Bone health in persons with haemophilia: a review
Abstract
Bone density is a measure of equilibrium between bone formation and bone resorption; any imbalance in these two processes might lead to osteopenia and osteoporosis. Osteoporosis in general has been considered to be an important cause of morbidity in both men and women and more so in haemophilia and other bleeding disorders. Specific triggering factors in persons with haemophilia (PWH) are abnormal liver function because of viral infection, bleeding and inflammation, lack of physical and athletic activities, low body weight and others. Although the pathogenesis of osteoporosis is not clear, it has been considered as a severe comorbidity in PWH in both developing and developed countries. This is more evident in developing countries where there is no free access to factor concentrates, and primary prophylaxis is beyond the reach of vast majority of the patients. Only few references are available in the literature on the prevalence and management of osteoporosis in haemophilia population. This review summarises the prevalence, plausible mechanisms and management options of this important morbidity in PWH.
Till recently, the major emphasis in the management of persons with haemophilia (PWH) in developed and developing countries has been to minimise or stop spontaneous bleeding in severe haemophilia patients and to manage various complications that arise because of recurrent bleeding in joints, muscles and in many other organs. With the availability of free factor concentrates and improvement in haemophilia care, there is dramatic improvement in the life expectancy and quality of life of PWH. However, a large number of PWH are at an increased risk of fracture of bones including long bones following trivial injury 1-3 suggesting that bone health in PWH may be poor. Bone health of these patients indeed has become a major concern for the world haemophilia community.
PWH in different parts of the world are exposed to varied health care, nutritional support and physical activities, which also has an impact on the pathogenesis of bone disease 4, 5. As such, in different parts of the world, studies unravel different sets of risk factors causing bone disease in PWH 6. The knowledge of prevalence of osteoporosis and understanding its pathophysiology is important to undertake suitable measures to prevent this morbidity.
Materials and methods
All relevant articles published till June 2011 were searched in MEDLINE, EMBASE, Cochrane using combinations of following key terms – haemophilia, factor VIII deficiency, factor IX deficiency, congenital coagulation disorders, osteopenia, osteoporosis, fracture, bone mineral density (BMD) with no language restriction. Articles were carefully studied independently by both authors and the references to be included were mutually agreed upon. A total of 211 relevant papers could be extracted. For the present review, emphasis was given to (i) unusual case reports, which points to potential pathogenesis of the condition (ii) case series where the parameters of bone health were actively investigated by different imaging techniques and biochemical parameters (iii) case series where fractures were described in patients with haemophilia and comments have been made on the healing process and intraoperative assessment of bone health (iv) case series where bone health has been correlated with chronic liver disease, chronic arthritis, viral hepatitis and vitamin D metabolism. Reviews and meta-analysis of bone health in haemophilia were also looked into, and only one meta-analysis 7 could be found, which also is included in the present review.
Fractures in haemophilia
Several case reports on fractures in PWH and various management strategies have been published 1, 8-10. Although the actual incidence of fractures in PWH has not been reported, the general opinion is that there is an increased prevalence of fracture in PWH. The factors predisposing to fracture are arthropathy, reduced BMD and poor musculature 2. These fractures heal well, although subperiosteal callous formation is poor. Fractures have also been reported in patients with moderate haemophilia, who generally do not present with spontaneous bleeding 8. However, it was not possible to understand from the literature, whether the fracture is more common in severe haemophilia A than severe haemophilia B or it is equally distributed between the two disorders. The pooled data show that fracture is 10–11 times more prevalent in patients with haemophilia A than in haemophilia B. Moreover, fractures have not been reported in severe Von Willebrand disease, where there is severe deficiency of factor VIII because of deficient Von Willebrand factor (VWF).
Osteoporosis and osteopenia in persons with haemophilia
Several studies have published increased prevalence of decreased BMD amongst PWH 6, 11-19. The prevalence of osteopenia and osteoporosis has been variable in these studies. However, low bone mass (osteopenia and osteoporosis combined) was found in 22–84% of all these patients. In general, the prevalence of osteoporosis has been found to be low in paediatric population 11. Gerstner et al. showed positive correlation between increase in bone loss in PWH with low 25-hydroxyvitamin D level, low body mass index (BMI), lower active score, decreased range of joint motion, history of inhibitor development and increased age of the patient 6. Almost in all the studies, BMD was studied in L1-L4 vertebrae (axial bone loss) and in the neck of the femurs (appendicular bone loss). The technology used to study BMD was DEXA in all the studies. Nair et al. 12 studied BMD using DEXA scan in 50 consecutive patients with severe haemophilia between 20 and 50 yr of age. These 50 patients consisted of both severe haemophilia A (42 patients) and haemophilia B (eight patients); 50% of these patients showed osteoporosis at lumbar spine, while in 38% the osteoporosis was in intertrochanteric area and in 32% osteoporosis affected the hip or head of the femur. 19/50 patients had hepatitis C and degree of osteoporosis was not different between hepatitis C affected and unaffected PWH. BMI of all PWH, studied by Nair et al. was much lower compared to age-matched normal controls (i.e. 19.16 ± 3.8 kg/m2 23.62 ± 2.79 kg/m2), although absolute BMD was significantly lower in PWH compared to age-matched control population (0.82 ± 0.14 g/cm2 vs. 0.93 ± 0.11 g/cm2). Barnes et al. 13 compared BMI-matched PWH and normal healthy controls and showed significantly reduced BMD in PWH. Mansouritorghabeh et al. 14 showed that BMD is significantly reduced even in cases of severe haemophilia B and the effect on BMD is not dependent on the severity of haemophilia 13, 14. PWH with low BMD have reduced bone formation activity, while no difference was found with regard to bone resorption markers 15. Other studies have shown that patients with haemophilia have short height, low body weight and low activity levels, which along with other comorbidities associated with haemophilia lead to lower bone mass 16. Abdelrazak et al. 17 showed that the reduction in BMD is not dependent on age and body size. Naderi et al. 18 have reported that bleeding into the joints is more pronounced in osteoporotic patients as compared to osteopenic or normal healthy controls. Wallny et al. 19 have reported osteopenia and osteoporosis in 69% of patients with severe haemophilia; 55 out of 62 patients also had haemophilic arthropathy.
Lorio et al. 7 in a recent meta-analysis involving all the significant studies in this area till 2010 showed significantly lower BMD in both adult (101 cases with 101 controls) and in paediatric cases of PWH (111 paediatric PWH with 307 controls). Lumbar BMD was more severely affected. Tlacuilo-Parra et al. 15 showed low BMD in paediatric PWH opined that inactivity is one of the important risk factor. Gallacher et al. 20 reported a reduction in BMD in patients with severe haemophilia but reported no difference in the markers of bone resorption between patients with haemophilia and controls. The prevalence of osteoporosis/osteopenia in some of the studies published in the literature is shown in Table 1.
| Reference | Number of patients | Age (yr) Median (range) | Number of age-matched controls | Type/Severity | BMD lumbar spine (L2-L4) | Other findings | HIV/HBsAg/HCV status |
|---|---|---|---|---|---|---|---|
| Christoforidis et al. 11 | 26a | 12.08 (4.94–18) | 13 | HA/severe 17, moderate 9 | 6 had reduced BMD | Osteoprotegerin decreased, sRANK-L and OC increased in patients |
HCV-1 HIV-nil |
| Gerstner et al. 6 | 30 | 41.5 (18–61) | Nil | HA-22 severe, 3 moderate and HB- 4 severe, 1 moderate | Reduced BMD in 21 patients; 8 had osteoporosis and 13 osteopenia | 25-hydroxyvitamin D levels were significantly lower in patients with osteoporosis |
HCV-25 HIV-11 |
| Nair et al. 12 | 50 | 29.53 (20–50) | 50 | 42 HA, 8 HB/all severe | Reduced BMD in 50% of the patients | Nil | HCV-19 |
| Gallacher et al. 20 | 19 | 18–69 | 19 | HA/severe | Mean BMD in patients was 1.109 ± 0.042 g/cm2 vs. 1.234 ± 0.027 g/cm2 in controls | Femoral neck density was also lower at 0.877 ± 0.034 g/cm2 vs. 1.067 ± 0.032 g/cm2; P < 0.0005 | HCV-18 |
| Barnes et al. 13 | 19a | 12.2 (5.73–18.5) | 215 | HA/severe | Mean BMD in patients was 0.102 g/cm3 vs. 0.113 g/cm3 in controls | No difference in markers of bone resorption | HCV-8 |
| Tlacuilo-Parra et al. 15 | 62a | 10.02 6-10, 2, 11-15 | 62 | HA/21 mild, 24 moderate and 24 severe | 24 had low BMD vs. 10/62 in controls | Reduced osteoblastic bone formation activity; no differences with regard to bone resorption markers | Nil |
| Abdelrazik et al. 17 | 30a | 4.97 ± 3.64 | 30 | HA/severe | BMD 0.48 ± 0.13 g/m2 for patients with haemophilia vs. 0.55 ± 0.14 g/m2 for controls | BMD Z-score: 0.68 ± 0.44 for patients with haemophilic vs. 0.19 ± 0.14 for controls | None |
| Mansouritorghabeh et al. 14 | 14 | 30.57 (19–55) | 14 | HB/severe | 5 had osteoporosis | ALP and SGPT increased in patients vs. controls | HCV-11 |
| Naderi et al. 2012 18 | 40 | 27.73 (20–56) | Nil | 30 HA;10HB | Not carried out | 3 had osteoporosis; 20 had osteopenia based on femur densitometry | HCV-11 |
| Wallny et al. 2007 19 | 62 | 41.4 (range, 20–66) | Nil | Severe HA | 27 had osteopenia; 16 had osteoporosis | – |
HCV-48 HIV-25 |
- BMD, bone mineral density; OC, osteocalcin; ALP, alkaline phosphatase.
- a Paediatric patients.
Bone health in haemophiliacs who are on primary prophylaxis
It has often been argued, but not conclusively proved that all complications of osteoporosis and osteopenia in haemophilia can be simply explained by repeated bleeding in the joints. If this was true, then the frequency of osteoporosis/osteopenia should not have been more in patients with haemophilia who are on primary prophylaxis with minimal or no joint bleeds. Khawaji et al. 21 showed that long-term prophylaxis in severe haemophilia indeed tends to preserve BMD. However, others 22 could not duplicate the data and it is felt that even patients on prophylaxis over long periods get microscopic bleeding into joints as shown by MRI scan. Very few studies are available on BMD in PWH who develop inhibitors 23. As the development of inhibitor is associated with more extensive haemorrhage and subsequent inflammation of the joints, severe osteoporosis in this condition is expected.
Osteoporosis and body mass index
Several studies have shown PWH have lower BMI than age-matched controls 6, 12. The meta-analysis by Lorio et al. 7 showed that BMD in both adult and paediatric PWH is significantly lower. Although a significant reduction in BMI was found in PWH as compared to age-matched controls, the decrease in BMD did not correlate with reduced BMI nor with HCV infection. Osteoporosis with low BMI in related disorders has also been reported 25, 26.
Pathophysiology of osteoporosis in haemophilia
The pathophysiology of the development of osteoporosis in PWH is not exactly known. The study of osteoporosis in PWH is only a recent development, and few studies that are reported involve PWH who differ by age, extensiveness of bleeding, nutritional supplement, infection and physical activities. The earliest study by Gallacher et al. 21 on the association of osteoporosis with severe haemophilia A consisted of PWH mainly with HCV infection and those who were not on primary prophylaxis. They showed reduced levels of 25-hydroxyvitamin D in these patients and reported HCV to be a significant cause of osteoporosis in PWH. Significant osteoporosis in hepatitis B- or C-infected patients without cirrhosis has also been well documented 27. In developing countries like India, a large number of PWH with severe disease are still being treated with fresh frozen plasma (FFP) and cryoprecipitate because of which the prevalence of viral infections has not reduced drastically as compared to their western counterparts. Both HIV infection and antiretroviral therapy are known to be causing osteoporosis 28, 29. Treatment with interferon-α is also known to cause osteopenia and osteoporosis 30.
In patients with chronic liver disease or cirrhosis, low BMD because of vitamin D deficiency, hypogonadism and reduced insulin like growth factor 1(IGF-1) has also been reported 31. The role of vitamin K in maintaining BMD 32 and reports of osteoporosis in patients who are on vitamin K antagonists 33 also point to the role of vitamin K in maintaining bone density. Some of the vitamin K-dependent proteins like osteocalcin are also one of the constituents of bone.
Chronic viral infection has also been shown to deregulate RANK ligand (RANKL) pathway, which in turn results in osteopenia/osteoporosis 34, 35. Osteoporosis has been associated with several haematological disorders associated with either internal bleeding or blood loss. The aetiology of osteoporosis in haematological disorders as a consequence of chronic haemorrhage and blood loss has been discussed in the back ground of an interesting hypothesis that the excessive demand for blood cell production in the haematopoietic system because of repeated bleeding and blood loss plays a significant role in the pathogenesis of osteopenia 36. This is evident in children who remain active and achieve peak bone density with vitamin D/Ca++ supplementation are less prone to develop osteoporosis later in life. In addition, HCV, HBV and HIV infection in some of these patients play an additive role.
Ranta et al. 22 in a case–control study of children with severe haemophilia analysed whole body spinal and axial skeleton bone density, controlling many of the variables described above and several other parameters of calcium metabolism including urinary calcium adjusted to urinary creatinine. PWH showed significantly increased calcium levels in the presence of normal serum vitamin D and PTH levels. Markers of bone catabolism were also within normal limits. These patients were haemophilic children mostly on primary prophylaxis and were exposed to adequate activity, calcium and vitamin D. Hence, haemophilia in some way was associated with hypercalcinuria without excessive destruction of bone. Idiopathic hypercalcinuria in unrelated situations have been associated with osteopenia in 1/3 of the children studied 37.
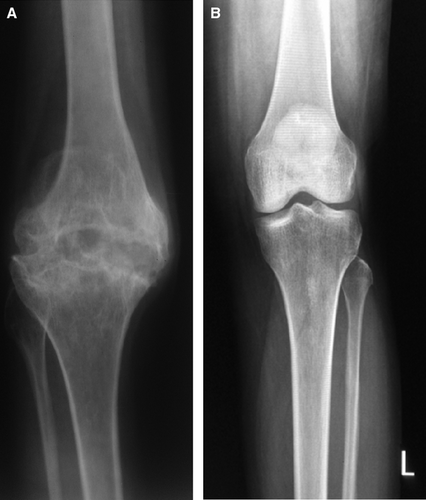
One of the most interesting findings in a recent study showed the effect of factor VIII and VWF on osteoclastogenesis. It was shown that the factor VIII–VWF complex can be physically associated with osteoprotegerin (OPG). OPG is an anti-osteoclastic protein and a soluble receptor for the proapoptotic protein TRAIL (tumour necrosis factor-related apoptosis-inducing ligand). Together with OPG factor, VIII–VWF inhibited RANKL-induced osteoclastogenesis 38. If this biological effect is significant, then a pathobiological role of factor VIII deficiency in the causation of osteoporosis even in the absence of severe haemorrhage or inflammation (synovitis) may be found. However, this mechanism neither explains hypercalcinuria without bone destruction in haemophilia nor does it explain the increased prevalence of osteoporosis even in patients with haemophilia B 14. Figure 1 shows radiological view of the knee joints showing arthropathy in a patient with haemophilia with highly reduced BMD and that of a normal individual with normal BMD.
Management of osteopenia in severe haemophilia
Table 2 shows drugs commonly used for the management of patients with osteoporosis. Presently, management of osteoporosis in PWH is predominantly guided by the management strategy of postmenopausal osteoporosis, although it may not be totally applicable to PWH. Even though the management of haemophilia has been improving across the world with the practice of primary prophylaxis, osteoporosis/osteopenia could still be a challenge for Physicians and Haematologists. Primarily, this can be improved by adequate exercise with an aim to develop maximum peak BMD in early adolescence. Studies have shown that vitamin D deficiency is pervasive not only in developing countries but even in developed countries 39-41. Adequate vitamin D prophylaxis with a target serum 25 hydroxy vitamin D levels above 30 nm can improve the bone health in PWH. Oral vitamin D can be combined with 1–1.5 g oral calcium. Role of vitamin K in the pathogenesis of osteoporosis in patients who are on oral anticoagulants (vitamin K antagonists) therapy argues in favour of its important role in bone development 42, 43. Vitamin C is another vitamin supplement essential for the development of collagen and ground substance, which may improve the bone health in PWH 44. Young haemophilics should avoid parathyroid hormone, Raloxifene or bisphosphonates for osteoporosis. However, elderly haemophilics may be treated with these drugs with vitamin supplementation and appropriate physical exercise. Newer drugs like Denosumab, a human monoclonal antibody against RANKL, which is an essential regulator of osteoclasts, has been found to be highly effective in postmenopausal women 45. This may have significant role in the management of severe osteoporosis in elderly haemophilics.
| Drugs | Mechanism of action |
|---|---|
| Calcium + vitamin D derivatives + vitamin K | Vitamin D increases muscle strength in addition to bone strength; Vitamin K improves carboxylation efficiency of osteocalcin |
|
Bisphosphonates Zoledronic acid Aamidronate Alendronate Ibandronate Risedronate Etidronate Clodronate |
High affinity for bone; released from the bone matrix upon exposure to acid and enzymes secreted by an active osteoclast |
| Calcitonin | Acts on the calcitonin receptor on osteoclasts to decrease their activity |
| Selective oestrogen receptor modulators (SERM) | Bind to oestrogen receptors and have tissue-specific effects that either mimic or antagonise the actions of oestrogen |
| Oestrogen replacement therapy | |
|
Synthetic steroid hormone Tibolone |
Binding to the oestrogen receptor |
|
Parathyroid hormone PTH analogues |
Anabolic effects on osteoblasts when used within physiological concentration |
| Strontium ranelate | Stimulates bone formation and decreases bone resorption |
|
Monoclonal antibodies – Inhibitors of RANK signalling Denosumab |
Binds to RANK Ligand (RANKL), a soluble cytokine produced by osteoblasts and prevents the osteoclast activation induced by the binding of RANK-L to its receptor RANK, thereby decreasing bone resorption |
| Cathepsin K inhibitors | Inhibit cathepsin K, which is an enzyme secreted by osteoclasts to increase bone resorption |
|
Tryptophan hydroxylase inhibitor LP-533401 |
Increases bone mineral density (shown in experimental animals) |
|
Antisclerostin monoclonal antibody AMG 785 |
Neutralises osteoblast-inhibiting protein sclerostin |
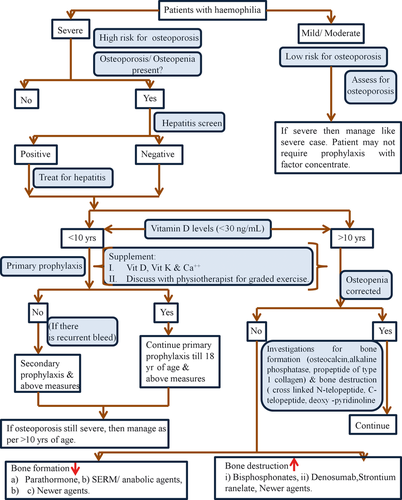
Some of the major advances in osteoporosis management include development of calcitonin drugs like Ronacaleret, a calcium-sensing receptor antagonist that stimulates release of hormones from the parathyroid glands 46. Cathepsin K inhibition is a novel approach to osteoporosis treatment. Odanacatib, a cathepsin k inhibitor has been shown to reduce bone resorption 47. Selective oestrogen receptor modulators (SERMs) like Raloxifene, lasofoxifene, bazedoxifene have also been found to be highly effective in the management of osteoporosis and fractures in postmenopausal women 48, 49. Significance of all these agents in the management of postmenopausal osteoporosis has been discussed elsewhere, but the role of these agents needs to be studied in PWH through randomised trials. Figure 2 shows the suggested algorithm for the management of haemophilia patients with osteoporosis.
There are some interesting observations on the prevention of osteoporosis from basic research. Sclerostin, a protein secreted by osteocytes, inhibits osteoblast function and hence inhibits bone formation. An antibody to sclerostin has been shown to improve BMD in preclinical experiments on mouse models. Reports of the phase I clinical trials of an anti-sclerostin monoclonal antibody (AMG 785) show that the bone protective effect seen in preclinical studies of this agent is reproduced in clinical trials 50. Increased bone density in cases of Van Buchem disease and sclerosteosis has been found to be due to reduced sclerostin concentration, and these clinical conditions suggest utility of anti-sclerostin antibody to improve bone density. Recent studies have linked serotonin production from gut enterochromaffin cells with osteoporosis 51. Thus, blocking serotonin production from these cells using tryptophan hydroxylase 1 (Tph1) inhibitor could improve BMD. This has been shown in experimental animals by using the compound LP-533401, which is not known to be crossing blood–brain barrier, thus having no impact on the synthesis of brain serotonin 52. In the same way, the serotonin receptor on osteoblasts could also be targeted.
Conclusion
Osteoporosis in haemophilia is an important challenge and is an ongoing epidemic. The causes of osteoporosis are diverse; the aetiopathogenesis is complex and these are likely to vary in different age groups and different geographical regions of the world where general management strategies of PWH are extremely variable. It is associated with lack of adequate exercise, multiple haemorrhage and inflammation, low vitamin D level and low BMI. The severity of the disease increases with HCV, HBV or HIV infection. Each individual case needs to be considered and treated on his own merits and demerits, and a judicious combination of various drugs will achieve the desired results. The simple approaches like participation in outdoor activities and sports, physical exercise, replacement with adequate vitamin D along with calcium and vitamin K, timely treatment for haemarthrosis, primary prophylaxis can be prescribed for all. Randomised trials for the management of osteoporosis in PWH are urgently required.




