24 h and lysed. Western blot assay was performed. The figure shows a representative of three blots.
To assess the relevance of previous findings in a more physiological setting, we have used normal human epidermal keratinocytes (NHEK) as our study model. Western blot analysis carried out with primary keratinocytes stimulated with IL-17A revealed decreased expression of filaggrin monomer (Fig. 2b). We have also confirmed all these findings with another filaggrin monomer–specific antibody (SPM181) and N-terminal domain–specific antibody (H-300), data not shown.
In summary, we show that IL-17A downregulates filaggrin expression in keratinocytes. This suggests that the exposure of epidermis to the cytokine could result in an increased barrier dysfunction in a disease setting.
Discussion
It has been reported that Th17 cells are increased in the peripheral blood and accumulate in the acutely involved skin of patients with both AE and psoriasis (39,41). While Th17 bias reported seems to be much greater in psoriasis (43), significant upregulation of IL-17 has been detected in skin samples of both dermatoses compared with the healthy skin (44). Although the secretion of IL-17 is mainly attributed to CD4+ T cell clones, it seems that a small proportion of CD8+ cells and other cells are also capable of producing this cytokine in both diseases (53,54). It has also been noted that the frequency of both circulating and skin-residing IL-17+ T cells correlate with the clinical severity (41,42,55). While this population is observed to be attracted to skin especially at the acute stages of inflammation, it was suggested to promote the secretion of profibrotic cytokines, TGFβ and IL-11, with the effects also observed in chronically involved skin (40). This evidence implies a pathogenic function of Th17 cells in diseases characterized by barrier dysfunction.
A study by Teunussen et al. reported that IL-17A upregulates both HLA-DR molecules and ICAM-1 in keratinocytes, thus contributing to the formation of a functional immunological synapse and enhancement of antigen presenting function of these cells (54). Furthermore, changes in the secretory profile, especially stimulation of IL-6 and IL-8 production, were also noted. In addition to this, another study reported that IL-17 also enhances the secretion of MIP-3α, especially in combination with IL-22 (56). Neutrophil chemoattractants of the CXCL family, as well as CCR6+ lymphocyte chemoattractant CCL20, were also upregulated in keratinocytes after stimulation with IL-17 (57). In this respect, this cytokine could enhance inflammation during early stages.
We were interested to see whether stimulation of keratinocytes with IL-17 could influence genes associated with functional epidermis. The results of our microarray study indicate that indeed IL-17 affects the expression of genes involved in the formation of skin barrier. Specifically, we have observed a broad downregulation of the genes encoding proteins involved in cellular adhesion under stimulation with the cytokine.
A reduction in profilaggrin/filaggrin expression that we observed in the presence of IL-17 corresponds to the downregulation of this transcript that has been previously reported by Sugiura et al. (58) in their microarray study of atopic lesional skin.
The analysis of ontology terms enrichment carried out based on our data suggested that epidermal differentiation, formation of cornified envelope and expression of S100 proteins are disturbed by the treatment. This corresponds to studies that reported similar changes (43,58–61) showing alterations of gene expression in skin samples from psoriatic and/or atopic patients. Interestingly, IL-17 on its own did not seem to affect transcription of other important late keratinocyte proteins (involucrin, loricrin and transglutaminases), which appear to be similar to the work of Mee et al. (60) in the study of psoriasis skin. However, others reported differential regulation of those markers in both atopic skin disease and psoriasis (61,62). A caveat of in vitro assays using cell lines and/or primary cells are that the changes observed may not reflect those in vivo within a stratified squamous epithelium.
IL-17 is chronically present during skin inflammation (63), e.g. abundantly secreted by immune cells, especially when exposed to Staph. aureus or allergens (37,38,64). We therefore believe that constant stimulation of keratinocytes within acute skin lesions results in an effect on the barrier formation. While it is important to consider that concentrations of the cytokine we used could enhance the effect compared with pathophysiological situation, there is a possibility that IL-17, together with other inflammatory cytokines, contributes to the barrier dysfunction.
Furthermore, as our microarray data suggest that the profilaggrin-processing machinery is also affected, an influence on the formation of active filaggrin monomers and then their degradation leading to altered formation of NMF should not be underestimated. Recently, a downregulation of caspase 14, another enzymes thought to be involved in filaggrin processing, has been reported in patients with both AE and psoriasis (65). In addition, expression of this protein seems to be reduced under Th1/Th2 cytokine stimulation. However, our microarray data did not reveal differential transcription of caspase 14 under IL-17 influence (data not shown), which suggests that this cytokine may not be important in the regulation of this particular enzyme. Interestingly, we could not detect the changes in the expression of filaggrin 2 gene and S100 fused-type proteins, suggesting that expression of these genes is differentially regulated. Western blot analysis of HaCaT cell cultures confirmed that the changes we observed are translated to the protein level. In addition, these results were also replicated in primary keratinocytes, which indicates that the findings are reproducible in normal epidermal cells. However, we cannot exclude that the alterations in expression we observed were mediated indirectly, by any other mediators upregulated following IL-17 stimulation; in fact, it is possible that various inflammatory stimuli present in AD lesions can affect filaggrin expression.
Recently, a downregulating effect of some Th cell cytokines, including IL-17 and IL-17 family member, IL-25, on filaggrin gene transcription has been observed in keratinocytes (66,67). Both groups used a solely Q-PCR approach, not microarrays, so they were not able to evaluate a broader spectrum of genes relevant to filaggrin expression nor confirmed the identified alterations at the protein level. Noh et al. (67) suggested that membrane-associated protein 17 (MAP17) is a central regulator of filaggrin expression by cytokines. However, the transcription of this gene was not altered according to our microarray data suggesting that this factor may be redundant. Moreover, the study did not investigate other genes that are important in the generation of filaggrin, such as processing enzymes, which are likely to influence expression at the protein level. Furthermore, according to the study of Noh et al., not all cytokines that increased MAP17 expression altered filaggrin transcription. In addition, the authors also observed a decrease in filaggrin mRNA levels in IFNγ-stimulated cultures, contrasting with previously published work (22) and our own unpublished data. This incoherence further suggests that regulation of filaggrin production most likely includes post-transcriptional modulation as we have shown in the current study of changes in the expression of filaggrin-processing enzymes. Thus, it is possible that changes in the expression of many genes including profilaggrin- and profilaggrin-processing enzymes together contribute to a significant reduction of filaggrin at the protein level, as has been found in the current study. We have not observed significant changes in microRNA expression in keratinocytes following IL-17 incubation (data not shown). However, there are likely to be many other factors influencing keratinocyte expression of filaggrin, and we believe that an extensive assessment of the alteration in expression at both mRNA and protein levels is essential for full understanding the outcome of this stimulation.
However, filaggrin expression is not the only factor that can affect the formation of functional epidermal barrier (17). Therefore, we have extended our study to investigate whether IL-17 could also alter the expression of other components involved in the maintenance of barrier function. The results of our study also suggest that IL-17 affects the expression of other important components of epidermal barrier, i.e. tight/gap junctions and adhesion molecules. This suggests a possibility of generalized effect of the cytokine on cell adhesion which may contribute to the histological appearance of spongiosis. While this could be a mechanism enabling for access of effector molecules and cells during inflammatory state in the skin, such an insufficient barrier could also explain an increased penetration of allergens and infectious agents through the skin. Interestingly, correlation of altered expression of one of the junctional proteins, claudin-1, with both AE and psoriasis has been reported (36,68).
Interestingly, a murine model of AE, filaggrin-deficient flaky tail mouse, exhibits Th17-dominated skin inflammation from an early age, even before increased IL-4 expression levels are observed. Moreover, the animals seem to be permissive to epicutaneous sensitization with protein antigen, as demonstrated by antibody and cytokine secretion (69). Likewise, Th17 responses seem to be involved in skin pathology in the animal model of psoriasis (70).
Th17 cells seem to be effectively induced by epicutaneous allergen exposure in mice in vitro (71) and in vivo, which is followed by an increase in serum cytokine levels (72). Interestingly, other routes of antigen administration do not seem to result in such a Th17 bias in the animals, implying that crossing the skin barrier is especially important for the Th17 cell priming. Interestingly, the transepidermal route of sensitization led to airway inflammation even in the absence of IL-4 and IL-13 (73). Moreover, Staphylococcus aureus, which colonizes the skin of the majority of patients with AE, seems to promote IL-17 expression in the skin (74).
We therefore believe that IL-17 affects expression of filaggrin and transcription of genes that are involved in maintenance of epidermal tightness and is an important participant of a cycle of inflammation that is observed in AE; increased antigen penetration results in Th17 priming which, in turn, further deteriorates barrier function of epidermis in a positive feedback loop. Interestingly, the studies by He et al. (72,73) also demonstrated that the epicutaneous sensitization has the potential to evoke IL-17-dependent lung inflammation in mice. This suggests a relationship between the inflammatory processes at distant sites, implying that IL-17 could also play a role in inducing other atopic manifestations in predisposed patients after cutaneous exposure to environmental allergens. Moreover, to further support the relationship between Th17 cells and atopy, a recent study reported that stimulation of B cells with IL-17 leads to an increase in IgE production by enhancement of class switching (75). All these results, together with documented here effect of the cytokine on filaggrin expression and barrier function, would imply a prominent role of IL-17 in atopy.
An important consideration that has to be taken into account when understanding the impact of IL-17 on condition of the skin is related to the phenotype of patients suffering from hyper IgE syndrome. The disease is dependent on mutations in STAT3, demonstrated as a regulator of Th17 polarization (76) has been identified in this group of patients (76). However, while an eczematous like rash is often observed in hyper IgE syndrome, it has been documented that clinical manifestation differs from classic atopic dermatitis, especially regarding the dryness of the skin, intensity of pruritus and atopic associations (77), meaning that it is difficult to compare the dermatoses which may be characterized by different underlying pathomechanisms.
As described above, while there is a lack of apparent correlation with the loss-of-function mutations and psoriasis (78–81), reduced filaggrin expression has been reported in the disease (78–80). Interestingly, the expression seems to increase after treatment; filaggrin was therefore proposed to be a marker of remission (78). However, the signs of atopy, which would potentially indicate increased antigen exposure through compromised epidermal barrier, are not frequently observed in psoriasis. While smaller studies documented increased sensitization to environmental allergens and total IgE levels in serum in this group of patients (82–84), these reports are scarce and clinical practice does not support the common existence of psoriatic/allergic phenotype. It is possible that other cytokines that are present in atopic skin contribute to other aspects of atopic skin biology including downregulation of antimicrobial peptides.
It is possible that the combination of genetically determined and acquired filaggrin insufficiency leads to a greater and more prolonged filaggrin downregulation in the setting of AE, compared to psoriasis in which filaggrin mutations have not been observed.
Clinical studies are currently underway to evaluate anti-IL-17 treatment in psoriasis. Certainly, based on presented here evidence, IL-17-mediated decrease of epidermal barrier function is likely to enhance inflammation observed in atopic skin disease. Therefore, atopic patients could potentially also benefit from such treatment.
Acknowledgements
DGO designed and performed the research, analysed data and wrote the paper, ALS, MS and CC performed the WB experiments, ST analysed the microarray data, GSO designed the research, analysed data and wrote the paper, TMP provided skin samples, and TAK isolated primary cells. We would like to thank Dr N. Fusenig for HaCaT keratinocytes, Dr M. E. Polak for the advice regarding growing primary lines and Miss Chiwan Chiang for the technical assistance. Funding was gratefully received from the Medical Research Council, Barrie Trust, British Skin Foundation and NIHR Biomedical Research centre programme.
Conflict of interests
The authors have declared no conflicting interests.




