Pemphigus mouse model as a tool to evaluate various immunosuppressive therapies
Abstract
Abstract: Pemphigus vulgaris (PV) is an autoimmune bullous disease caused by immunoglobulin G (IgG) autoantibodies against desmoglein 3 (Dsg3). We have generated an active disease mouse model for PV by adoptive transfer of Dsg3−/− lymphocytes. In this study, we investigated the benefits and limitations of this model as a tool to evaluate various immunosuppressive therapeutic strategies. We used the following three measurements to evaluate the effects of the drugs during the time course: Dsg3 enzyme-linked immunosorbent assay scores that represent the level of production of anti-Dsg3 IgG, body weight loss that reflects the severity of oral erosions and PV score that reflects the extent of skin lesions. We examined various immunosuppressive agents currently used to treat patients with PV model mice in preventive protocol. Cyclophosphamide almost completely suppressed the production of anti-Dsg3 IgG, development of body weight loss and the appearance of the PV phenotype in contrast with the control group without the drug. Azathioprine, cyclosporin A and tacrolimus hydrate also showed suppressive effects to various degrees. However, methylprednisolone and dexamethasone failed to show significant effects in contrast to the findings reported in humans. Knowing the advantages and limitations of this model will provide an important foundation for the future evaluation and development of novel therapeutic strategies.
Abbreviations:
-
- AZA
-
- azathioprine
-
- CPA
-
- cyclophosphamide
-
- CsA
-
- cyclosporin A
-
- DEX
-
- dexamethasone
-
- Dsg3
-
- desmoglein 3
-
- ELISA
-
- enzyme-linked immunosorbent assay
-
- FK506
-
- tacrolimus hydrate
-
- mDsg3
-
- mouse Dsg3
-
- m-PSL
-
- methylprednisolone
-
- OD
-
- optical density
-
- PBS
-
- phosphate-buffered saline
-
- IgG
-
- immunoglobulin G
-
- PV
-
- pemphigus vulgaris
Introduction
Many tissue-specific autoimmune diseases are rare and their treatment protocols are largely dependent on the physician’s experience. Consequently, the protocols have regional variations and it is difficult to reach a global or even a local consensus on treatment. The low incidence of the diseases also makes it difficult to perform large-scale randomized controlled clinical studies to achieve evidence-based medicine. Heterogeneity among the patients with tissue-specific autoimmune diseases and limited access to ex vivo specimens of immune-mediated cells with an appropriate set of healthy controls are additional obstacles to the evaluation of conventional and development of new therapeutic regimens.
Pemphigus is a tissue-specific autoimmune disease that targets the skin and mucous membranes. Pemphigus has two major subtypes, pemphigus vulgaris (PV) and pemphigus foliaceus, which are caused primarily by immunoglobulin G (IgG) autoantibodies against desmoglein 3 (Dsg3) and Dsg1, respectively (1). PV is characterized clinically by painful oral erosions and flaccid blisters of the skin, and histologically by blister formation just above the basal cell layers because of the loss of cell-cell adhesion of keratinocytes. PV can be fatal without appropriate treatment. Circulating anti-Dsg3 IgG autoantibodies are measured by enzyme-linked immunosorbent assay (ELISA) using recombinant Dsg3, and their titres are closely correlated with the disease activity (2–4), and epitopes on Dsg3 are correlated with clinical phenotype or severity (5,6).
We have recently developed an active disease mouse model for PV by adoptive transfer of lymphocytes from immunized or naïve Dsg3−/− mice to mice expressing Dsg3 (7–10). Immunological tolerance against Dsg3 should not be acquired in Dsg3−/− mice because immunomodulatory cells are never exposed to Dsg3 during development of the immune system. In Rag2−/− recipient mice, anti-Dsg3 IgG was stably produced, bound in vivo to the cell surface of keratinocytes, and caused suprabasilar acantholysis in the oral mucosa and oesophagus. As a result, the mice showed weight loss because of the inhibition of food intake. The recipient mice also developed patchy hair loss or telogen hair loss. The antibody production was stable and observed over 6 months after adoptive transfer.
In the present study, we used this model in preventive protocol to evaluate the effects of various immunosuppressive reagents that are currently used to treat patients with PV. We examined four immunosuppressive agents, cyclophosphamide (CPA), azathioprine (AZA), cyclosporin A (CsA) and tacrolimus hydrate (FK506) and two glucocorticoids, methylprednisolone (m-PSL) and dexamethasone (DEX). We monitored the level of circulating anti-Dsg3 IgG by ELISA as a causative factor of the disease and body weight loss as well as PV score as the phenotype of the disease along the time course. The PV mouse model represents a valuable tool for evaluation of various therapeutic strategies in preclinical studies.
Materials and Methods
Adoptive transfer of splenocytes
Dsg3−/− mice were obtained by mating male and female Dsg3+/− mice, which have a mixed genetic background of 129/SV (H-2b) and C57BL/6J (H-2b). Splenocytes were obtained from Dsg3−/− immunized with the recombinant extracellular domain of mouse Dsg3 (mDsg3) (7). Eight-to-ten-week-old Dsg3−/− mice were primed by subcutaneous injection of 10 μg of purified mDsg3 in complete Freund’s adjuvant, boosted once a week for 2 weeks by subcutaneous injection of the same dose of mDsg3 with incomplete Freund’s adjuvant, and boosted twice more for 2 weeks by intraperitoneal injection of mDsg3 without any adjuvant. Approximately 1.5 × 107 splenocytes from Dsg3−/− mice immunized with mDsg3 were adoptively transferred into 8-to-10-week-old C57BL/6 Rag2−/− mice (Central Institute for Experimental Animals, Tokyo, Japan) via the tail vein. These mice were maintained under specific pathogen-free conditions in our animal facilities. All mouse studies were approved by the Animal Ethics Review Board of Keio University.
Administration of various drugs to pemphigus model mice
To examine the effects of various drugs, treatment was initiated from 2 days before adoptive transfer and continued for 4 weeks, and the mice were evaluated for 8 weeks. Five PV model mice were treated along with five untreated controls. We used DEX (Decadron®; Banyu Pharmaceutical, Tokyo, Japan), m-PSL (Solu-Medrol®; Pfizer, Tokyo, Japan), AZA (Imuran®; GlaxoSmithKline, Uxbridge, Middlesex, UK), CPA (Endoxan®; Shionogi, Tokyo, Japan), CsA (Sandimmun®; Novartis Pharma, Basel, Switzerland) and FK506 (Prograf®; Fujisawa Pharmaceutical, Tokyo, Japan). DEX was given intraperitoneally at a dose of 10 mg/kg daily and m-PSL was given intraperitoneally at a dose of 100 mg/kg daily. AZA was given orally at a dose of 15 mg/kg 3 days per week. CPA was given intraperitoneally at a dose of 40 mg/kg 3 days per week. CsA was given intraperitoneally at a dose of 40 mg/kg daily and FK506 was given intraperitoneally at a dose of 5 mg/kg daily (Table 1).
| Drugs | Doses (mg/kg) | Frequency | Duration (days) | Route | Efficacy* | ||
|---|---|---|---|---|---|---|---|
| ELISA (index) | Body weight loss (g) | PV score (points) | |||||
| CPA | 40 | Three times a week | 35 | Intraperitoneal | <0.001 (day 14) | <0.001 (day 14) | <0.001 (day 14) |
| AZA | 15 | Three times a week | 28 | Oral | 0.007 (day 11) | NS | 0.014 (day 14) |
| CsA | 40 | Daily | 28 | Intraperitoneal | 0.011 (day 11) | 0.018 (day 14) | 0.008 (day 14) |
| FK506 | 5 | Daily | 28 | Intraperitoneal | 0.030 (day 14) | NS | NS |
| m-PSL | 100 | Daily | 28 | Intraperitoneal | 0.030 (day 14) | NS | 0.037 (day 28) |
| DEX | 10 | Daily | 28 | Intraperitoneal | NS | NS | NS |
- AZA, azathioprine; CPA, cyclophosphamide; CsA, cyclosporin A; PV, pemphigus vulgaris; FK506, tacrolimus hydrate; ELISA, enzyme-linked immunosorbent assay; m-PSL, methylprednisolone; DEX, dexamethasone; NS, not significant.
- *Statistically significant compared with control mice without drugs.
- P-values were determined by Mann–Whitney U-test.
- P-values are listed when there was significant improvement in each category.
As a route of administration of these agents, we mostly used intraperitoneal injection because it is technically difficult to apply the agents orally to model mice that have mucosal erosion from mouth to oesophagus in the first place. Insertion of stomach sonde may induce some trauma, which excerbate the disease activity. It is also technically difficult to apply these agents intravenously to mice ‘daily’ because of scaling in the tail or induction of erosion on ocular mucous membranes.
ELISA
Circulating anti-Dsg3 IgG was measured by ELISA using recombinant mDsg3 as a coated antigen as described previously (7). Each sample was diluted 5000-fold and 10000-fold in duplicate. Standard serum obtained from a PV model mouse was used as a positive control, and serum from C57BL/6 Rag2−/− before adoptive transfer was used as a negative control. ELISA scores were obtained as index values with the following rules: index values = 100 × (OD450 of the sample − OD450 of the negative control)/(OD450 of the positive control − OD450 of the negative control), where OD is the optical density. When the OD score exceeded 1.5, the serum sample was further diluted and the index value was multiplied by the dilution factor. When PV phenotype becomes more severe, mice die because of it. To avoid apparent improvement by ignorance of scores of dead mice, we used the ELISA index values at the time of death as virtual scores until the observation period was completed.
PV score
To evaluate the disease activity of model mice, we considered the PV score, which was calculated by expressing the phenotype numerically as described previously (9). We also counted a score of 1 for erosion, 1 or 0.5 for hair loss and 1 for elongation of the lower fore teeth. The maximum total score was 11. When mice died, the PV scores at the time of death were used as virtual scores thereafter.
Result
Intertest variation of the production of anti-Dsg3 IgG and development of the PV phenotype in PV model mice
We began administering various immunosuppressive agents before adoptive transfer of immunized Dsg3−/− splenocytes. To evaluate the effects of the immunosuppressive agents, we used three measurements: ELISA scores to reflect the level of circulating anti-Dsg3 IgG; body weight loss, which is caused by inhibition of food intake and reflects the extent of oral erosions; and PV score, which mainly reflects the extent of the skin lesions (9). All of these measurements were obtained without sacrificing the mice, and therefore the time courses could be examined and compared.
We first examined the inter test variation of the PV model mice with these three measurements. All the recipient mice that received immunized Dsg3−/− splenocytes produced anti-Dsg3 IgG and developed the PV phenotype (incidence: 100%, n = 35). We compared the time courses of Dsg3 ELISA score, body weight loss and PV score among seven experiments (n = 5 for each experiment), which were performed at different times without any immunosuppressive agent (day 0 as the day for adoptive transfer of Dsg3−/− splenocytes) (Fig. 1). The production of anti-Dsg3 IgG was detected on day 7, reached a plateau on day 14, and was maintained throughout the time course with slightly lower levels than the peak production on day 14 (Fig. 1a). The peak level of anti-Dsg3 IgG production showed some variations among experiments, but there were no significant variations among mice in the same experiment group. The weight loss became apparent on day 7, showed a progressive phase between days 7 and 14, and stabilized after day 14 (Fig. 1b). The PV score started to rise on day 7 to 10, increased continuously until about day 28, and reached a plateau (Fig. 1c), although the plateau PV scores showed some variations as found in the circulation levels of anti-Dsg3 IgG and body weight loss.
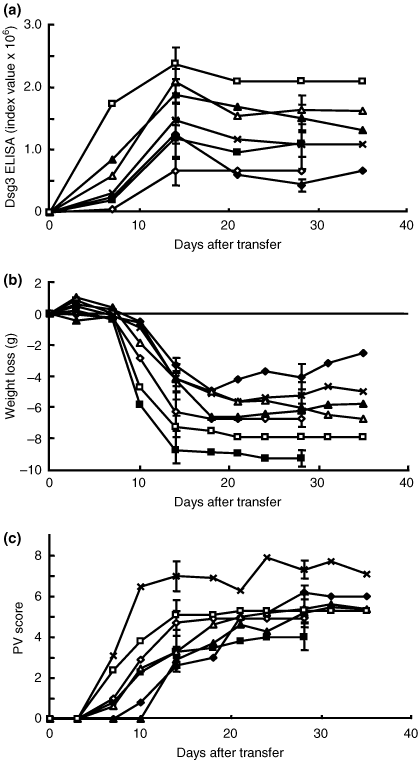
Intertest variations of pemphigus vulgaris (PV) model mice. The desmoglein 3 enzyme-linked immunosorbent assay score (a), body weight loss (b) and PV score (c) are plotted along the time course of the experiment. Each experiment consisted of five PV model mice observed for 35 days except for two experiments in which animals were observed for 28 days (open rhombi, closed squares). Although there were some variations in the degree of each measurement, the patterns of the time courses were similar among the experiments.
These findings indicate that the generation of PV model mice was quite reproducible and that the mice showed similar time courses of anti-Dsg3 IgG production as well as the PV phenotype, although some variations were detected in the level of anti-Dsg3 IgG production and severity of the disease phenotype among different experiments. Thus, it is quite feasible to use PV model mice to evaluate the efficacy of various immunosuppressive agents. However, it is important to prepare a control group without immunosuppressive agents for each experiment to compensate for the intertest variations of the peak or plateau levels of the three measurements.
Evaluation of CPA
To examine the effects of CPA with the PV mouse model, CPA (40 mg/kg) or phosphate-buffered saline (PBS) as a control was injected intraperitoneally into Rag2−/− recipient mice (n = 5) 3 days per week beginning 2 days before adoptive transfer with immunized Dsg3−/− splenocytes. Administration of CPA was continued for 5 weeks and discontinued afterwards. The dose was chosen based on previous mouse studies as well as pulse treatment in humans (11,12). The mice were evaluated based on anti-Dsg3 IgG production, body weight loss and PV scores for 119 days.
All of the mice injected with PBS showed anti-Dsg3 IgG production that reached a sharp peak on day 14 and became persistent thereafter at about half the peak level until day 119 (Fig. 2a). All the control mice developed body weight loss, which became apparent on day 10, followed by a progressive phase until day 17, and remained stable for as long as day 119 (Fig. 2b). The PV score became positive on day 10 and increased rapidly until day 28, followed by a stable plateau phase (Fig. 2c). A representation of the gross phenotype of these mice is shown Fig. 2c.
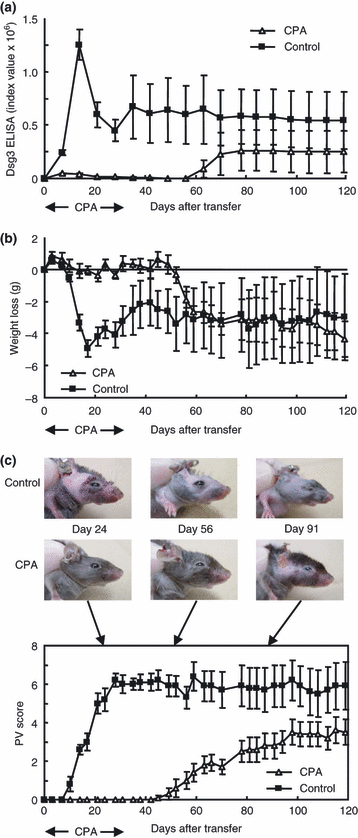
Evaluation of cyclophosphamide (CPA) in pemphigus vulgaris (PV) model mice. The time courses of changes in the desmoglein 3 (Dsg3) enzyme-linked immunosorbent assay score (a), body weight loss (b) and PV score (c) are shown (CPA, open triangles, n = 5; control phosphate-buffered saline, solid squares, n = 5). The representative gross phenotype is also shown (c). CPA was administered 3 days per week from 2 days before adoptive transfer to 35 as indicated by arrows. CPA inhibited the production of anti-Dsg3 immunoglobulin G (IgG) and the development of PV phenotype during the period of CPA administration. After CPA was discontinued, the mice began to produce anti-Dsg3 IgG with the appearance of the PV phenotype (c).
In contrast, injection with CPA suppressed anti-Dsg3 IgG production almost completely as long as CPA was given until day 35 (Fig. 2a). The mice that were given CPA did not show apparent body weight loss (Fig. 2b), and the PV score was flat without any apparent hair loss or erosion during these periods (Fig. 2c). However, once CPA was discontinued, these mice began to produce anti-Dsg3 IgG on day 56 (about 3 weeks after discontinuation of CPA), although the level was constantly lower than those of mice receiving PBS (Fig. 2a). The body weight loss became apparent slightly earlier on day 52 and showed a rapid drop between days 52 and 56 (Fig. 2b). The PV score turned positive on day 45 and increased gradually thereafter. Although the antibody level was significantly lower than that in the control mice (P < 0.001), the body weight loss and PV scores at plateau levels were almost the same as in the control mice.
These findings indicate that the PV model mice responded well to CPA, which has been used as an effective immunosuppressive agent for the treatment of pemphigus, and that the phenotype of the PV model mice was reversible once the treatment was discontinued with the dose administered in this experiment.
Evaluation of AZA
To examine the effects of AZA, 15 mg/kg of AZA or control sodium carboxymethyl cellulose solution was given orally to Rag2−/− recipient mice three times per week starting from 2 days before adoptive transfer (n = 5). The dose was chosen based on the findings of a previous study in mice (13) and a preliminary study in which administration at 30 mg/kg/day every day caused severe anaemia after 3 weeks of treatment (data not shown). The plateau level of anti-Dsg3 IgG production was significantly suppressed in mice that were given AZA compared with the controls (Fig. 3a; P = 0.007, day 11). The PV score was also significantly suppressed (Fig. 3c; P = 0.014, day 11; P = 0.030, day 28). However, the weight loss was not significantly different between the two groups throughout the time course (Fig. 3b).
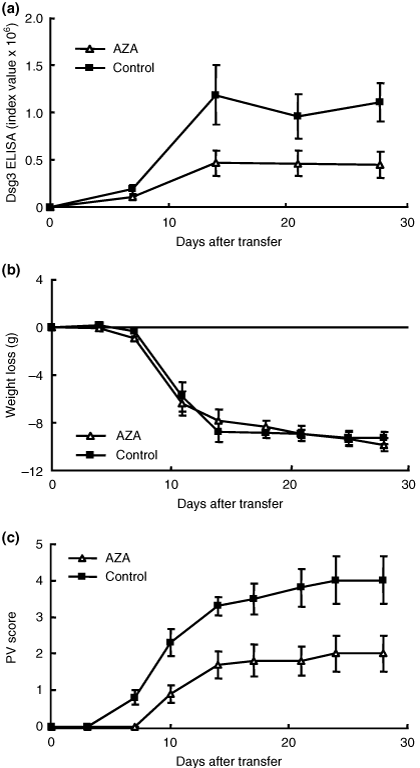
Evaluation of azathioprine (AZA) in pemphigus vulgaris (PV) model mice. The time course of changes in the desmoglein 3 (Dsg3) enzyme-linked immunosorbent assay score (a), body weight loss (b) and PV score (c) are shown (AZA, open triangles, n = 5; control, sodium carboxymethyl cellulose solution; solid squares, n = 5). AZA was administered 3 days per week from 2 days before adoptive transfer to 28. AZA significantly suppressed the production of anti-Dsg3 immunoglobulin G and development of PV phenotype (a; P = 0.007, day 11) (c; P = 0.014, day 11), but there were no significant differences in body weight loss throughout the experimental period (b).
Evaluation of CsA
To examine the effects of CsA, 40 mg/kg of CsA or control PBS was given intraperitoneally to Rag2−/− recipient mice every day starting from 2 days before adoptive transfer (n = 5). This dose was chosen based on the results of a previous study with MRL/Mp-lpr/lpr mice (14). In this experiment, all the control mice died by day 18 because of the severity of the phenotype. On day 14, the level of anti-Dsg3 IgG production was significantly lower in mice that were given CsA than in the controls (Fig. 4a; P = 0.011, day 11), and the body weight as well as PV score were also significantly lower in treated mice than in controls (Fig. 4b; P = 0.018, day 14) (Fig. 4c; P = 0.008, day 14).
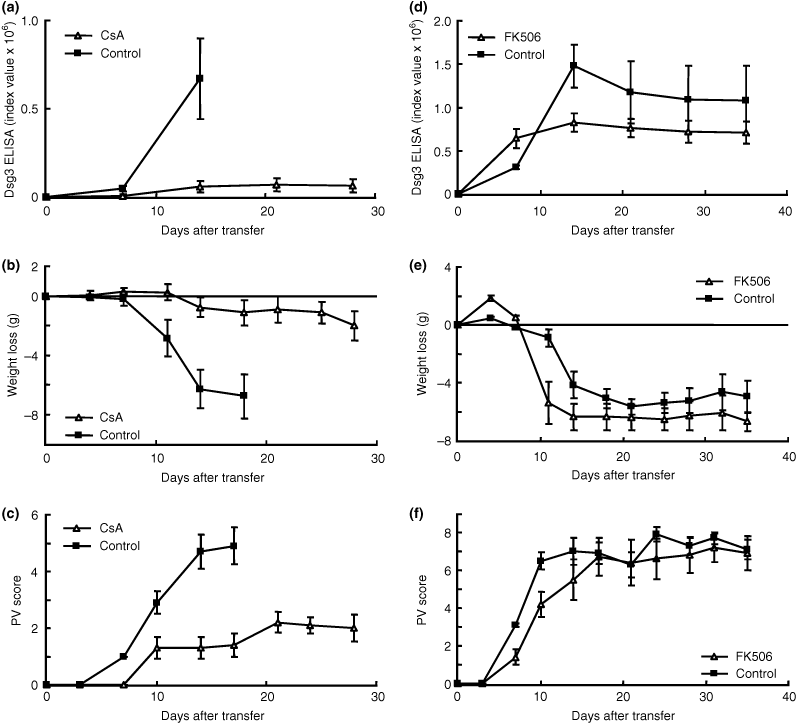
Evaluation of cyclosporin A (CsA) and tacrolimus hydrate (FK506) in pemphigus vulgaris (PV) model mice. The time courses of changes in the desmoglein 3 (Dsg3) enzyme-linked immunosorbent assay (ELISA) score (a), body weight loss (b) and PV score (c) are shown [CsA, open triangles, n = 5; control phosphate-buffered saline (PBS), solid squares, n = 5]. CsA was administered daily from 2 days before adoptive transfer to 28. CsA significantly suppressed the production of anti-Dsg3 Immunoglobulin G (IgG) and development of the PV phenotype (a; P = 0.011, day 11) (b; P = 0.018, day14) (c; P = 0.008, day 14). The time courses of changes in the Dsg3 ELISA score (d), body weight loss (e) and PV score (f) are shown (FK506, open triangles, n = 5; control PBS, solid squares, n = 5). FK506 was administered daily from 2 days before adoptive transfer to 28. FK506 significantly suppressed the production of anti-Dsg3 IgG (d; P = 0.030, day 14), but there were no significant differences in body weight loss or development of the PV phenotype along the time course of the experiment (e, f).
Evaluation of FK506
To examine the effects of FK506, FK506 (5 mg/kg) or control PBS was injected intraperitoneally into Rag2−/− recipients every day starting from 2 days before adoptive transfer (n = 5). This dose was chosen based on a previous study on experimental allergic encephalomyelitis (15). The level of anti-Dsg3 IgG production was significantly lower in mice that were given FK506 than in controls (Fig. 4d; P = 0.030, day14). However, FK506 did not show any significant suppressive effect on body weight loss or PV phenotype development at any time point during the experimental period (Fig. 4e, f).
Effects of m-PSL and DEX
The effects of two glucocorticoids m-PSL and DEX, which are used as standard treatment for pemphigus, were examined. In one experiment, m-PSL (100 mg/kg) or PBS as a control was injected intraperitoneally into Rag2−/− mice every day from 2 days before adoptive transfer of splenocytes from immunized Dsg3−/− mice (n = 5). In another experiment, DEX (10 mg/kg) or PBS as a control was injected intraperitoneally in the same way. These doses were chosen because our preliminary studies based on previous reports with 0.4 mg/kg/day of DEX and 5 mg/kg/day of PSL (16–18) did not show apparent effects in our model (data not shown). The level of anti-Dsg3 IgG production and PV score were significantly lower in mice that were given m-PSL than in controls throughout the time course of the experiment (Fig. 5a; P = 0.030, day 14) (Fig. 5c; P = 0.037, day 28). However, the body weight loss was not significantly different between the two groups (Fig. 5b). The level of anti-Dsg3 IgG production was lower in mice that were given DEX than in controls throughout the time course of the experiment, although the difference was not statistically significant (Fig. 5d; P = 0.072, day 21). DEX did not show any significant suppressive effect on body weight loss or PV scores throughout the experimental period (Fig. 5e; P = 0.15, day 21) (Fig. 5f; P = 0.48, day 21). These findings indicated that glucocorticoids failed to show dramatic immunosuppressive effects in PV model mice, as seen in patients, and that the PV model mice are not ideal to evaluate the effects of glucocorticoids.
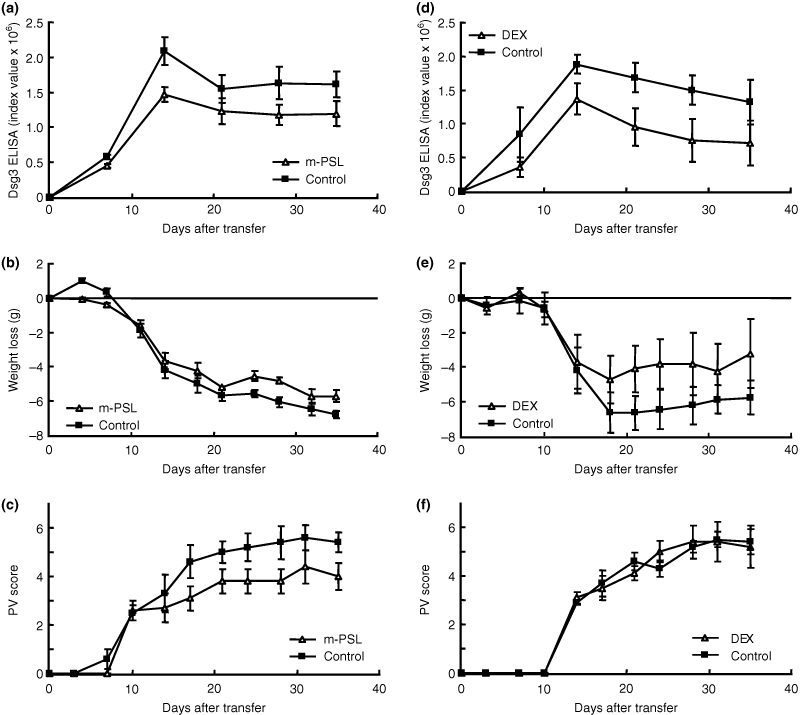
Effects of methylprednisolone (m-PSL) and dexamethasone (DEX) in pemphigus vulgaris (PV) model mice. The time courses of changes in the desmoglein 3 (Dsg3) enzyme-linked immunosorbent assay (ELISA) score (a), body weight loss (b) and PV score (c) are shown [m-PSL, open triangles, n = 5; control, phosphate-buffered saline (PBS), solid squares, n = 5]. M-PSL was administered daily from 2 days before adoptive transfer to 28. M-PSL significantly suppressed the production of anti-Dsg3 immunoglobulin G (IgG) and development of the PV phenotype (a; P = 0.030, day 14) (c; P = 0.037, day 28), but there were no significant differences in body weight loss along the time course of the experiment (b). The time courses of changes in the Dsg3 ELISA score (d), body weight loss (e) and PV score (f) are shown (DEX, open triangles, n = 5; control PBS, solid squares, n = 5). DEX was administered daily from 2 days before adoptive transfer to 28. DEX did not significantly suppress the production of anti-Dsg3 IgG, body weight loss or development of the PV phenotype (d,e,f).
Discussion
In this study, we used a PV mouse model to evaluate various immunosuppressive agents in current use in our daily practice to treat patients with PV. The main purpose of this study was to investigate the benefits as well as the limitations of this PV mouse model as a tool to evaluate immunosuppressive therapeutic strategies, including those that may be developed in the future. We administered the drugs before the development of PV phenotype. Therefore, this evaluation was based on preventive rather than treatment effects.
We used three measurements to evaluate the effects of the drugs: Dsg3 ELISA scores that represent the level of anti-Dsg3 IgG production, body weight loss that reflects the severity of oral erosions and PV score that mainly reflects the extent of skin lesions. These measurements were obtained without sacrificing the mice, and therefore the time course of each measurement could be studied. The majority of mice followed a similar time course in the measurements, even among the experiments performed at different time-points (Fig. 1). The peak levels of anti-Dsg3 IgG production, weight loss and PV score did not show significant variation among mice in the same experiments. However, these peak levels were different among different experiments, although the immunization schedule and number of splenocytes transferred were the same. The precise reasons for this are not known, although it is possible that the mixed 129/SV (H-2b) and C57BL/6J (H-2b) genetic background of the Dsg3−/− mice used in this study may have yielded slight variations in their immune reactions. Therefore, it is important to include a control PV mouse without any therapeutic agents in parallel for each experiment as was performed in this study.
Several mouse models for autoimmune diseases have been used to evaluate immunosuppressive therapies, including NZB/W F1 mice and MRL/Mp-lpr/lpr mice for systemic lupus erythematosus, experimental autoimmune encephalomyelitis, multiple sclerosis, etc. (19–22). There are several advantages to using PV model mice over the mouse models developed previously. In PV model mice, the pathogenic role of anti-Dsg3 IgG is quite established in the pathogenesis of pemphigus, while anti-nuclear antibodies are merely a marker of the disease. Anti-Dsg3 IgG interferes with the adhesive function of Dsg3 in mucous membranes and the skin, and leads to a loss of cell-cell adhesion with resultant blister formation (23–25). Therefore, the titres of circulating anti-Dsg3 IgG are strongly correlated with the disease activity, as observed in patients with PV (2). Furthermore, the phenotype can be easily evaluated and scored without sacrificing the mice because it appears on the skin. The body weight can also be measured easily.
We were surprised that glucocorticoid did not show dramatic effects in suppressing the development of the PV phenotype. We began to use a lower dose of steroid (1 mg/kg/day, data not shown) without any apparent effects, and therefore we increased the doses and tested 100 mg/kg of m-PSL or 10 mg/kg of DEX, which were 100 times the standard doses used in humans. Although m-PSL and DEX suppressed the anti-Dsg3 IgG production to a certain extent, DEX failed to yield significant differences in weight loss or PV score. These findings suggest that the PV mouse model is not ideal for evaluating the effects of systemic steroid therapy, in contrast to our experiences in humans. It was shown previously that mice have a much higher clearance rate for drugs than humans (26). Steroid metabolism and steroid-induced signals related to antibody production may be different between mice and humans. On the other hand, although the therapeutic effects of m-PSL were reported in passive transfer model with neonatal mice through up-regulation and changes in post-translational modification of keratinocyte adhesion molecules (27), those effects were not potent enough to block the blister formation in our studies. The precise reasons for the unresponsiveness of the PV model mice to the steroid remain to be elucidated.
In the PV mouse model, splenocytes from Dsg3−/− mice are adoptively transferred to Rag2−/− mice which have lymphopenic condition because of lack of mature B and T cells (7). Therefore, homeostatic proliferation of the transferred lymphocytes are expected to occur in the early phase of anti-Dsg3 IgG production when various immunosuppressive agents were given and this condition is only observed in the PV mice, but not in patients with PV (28–30). Although it is not clear how homeostatic proliferation affected the outcome of immunosuppressive agents in our study, this point should be considered when we draw any conclusion on the evaluation of the efficacy of the tested agents in the PV mouse model. Further studies are necessary to elucidate the exact impact of homeostatic proliferation in the PV mouse model.
In this study, we evaluated the effects of four other immunosuppressive agents that are used as steroid-sparing agents, together with glucocorticoid. However, we did not test the combined effects of these immunosuppressive agents and glucocorticoid because, as discussed above, the steroid did not show significant effects. CPA is a derivative of nitrogen mustard and acts primarily as a DNA cross-linker. AZA is an antimetabolite agent and acts to inhibit purine synthesis. CsA is a peptide produced by fungus and binds to cyclophilin, and this complex inhibits calcineurin to suppress T cell function. FK506 is a 23-membered macrolide lactone and binds to immunophilin creating a complex, which specifically inhibits T cell function.
There have been few reports of monotherapy in PV patients. CPA is used as intravenous pulse monotherapy (31,32). AZA monotherapy has also been reported (33). CsA is a useful adjuvant therapy with steroid-sparing effects in PV, but CsA monotherapy has not been reported (34,35). FK506 was reported with regard to topical use only. A case of PV was reported in which a recalcitrant area of erosion on the cheek recovered only when topical tacrolimus was used in addition to systemic therapy (36). In this study with PV model mice, CPA demonstrated the strongest effects as monotherapy among the drugs tested in the given doses, which was consistent with the clinical findings in patients to the effect that CPA pulse therapy is one of the most potent immunosuppressive treatments for PV.
We are aware of the obvious limitations of the PV mouse as a model of PV as discussed above, but feel strongly that when used appropriately it will continue to be a crucial tool for improving our understanding and treatment of this devastating disease. Knowing the advantages and limitations of this model will provide an important foundation for the future evaluation and development of novel therapeutic strategies.
Acknowledgements
We thank Ms Yoshiko Fujii for the preparation of recombinant proteins and Ms Hiromi Itoh for excellent animal care. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Health and Labour Sciences Research Grants for Research on Measures for Intractable Diseases; the Ministry of Health, Labor and Welfare of Japan and Keio Gijuku Academic Development Funds.




