Exploring the role of stem cells in cutaneous wound healing
Abstract
Abstract: The skin offers a perfect model system for studying the wound healing cascade, which involves a finely tuned interplay between several cell types, pathways and processes. The dysregulation of these factors may lead to wound healing disorders resulting in chronic wounds, as well as abnormal scars such as hypertrophic and keloid scars. As the contribution of stem cells towards tissue regeneration and wound healing is increasingly appreciated, a rising number of stem cell therapies for cutaneous wounds are currently under development, encouraged by emerging preliminary findings in both animal models and human studies. However, we still lack an in-depth understanding of the underlying mechanisms through which stem cells contribute to cutaneous wound healing. The aim of this review is, therefore, to present a critical synthesis of our current understanding of the role of stem cells in normal cutaneous wound healing. In addition to summarizing wound healing principles and related key molecular and cellular players, we discuss the potential participation of different cutaneous stem cell populations in wound healing, and list corresponding stem cells markers. In summary, this review delineates current strategies, future applications, and limitations of stem cell-based or stem cell-targeted therapy in the management of acute and chronic skin wounds.
Introduction
Adult stem cells (ASCs) are considered to be the source for replenishing lost cells during wound healing, and therefore are recognized as key players in tissue regeneration. To name but a few, hepatic regeneration (1–3), lung regeneration (4), renal repair (5), corneal epithelial healing (6), neuroregeneration (7), cardiomyopathy (8) and tissue repair in ischemic injury (9) serve as exemplary processes for which this has been shown besides cutaneous wound repair (10–16). In all instances, tissue-specific ASCs provide daughter cells to repopulate the lost or injured tissues by differentiation and/or by releasing paracrine signalling molecules to recruit inflammatory cells and tissue progenitor cells (17,18). As ASCs have been identified in almost every tissue type in the human body and as their exploitation for regenerative medicine purposes invokes considerably fewer ethical concerns than the use of embryonic stem cells (19), and therapies involving autologous ASCs do not pose risk: there has been a rapid growth in the field of basic ASC research, and its clinical applications to regenerative medicine.
As skin has multiple important functions, such as acting as the barrier to foreign pathogens, regulating body temperature, supplying sensation and preventing dehydration of the body (20–25), normal wound healing is a critical survival factor, both in the individual and the species as a whole. In current medical practice, aberrations of cutaneous wound healing consumes substantial resources and often requires major, long-term medical attention (26). Severe scarring disorders not only affect the patients aesthetically but also psychosocially (27–29). Burn injury presents another type of insult to the skin which not only can be potentially lethal, but whose depleting effects on local tissue stem cells may result in the inability of the wound to repair itself, thus increasing the need to surgically graft borrowed skin from another body site to restore the functions of the skin (30,31).
Chronic wounds severely affect a patient’s quality of life (32) and generate enormous medical costs (26,33,34). Such wounds are frequently linked to old age (32), coinciding with a poorer reservoir of fully functional stem cells (35–38). Both chronic wound and keloid scarring may recur, suggesting differences in the intrinsic healing ability of stem cells between patients who heal poorly and those who heal more aggressively. Both hypertrophic and keloid scarring are characterized by hyperproliferation of wound fibroblasts (39,40) and overproduction of extracellular matrix (ECM) proteins (41–43). In addition, keloid scarring is characterized by its invasive growth into the surrounding skin and high risk of recurrence following any treatment modality (29,44–46).
A more complete understanding of the contribution of ASCs and their regulatory controls to normal and impaired wound healing, therefore, appears vital for a better control of wound repair, and is likely to promote novel treatment strategies for improved wound healing. In this review, we give an in-depth synthesis of the salient concepts, cover the different types of stem cells – both local and circulatory – that have been implicated in cutaneous repair. We present the potential sources of these stem cells, and discuss some of the pertinent concepts proposed to date regarding the mechanisms through which stem cells are thought to contribute to wound healing. Perspectives on research leading to better understanding of the role of stem cells and their application in cutaneous healing, leading to novel therapies for clinical management of difficult wounds will be discussed. We finally conclude by delineating selected avenues towards optimizing clinical management of cutaneous wound healing disorders by manipulating stem cell populations.
Main events in wound healing
The purpose of skin wound healing is to restore a skin defect and to regain, at least in part, lost integrity, tensile strength and barrier function of the skin (47). This wound healing process is recognized to consist of the following phases: the inflammatory, proliferative and tissue remodelling leading to scar formation (48).
Upon injury and damage to cutaneous blood vessels, platelets are exposed to the ECM proteins, which would then lead to immediate coagulation and fibrin clot formation (24). Mediated by diffusible factors released by the platelets and mast cells, such as platelet-derived growth factor (PDGF) (49) and tumour necrosis factor-α (50), leucocytes migrate to the wound. These cells migrate from the peripheral blood via diapedesis (51) (Fig. 1) into the wound site. Aside from eliminating microbes and debris, these inflammatory cells initiate repair and angiogenesis as the wound exits its inflammatory phase.
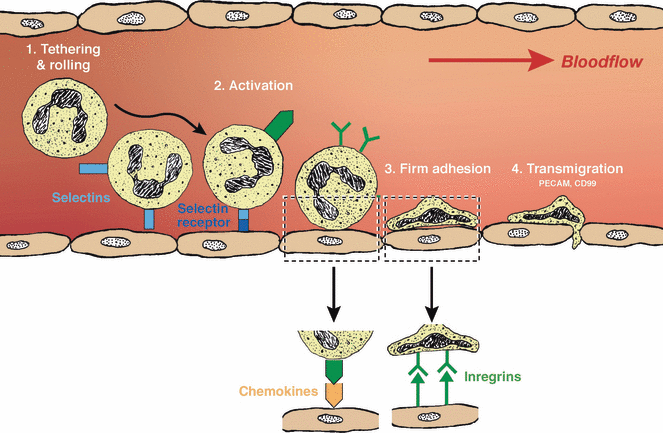
Illustration of the diapedesis process. Neutrophils begin diapedesis by tethering and rolling on the inner wall of the blood vessel, which induces selectins expression. The binding of the selectins on the neutrophils to their receptors on the endothelial wall activates chemokine expressions. On binding their chemokines to the chemokine receptors, integrin expression is induced. The integrins binding to the endothelium lead to firm adhesion of neutrophil to the blood vessel. Mediated by proteins such as platelet/endothelial cell adhesion molecule and CD99, transmigration across the endothelial takes place and neutrophils are able to move from the blood stream to the extracellular matrix.
During the subsequent proliferative phase, granulation tissue (GT) deposition provides a provisional wound bed for reepithelialization. Neovascularization occurs to support the newly formed tissue and to transport circulatory cells to the wound (52). Mast cells, which are required for early closure of normal wound healing (53), have been reported to promote the proliferation of fibroblasts, endothelial cells (ECs), and keratinocytes during the proliferative phase (54–56). For wound healing to progress, bidirectional interactions between keratinocytes and fibroblasts are essential. Through paracrine signalling, keratinocytes release interleukin (IL)-1 (57), which induces fibroblasts to secrete cytokines and growth factors important in wound repair, such as keratinocyte growth factor, fibroblast growth factor (FGF)-7, IL-6, granulocyte-macrophage colony-stimulating factor and hepatocyte growth factor (HGF) (57–59). These factors in return promote keratinocyte proliferation, creating a paracrine loop (57–62).
Although the desirable outcome of coordinated healing is to regenerate tissue with a similar structure and comparable functions to intact skin (47), dermal wounds are often repaired by collagen deposition and tissue remodelling. Collagen I, the most abundant ECM component, is the main component of scar tissue. The co-alignment of collagen bundles and fibroblasts in GT plays a key role in forming normal flat scars (63,64). For final wound closure, myofibroblasts exert contractile forces (65). Following complete wound closure, tissue remodelling takes place below the epidermal surface and can take up to a year or longer to complete (66). In adults, wound healing is generally viewed as ideal if only a mature, non-erythematous flat linear scar is the final outcome (63).
Stem cells – key players in different phases of wound healing
Inflammatory cell precursors from bone marrow, hair follicle and dermis
The obvious source for the leucocytes that migrate to the wound site during the early, inflammatory phase are bone marrow (BM)-derived haematopoietic stem cells (HSC), which have long been recognized to give rise to all blood cell lineages (67). BM-mesenchymal stem cells (MSC) have been shown to participate in this stage of wound healing, as they direct the haematopoietic progenitor cells (HPC) to differentiate into dendritic cells via the Notch signalling pathway (68). Through this interaction and allowing myelopoiesis in the early inflammatory phase, the demand for leucocytes is at least partly fulfilled.
It is much less clear to what extent the resident progenitor/stem cells participate in the early, inflammatory phase of wound healing. Interestingly, the intermediate bulge region of the epithelium of murine hair follicles (HFs) harbours HPC that are morphologically and immunophenotypically (CD45+/Lineage−(Lin−)/c-Kit+) almost identical to those from the BM and circulating blood of foetal mice (69). The connective tissue sheath (CTS) of murine HFs has been established as an extramedullary source of HSC (70), and is a local reservoir of connective tissue-type mast cells for which it harbours precursor cells (69). As shown by transplantation of BM from green fluorescent protein (GFP) positive (+) transgenic mice to wild-type mice, these mast cell precursors seem to have derived from the BM but seek the HF as a niche. In response to stem cell factor (SCF), a mast cell proliferative factor (71), produced locally by HF cells (72,73), these mast cell precursors may mature in the CTS, from where it is conceivable that their more differentiated progeny then participates in cutaneous wound healing, whenever the CTS is injured, which happens regularly in conjunction with skin trauma (74). Mast cell precursors that express HSC markers have also been isolated from the dermis of adult and neonatal mice (75). These Lin- dermal cells can generate large numbers of mast cells when cultured in the presence of SCF and IL-3, another mast cell maturation promoting factor (71). It is possible that these dermal mast cell precursors and other intradermally deposited HPC populations participate in cutaneous wound healing by supplying to the tissue macrophages, dendritic cells and mature mast cells (Fig. 2).
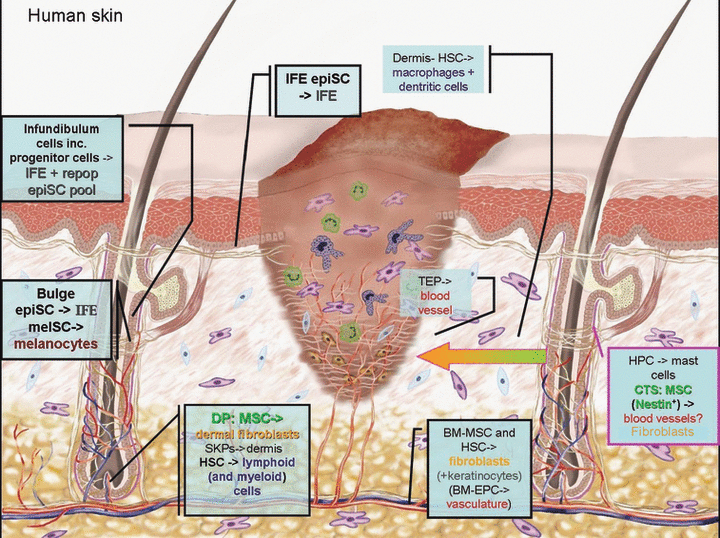
The stem/progenitor cell populations that contribute to cutaneous wound healing. Bars connecting to text boxes show where these populations normally reside in the skin and coloured texts describe the end differentiation products. Where there is a question mark, it means that the notion may be possible but has not been shown by empirical evidence. IFE, interfollicular epidermis; epiSC, epithelial stem cell; melSC, melanocyte stem cell; MSC, mesenchymal stem cell; SKPs, skin progenitor cells; HSC, haematopoietic stem cell; EC, endothelial cell; TEP, tissue endothelial progenitors; BM, bone marrow; EPC, endothelial precursor cells; HPC, haematopoietic precursor cells.
Reepithelialization – multiple players
Keratinocytes that reepithelialize the wound are derived from two populations of epithelial stem cells (epiSC) in the skin: the interfollicular epiSC located in the basal layer of the interfollicular epidermis (IFE) (76), and the HF bulge epiSC, located in the outer root sheath (ORS) of the permanent portion of the HF where the arrector pili muscle inserts (77–80) (Fig. 3). They are self-renewing and able to produce rapidly proliferating daughter cells – transient amplifying cells – that undergo several cell divisions before differentiating via Notch/p63 cross-talk (81) and leaving the basal compartment (76,82,83). In response to injury, these two epiSC populations provide keratinocytes that reconstruct the epidermal barrier (84,85). Knock-out mice of c-myc, a regulator of the exit of epidermal stem cell fate of IFE epics (86), display hindered reepithelialization because of the inability of epiSC in IFE to produce daughter keratinocytes (87).
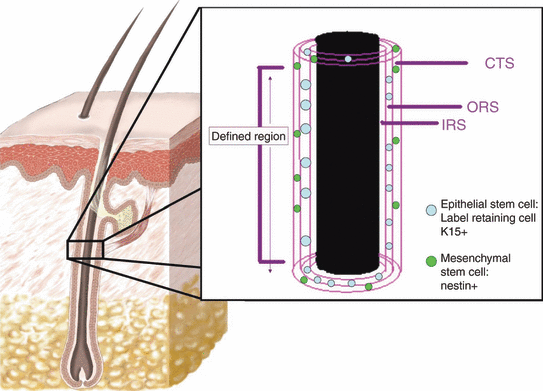
The bulge region of a human hair follicle. In the human hair follicle, the bulge is not protruding like a murine hair follicle bulge, therefore it is identified as the part of the outer root sheath (ORS) where the arrector pili muscle inserts into, or where the label-retaining or keratin 15 positive (K15+) cells, i.e. epithelial stem cells are found (176). In the enlargement picture, the three layers inner root sheath (IRS), ORS and connective tissue sheath (CTS) are depicted. While epithelial stem cells can be found in the ORS or the bulge region, mesenchymal stem cells, which are nestin+ are found in the CTS.
In mice, the HF keratinocytes from the upper infundibulum (a part of the distal ORS of the HF epithelium), which are distinguishable by their α6-integrinlow CD34− Sca-1− phenotype, are able to give rise to long-term stable IFE and pilosebaceous lineages, plus self-renew in vivo (88) – all together an indication of their capacity to function as epiSCs in the IFE (89). Although HF bulge epiSC are not necessarily required for epidermal survival (84), nor does its absence prevent reepithelialization (90), they seem to enhance the early stages of wound closure (90,91). In neonatal mouse models, ablating early HF epiSC led to severe impairment of IFE regeneration (91), whereas in adult mutant mice that lack HF development, there is an acute delay in wound closure (90).
Mesenchymal-to-epithelial transition (MET)
Mesenchymal-to-epithelial transition (MET) describes the phenomenon of a cell changing from a mesenchymal phenotype to an epithelial phenotype; the reverse of which is termed epithelial-to-mesenchymal transition (92). Both processes are important during embryonic skin development, tissue regeneration and wound healing (93). MET is not restricted to skin, as it is also required in, for example, the de novo development of a hair cell in ear (94). Mesenchymal multipotent progenitors in the murine dermis have been identified and were shown to be able to differentiate into keratinocytes (95). Therefore, it deserves to be explored whether some of these dermal (mesenchymal) progenitor cells help to reepithelialize the wound via MET in vivo. In addition, there exists a low level of MET among the BM-MSC population, as a small number of BM-MSC had been found in the wound epidermis in a mouse model (96). Its contribution to replenishing epithelial cells in normal wound healing through differentiation is however very limited, as BM-derived keratinocytes are relatively rare (96).
Cell fusion is another proposed explanation of the MSC plasticity (97,98). However, although cell fusion between MSC and epithelial cells occurred frequently ex vivo (99), transdifferentiation is believed to be the predominant event, in vivo (14,100). Nevertheless, other than via MET or cell fusion, these BM-derived cells are likely to contribute to epidermal wound healing by promoting epithelialization via paracrine signalling, as the application of exogeneous BM-MSC into diabetic mouse wounds was found to augment the level of cell division in the endogenous keratinocyte population (14).
Revascularization by resident and circulating endothelial cell precursors
Angiogenesis is the main mode of revascularization in normal wound healing (101) and primarily depends upon the sprouting of resident ECs. Recently, additional stem cell types with angiogenic ability have been identified – the so-called tissue-resident endothelial precursors (TEPs), which were isolated from murine adipose tissue and dermis (102), and pluripotent nestin expressing cells (103). Murine hair follicle nestin-GFP+ cells, derived from transgenic mice carrying a nestin second intron enhancer give rise to nascent blood vessels and Schwann cells in vivo. Moreover, after transplantation into mice with sciatic nerve and spinal cord crush injury they promote neuroregeneration. These nestin+ cells can be harvested and expanded from murine bulge explants by transgenic expression of GFP (104,105) (Table 1). Both TEPs and nestin+ cells have been differentiated into EC markers-expressing cells in vitro and reportedly form functional blood vessels that link to the existing capillary network in transplantation experiments (102,103). In humans, nestin+ bulge MSC are distributed within the HF mesenchyme (106–109) (Fig. 3). Human cutaneous nestin+ cells have the potential as an easily accessible and abundantly available source of adult, autologous progenitor cells. Coincidentally, newly formed blood vessels in human skin can be identified by nestin expression (110), suggesting a link between nestin+ HF-MSC and neovascularization in human skin.
| Cell type | Location | Expressed markers | Derived lineages | References |
|---|---|---|---|---|
| Epithelial stem cell | Bulge | K15 (h-preferential; m), K19(m), CD34(m), S100a4(m), S100a6(m) CD200(h), Sox9(m: early epiSC precursor) | All hair follicle lineages including sebaceous gland, and IFE | (77,91,146,177–179) |
| Epithelial stem/progenitor cell | Anagen: lower ORS; telogen: bulge and secondary germ | Lgr5(m) and partially overlaps with K15 and CD34 in the telogen phase | All hair follicle lineages | (180) |
| melSC | Bulge and sub-bulge | Dct, SOX10, PAX3, Mitf | Mature melanocytes | (135,136) |
| eNCSC | Bulge in mouse hair follicle | Nestin, Nanog, Oct4, CD34, Tcf3, S100a4, S100a6 | Neurons (peripheral type and GABA-ergic type), Schwann cells, smooth muscle cells, melanocytes adipocyte-like cells, chondrocytes | (103–105,181) |
| SKPs | DP | Intact follicle (in vivo): fibronectin; papillaspheres (in vitro): fibronectin,vimentin, nestin, musashi, Pax3, slug | Neurons, glial cells, Schwann cells, smooth muscle cells, adipocytes | (130) |
| MSC | CTS and DP | CD90, CD44, CD73 CD34-, nestin | Adipocytes, osteoblasts, chondrocytes, dermal fibroblasts, myocytes, neurons | (107,108,127,182) |
| HSC | CTS and DP | N/A | Erythroid and myeloid lingeages | (70) |
| HPC | Pre-dominantly in the intermediate bulge containing section; layer unspecified | CD45+, c-kit+, Lin− | Connective tissue-type mast cells | (69) |
- K, cytokeratin; CD, cluster of difference (cell surface marker); melSC, melanocyte stem cells; eNCSC, epithelial neural crest stem cell; SKPs, skin progenitor cells; IFE, interfollicular epidermis; DP, dermal papilla; CTS, connective tissue sheath; MSC, mesenchymal stem cell; HSC, haematopoietic stem cell.
- Where indicated by ‘m’ in brackets, the marker is a restricted marker in mouse, ‘r’ for rat and ‘h’ for human.
A portion of neovascularization (especially in deeper wounds) may occur through vasculogenesis, as suggested by the presence of BM-derived endothelial precursor cells (EPCs), a subset of HSC, in the wounds of irradiated mice transplanted with BM-derived EPCs (101,111). They were mainly detected in deeper skin layers – GT and adipose tissue (111). Their presence in wound is transient, likely because of the capillary regression event at later wound healing stages, leading towards scar tissue formation (111). The mobilization of stem cells from the BM is a dynamic process regulated by shear stress imparted by blood flow and the activation of metalloproteinases that induce the release of ‘kit ligand’ (112). Angiogenic factors including VEGF, HGF and angiopoietins are released from the injured tissue and BM-MSC recruited to the wound (14,113). Through the interaction with their receptors expressed on EPC and MSC, endogenous stem cells are encouraged to circulate, and thereby the recruitment of these cells to neo-angiogenic sites will accelerate the revascularization process (114,115).
Adipose-derived stem cells (ADSC), although not known to form nascent blood vessels in normal wound healing, are multipotent stem cells able to differentiate into EC and promote angiogenesis (13). They may facilitate angiogenesis in deeper wounds.
BM-derived stem cells, hair follicle and dermal MSC contribute to the final phase of healing
The origin of BM-derived fibroblasts in skin has long been debated. On the other hand, BM-derived fibrocytes infiltrate the wound at early time points of the wound healing process and express ECM proteins vimentin, collagen I and III (116,117). Clonal transplantation of HSC in mice confirmed that fibrocytes are able to differentiate into fibroblasts and myofibroblasts in several organs including skin (118). On the other hand, BM-derived mesenchymal cells (MC) were found to assume a fibroblast cell fate in vivo in mice during cutaneous wound healing and express the fibroblast-specific collagen I-α2 chain in vivo (64) and both collagen I and III genes in vitro (64,119) [whereas the skin-resident interfollicular dermal fibroblasts reportedly transcribe only collagen I (119)].
PDGF activated fibroblasts may attract BM-MSC to the wound site by releasing chemoattractants for the latter (120). Governed by the Wnt/β-catenin pathway, accumulated MSC are able to differentiate into fibroblasts (121). Furthermore, BM-derived MC are stably represented in dermal wounds throughout the repair process, especially during tissue remodelling (64,119). BM-MC thus greatly contribute to laying down collagen in the ECM along with the resident dermal fibroblasts, and may also help contracting the wound in vivo, as in vitro they demonstrate a contractile phenotype (119,122) and express alpha-smooth muscle actin (a myofibroblast marker) (120). As an HSC origin for fibroblasts/myofibroblasts does not exclude the possibility of an MSC origin of these cells, it is conceivable that BM-derived fibroblasts in the skin wound arise from both BM-HSC and MSC.
As a local source for replenishing dermal fibroblasts, the main dermal population, multipotent dermal MSC (95,107,123,124) are considered to be the primary candidates. They exhibit a high telomerase activity level compared with BM-MSC, which is linked to a high proliferative activity, thus a greater regenerative potential (108). Furthermore, murine dermal cells have the capacity to produce muscle-like structure (expression of smooth muscle actin and desmin) when injected subcutaneously into mice (95) suggesting the presence of myofibroblast precursor cells. HF-MSCs are a potential source of fibroblasts in normal wound healing. CTS cells express smooth muscle actin, in vivo and in vitro (125); transplantation of HFs with vital dye-labelled CTS cells into a murine wound bed resulted in CTS fibroblasts from the lower HF being incorporated into the wounded dermis (74). In intact mouse skin, HF cycling involves extensive, bidirectional trafficking of fibroblasts between the HF dermal papilla (DP) and the CTS (126); hence, it is conceivable that CTS fibroblasts may migrate into the interfollicular dermis under wound healing conditions during GT formation (74,127,128) (Fig. 2).
Although neurons are neither produced, nor do they reside in the skin, progenitor cells with neuronal potential have been identified in the skin: SKPs, eNCSC and human HF-MSC (Table 1). Though they are unlikely to be responsible for skin reinnervation as sensory neurons reside in dorsal root ganglia, these cells are highly plastic (104,105,129,130). Therefore, although it still remains to be proven, it is conceivable that these progenitor cells also contribute to GT formation during wound healing (e.g. SKPs have enormous potential to take part in dermal reconstruction, whereas eNCSC have at least been shown to participate in revascularization in murine skin, and the dermal repair action of CTS-MSC) (Fig. 2).
Repigmentation
After wound closure, a well-healed wound may eventually be repigmented (131,132) and may even reconstruct its appendages (133,134). The cell pool responsible for epidermal repigmenation is currently thought to be the amelanotic melanocytes in the HF-ORS, which derive from melanocyte stem cells (melSC) residing in the HF bulge and sub-bulge (135) (Fig. 2). Strong evidence generated from a number of mouse models (135–137) (Table 2) suggests that the melanocytes that repigment a regenerated epidermis are of follicular rather than of epidermal origin. This is corroborated by the clinical observation that epidermal repigmentation typically occurs in a regularly spaced, ‘spotted’ pattern (i.e. a follicular distribution), both in regenerated epidermis (138,139) (Table 2) and in repigmenting skin of patients with vitiligo (71).
| Evidence that hair follicle melSC are sources of melanocytes in IFE | References |
|---|---|
| Pulse chase experiment using BrdU demonstrated the melSC in the bulge as LRC and the melanocytes in the hair bulb and hair matrix to be non-LRC | (135) |
| Following conditional depletion of immature melanocytes, the TA daughter melanocytes can reverse cell fate to restablish melanocyte reservoirs on occupying new niches | (135) |
| In furred animals, the IFE is unpigmented thus a lack of melanocytes in the IFE, therefore in the evolutionary pathway the IFE is not a melSC niche | (136) |
| Follicular melanocytes are able to migrate up a HF to give it a characteristic colour, and migrate out into the surrounding melanocyte-deficient skin | (137) |
| Transplanted follicular melanocytes, labelled using PKH26 fluorescent cell linker dye, can be found in the healed wound. Repigmentation subsequently occurred | (137) |
| SEM and histological examination of the skin section of vitiligo patients showed that the melSC are present in the hair follicle bulge but absent in the epidermis | (138) |
| During the repigmentation of vitiliginous skin, HF melanocytes migrate and mature en route from the infundibulum to the orifice and to the surrounding IFE | (138) |
| Congenital melanocytic naevi patients whose skin was severely repigmented after dermabrasion and curettage treatment: histological samples clearly demonstrated that pigmented naevoid cells resided along the pilosebaceous unit | (139) |
- BrdU, 5-bromo-2-deoxyuridine; TA, transient amplifying; HF, hair follicle; IFE, interfollicular epidermis; LRC, label-retaining cells; SEM, scanning electron microscopy.
Hair follicle neogenesis
Although not yet demonstrated to occur spontaneously in wounded human skin, full-thickness excisional wounds in mice were found to heal with de novo HF generation, in a process that requires functional Wnt signalling (134). This is in keeping with earlier observations that there is skin appendage neogenesis in the antlers of adult deer (133) and in wounded rabbit skin (140).
In mice, the regenerated HFs establish their own epiSCs and undergo HF cycling. Interestingly, the de novo-generated HFs seem to arise from epithelial cells outside the bulge, suggesting that epidermal cells in the wound assume a HF stem cell-like phenotype and become susceptible to inductive signals that drive these keratinocytes into a HF-type epithelial differentiation pathway. This raises the intriguing possibility that wounding reverts the skin, at least to some extent, to an embryonic phenotype (27), i.e. to a signalling milieu that may facilitate tissue regeneration.
Importance of the hair follicle in wound healing
It has long been observed that, when the HF is still relatively intact post injury, epidermal sheets spread centrifugally from the HF infundibulum to reepithlialize the wound bed (90,131,141). Moreover, in deeper wounds, where the HFs are largely destroyed, the wound heals more slowly and only from the wound-edge (90,142). Wound healing also happens faster in skin areas rich in terminal HFs (such as the fully haired scalp, whereas wound healing is retarded in balding scalp) (25), and the rate of wound healing is found to be correlated with synchronized HF cycling in mice (it is maximal in anagen skin) (143). Apparently, epidermal cells from skin appendages, such as HFs, also quickly remove clotted blood and damaged stroma from the wound area (144). A link between the recovery of the number of DP and dermal reconstruction has been suggested, such that a smaller number of DP recovered correlates to increased scarring (145). In addition, epithelial and mesenchymal HF cells (e.g. ORS keratinocytes and CTS or DP fibroblasts of rats) alone are sufficient to generate skin equivalent in vitro (128), whereas injecting a combination of DP, CTS and ORS cells allows the de novo generation of HF in skin as demonstrated in a rat model (128).
These observations are easily reconciled with the current appreciation of the HF as a major local reservoir for several different progenitor cell populations known to play key roles in wound healing (Table 1). For instance, the major site for epiSCs shifts from the IFE in embryos to the bulge in adults, during development. This is shown by the shift in proliferative potential from the embryonic epidermal cells to adult follicle bulge cells, demonstrated by the number of colony-forming units isolated from the HF and the IFE, which decreases in the IFE dramatically after birth, but increases in the neonatal and adult bulge relative to the IFE (84). This shift also coincides with the reported expression of keratin-15 (HF bulge epiSC marker), initially found in the embryonic and neonatal IFE, but moves towards the adult HF bulge (146). Furthermore, it is known that, harvesting SKPs that have enormous potentials in the participation of wound healing is more problematic in adult human than harvesting them from foetal skin (130). However, they can be successfully isolated from the DP from adult human HF (130), supporting our argument that the HF is an important stem cell niche. Evidently, the HF and its CTS provide adequate environment/niches for maintaining both pluripotency and quiescence of various SC populations (147–149).
These findings make the HF a most attractive target for regenerative medicine, by exploiting it as an easily accessible and abundantly available ASC reservoir (127,130,150), and by exploiting its – under-investigated and poorly characterized – roles as promoter of cutaneous wound healing on multiple levels.
Clinical applications of stem cells to wound healing
On the basis of these findings into the potential role of ASCs in wound healing and tissue repair, a number of stem cell treatments have already been developed and put to clinical trial in recent years. Using ASCs presents with fewer ethical issues, the use of autologous stem cells minimizes the risk of infection and excludes the problem of graft-versus-host disease. Encouraged by positive results from stem cell-based treatment strategies in postinfarction myocardial repair (151,152), similar strategies have been applied to treat skin wounds, where positive effects on all phases of wound healing have been reported with various types of MSC and HF epiSC (10–14,16,137,153) (Tables 3 and 4). In addition, the implantation of HFs in Integra™ skin equivalent templates for transplantation onto a human patient’s burn wound accelerated reepithelialization, minimized skin graft failure by reconstituting the epiSC pool, and was felt to produce cosmetically satisfactory results (Table 4) (153). However, a number of criteria and considerations need to be carefully contemplated when choosing the most suitable treatment regimes and strategies, which are discussed below.
| Treatment | Species/model | Control | Results | References |
|---|---|---|---|---|
| Spraying autologous/allogeneic MSC with a mixture of fibrin/thrombin | db/db mice and wt mice with full-thickness wound | Db/+ littermates and db/db mice with fibrin spray alone | Prevention of ulceration and accelerated wound closure | (12) |
| Intradermal injection of BM-MSC round the wound or in Matrigel applied to wound bed | Semitransparent BALB/c and db/db mice with full-thickness excisional skin wounds | Neonatal dermal fibroblasts or vehicle treatment | Increased complete wound closures in db/db mice; enhanced reepithelialization, cellularity; increased vasculature and appendages; thicker and larger granulation tissue | (14) |
| BM-MSC systematically or locally applied | Streptozotocin-induced diabetic adult rats | Untreated adult rats | Immediate and insignificant increase in wound collagen and wound healing growth factors | (15) |
| Collagen gel solution with ADSC applied | Female nude mice, with two full-thickness dorsal wounds each | Collagen gel solution with no ADSC | Significant reduction of wound size and accelerated the reepithelialization from the wound edge | (16) |
| ADSC seeded in human ADM applied to skin wounds | Athymic nude mice with dorsal full-thickness excisional wound | No graft applied or unseeded ADM applied to wounds | Acceleration of wound closure; local persistence of applied ADSC and differentiation into epidermal, endothelilal cells and dermal fibroblasts | (13) |
- IV, intravenous; MSC, mesenchymal stem cell; hMSC, human MSC; db, diabetic; wt, wild type; ADSC, adipocyte-derived stem cells; ADM, acellular dermal matrix.
| Treatment | Patients | Control | Results | References |
|---|---|---|---|---|
| Spraying autologous MSC with a mixture of fibrin and thrombin | Patients with acute and chronic wounds | Fibrin spray alone without MSC applied to second or third wound in patients with >1 wounds | Strong correlation between the no. of cells and reduction of ulcer area; instant pain relief to acute wound patients; acceleration of wound closure and resurfacing; reduction in ulcer size or complete wound closure among chronic wound patients by 16–20 weeks; no adverse effects | (12) |
| Topical application of culture expanded autologous BM-MSC | Human chronic ulcer (>1 year) patients not responded to other previous treatments | Other conventional treatments as listed in publication | Wound closure, increase in cellularity and dermal rebuilding | (11) |
| Hair follicle micrografts into Integra dermal regeneration template applied to burn wound 12 days after burn | Human male patient suffering from a 12% full-thickness burn | No control, although compared with previous treatments without follicle micrografts | Accelerated reepithelialization induced by hair follicle stem cells; restoration of stem cell populations, achieved hair growth and skin maturation | (146) |
- MSC, mesenchymal stem cell.
Other than transplantation, recruiting endogenous stem cells to the site of injury presents an alternative (or complementary) treatment method. For example, intradermal injection of cutaneous T-cell attracting chemokines and chemokine (C-C motif) ligand-17 and ligand-21 resulted in a significant acceleration of skin regeneration via recruiting BM-derived keratinocyte precursors and BM-MSC, respectively (100,154). Increased MSC migratory rate is also inducible by HGF (155), basic fibroblast growth factor (bFGF) (120,156) and CXCL5 (120) in vitro (secretion of bFGF and CXCL5 is upregulated in PDGF-activated fibroblast) (15). Thus, HGF, bFGF and CXCL5 may be exploited as BM-MSC chemoattractants.
To enable a sustained, long-term and localized delivery of these diffusible factors, various forms of ECM-like material have been developed and examined as carriers. Binding HGF to a heparin-containing semi-synthetic ECM-like hydrogel, for example, prevented the rapid proteolysis that normally occurs to HGF in vivo and a slow release of HGF could be controlled (155). Applying bFGF to wounds using a synthetic ECM was also shown to accelerate wound repair in a dose-dependent manner in genetically diabetic mice (157). Although the chemotaxis of BM-MSC was not examined in this study, based on the finding that bFGF increases the migratory rate of MSC, it is conceivable that the acceleration in wound repair was attributed to BM-MSC. Topical application of VEGF, aside from locally upregulating growth factors important for wound repair, was also shown to accelerate diabetic wound healing by recruiting BM-derived cells that contribute to revascularization (158). However, caution must be exercised while applying VEGF to wounds, because VEGF has been shown to increase scarring in a recent report (159). In contrast, bFGF injected into patients with incisional wounds was shown to inhibit scarring (160).
Points to consider in stem cell-based wound healing treatments
Application of stem cells is advantageous over administering single biological diffusible factors because stem cells can interact with their environment and release multiple wound healing factors. Although improvement in wound healing has been demonstrated in many stem cell treatment reports, we still need to consider several issues when administering stem cells to wound patients. We must consider, first of all, whether the patients are suitable for stem cell treatments and which stem cell population would be the most appropriate.
The age of the patients should come into consideration, because in older patients the functionality of their stem cells decreases, even if the quantity does not necessarily decrease (35,36,161). In a mouse model, it has even been shown that BM-MSC from old mice inhibit rather than promote wound healing when applied to wounds in diabetic mice (161). Moreover, the aspiration of MSC from the BM is itself an invasive procedure, hence not recommended for older patients. As a result, the use of autologous ASC for treating older patients may be less suitable than applying allogeneic ASC. One must then consider the possibility of immuno-rejection that can be triggered by the transplantation of foreign cells. Fortunately, BM-MSC were found to interact with the dentritic cells, T cells and natural killer cells from the host to promote a more anti-inflammatory and tolerant phenotype (162). They do so by releasing chemokines and nitric oxide, thus reducing the risk of host-versus-graft disease during the transplantation of exogenous BM-MSC (162–165). Additionally, immune tolerance-promoting strategies should be investigated. For example, the temporal induced expression of indoleamine 2,3-dioxygenase in dentritic cells and monocytes by administering its activators, such as interferon-γ, has the potentials to reduce the risks of allogeneic reactions in stem cell transplantations via inhibition of T-cell response (166–169).
The mode of stem cells delivery should also be well thought-out. The stem cells should ideally be able to retain their stemness until delivered; and to maintain localized at the wound site, to enhance engraftment. For such purposes, an acellular dermal matrix was employed as a carrier for delivering ADSC to the wounds locally (13). This method successfully prevented systemic distribution of the ADCS, thus is likely to be a more effective method for targeting the wound site than via injection of stem cells (13).
In diabetic wounds, the long-term reconstitution of blood vessels is important for the success in sustained wound healing (170). As the mobilization and homing ability of BM-EPCs in diabetic patients were found to be severely impaired (171–173), although application of bFGF may have accelerated wound healing in diabetic models possibly through mobilizing BM-MSCs (157), for ensuring long-term revascularization, the direct application of EPCs to the wound may be more effective (14,171).
While stimulating GT formation is desired in a deep chronic ulcer, this may be counter-productive in an acute wound, where excessive GT formation could lead to excess scarring (174). Another category of patients who may not be suitable candidates of BM-MSC-based wound healing therapy are those who are susceptible to keloid scarring, as BM-MSC release VEGF (14) which is linked to excessive scarring (159). Recently, it has been suggested that human MSC may be involved in keloid pathogenesis as the keloid fibroblasts were shown to be chemotactic to hMSC in vitro and their co-culture leads to the display of keloid fibroblast phenotype in hMSC (175). Therefore, although hMSC may help reduce scarring and restore tensile strength in acute wounds (165), patients predisposed to keloid disease should perhaps avoid BM-MSC application to their wounds. So far, these keloid promoting effects discussed come from BM-MSC, but the same effects from transplanting other ASC like those from the skin, HF and adipose tissue have not been reported. Therefore, future studies should aim to find out whether these skin- or HF-derived stem cells (which are more accessible than the BM-MSC by any means) could be better alternatives. And if not, we must learn how they may be involved in the pathogenesis of wound healing disorders and how to rectify the problem.
Conclusions and perspectives
Exploiting and targeting various intracutaneous and bone marrow-derived stem cell populations for improved wound healing is a promising tissue repair strategy. Recent data have demonstrated the feasibility of autologous ASC therapy in cutaneous repair and regeneration. There are, however, many unresolved questions regarding experimental and clinical application of stem cells in wound healing, which need to be systematically addressed in future research.
This review has provided a critical overview of recent developments and highlighted the contribution of both BM-derived and resident, intracutaneous stem cells and emphasized the recognition of progenitor cell populations associated with the HF (147) in cutaneous wound healing. Nonetheless, the relative role and contribution of locally maintained progenitor cells to skin wound healing compared with BM-derived ASCs upon cutaneous wounding needs further elucidation.
In summary, this review has attempted to explore the potential role of stem cells in cutaneous wound healing and delineated strategies in current stem cell therapy and future perspectives in the management of acute and chronic cutaneous wound healing. Future research will focus on the biology of ASCs, in particular, revealing the signals that mediate cellular growth and differentiation and further explore the therapeutic merits of stem cells in different types of cutaneous wounds.




