Central serous chorioretinopathy
Abstract.
Central serous chorioretinopathy (CSC) is a disease of the retina characterized by serous detachment of the neurosensory retina secondary to one or more focal lesions of the retinal pigment epithelium (RPE). CSC occurs most frequently in mid-life and more often in men than in women. Major symptoms are blurred vision, usually in one eye only and perceived typically by the patient as a dark spot in the centre of the visual field with associated micropsia and metamorphopsia. Normal vision often recurs spontaneously within a few months. The condition can be precipitated by psychosocial stress and hypercortisolism. Ophthalmoscopic signs of CSC range from mono- or paucifocal RPE lesions with prominent elevation of the neurosensory retina by clear fluid – typical of cases of recent onset – to shallow detachments overlying large patches of irregularly depigmented RPE. The spectrum of lesions includes RPE detachments. Granular or fibrinous material may accumulate in the subretinal cavity. Serous detachment often resolves spontaneously. From first contact, counselling about the potential relation to stress and glucocorticoid medication is warranted. After 3 months without resolution of acute CSC or in chronic CSC, treatment should be considered. Resolution of detachment can usually be achieved in acute CSC by focal photocoagulation of leaking RPE lesions or, in chronic CSC, by photodynamic therapy. The effect of therapy on long-term visual outcome is insufficiently documented. Reattachment within 4 months of onset is considered a relevant therapeutic target because prolonged detachment is associated with photoreceptor atrophy. This suggests that the value of treatment depends upon proper selection of cases that will not resolve without therapy. Chronic CSC may be difficult to differentiate from occult choroidal neovascularization secondary to CSC. Patients with chronic CSC who receive glucocorticoid treatment for systemic disease can often be managed without having to discontinue this medication.
Introduction
Central serous chorioretinopathy (CSC) is one of several chorioretinal disorders characterized by serous detachment of the neurosensory retina and/or the retinal pigment epithelium (RPE). CSC is one of the 10 most common diseases of the posterior segment of the eye and a frequent cause of mild to moderate visual impairment. Spontaneous resolution is common in acute CSC with monofocal or paucifocal RPE changes. Lack of such resolution can often be managed by photocoagulation therapy directed at foci of RPE leakage. Recurrent or persistent detachment is often associated with more diffuse RPE change, which is also seen in relation to secondary subretinal neovascularization. In this article, we review the literature on CSC with emphasis on diagnosis, management, and major unresolved issues relating to this disease.
Methods
The purpose of this article is to review the scientific literature, with emphasis on publications made after the extensive review by Yannuzzi (1987). The survey was closed on 18 September 2006. Publications were retrieved using PubMed (http://www.ncbi.nlm.nih.gov/entrez) using the search terms ‘central serous’, ‘central serous retinopathy’ and ‘central serous chorioretinopathy’. The review includes papers of relevance, omitting duplications of previous findings, some small case reports and minor reviews. Additional publications that illustrate methodological principles or fundamental physiology were included as deemed relevant.
Nosographic history
CSC was first described by Albrecht von Graefe in 1866 (Yannuzzi 1987). The condition has been given various labels, including idiopathic central serous choroidopathy (Gass 1967), central serous pigment epitheliopathy, central serous retinopathy (Yannuzzi 1987) and the currently favoured term, central serous chorioretinopathy. Chronic CSC is also known as diffuse retinal pigment epitheliopathy.
Epidemiology
The epidemiology of CSC has not been systematically surveyed. The literature consistently reports a higher prevalence in men than in women in clinic-based patient populations, men accounting for 88% (Spitznas & Huke 1987), 83% (Castro-Correia et al. 1992), 79% (Wang et al. 2005), 73% (Tittl et al. 1999) to 72% (Spaide et al. 1996) of study populations. CSC is found from early adulthood and there is no apparent upper age limit (Schatz et al. 1992; Spaide et al. 1996). The peak prevalence is around 45 years. In women and in patients with chronic CSC, the peak prevalence tends to be higher (Gackle et al. 1998; Wang et al. 2005). Bilateral involvement has been reported in 40% of cases (Gackle et al. 1998). Among non-surgical retinopathies, CSC may rank fourth in incidence after age-related macular degeneration (AMD), diabetic retinopathy and branch retinal vein occlusion; CSC may be second only to AMD as the presumed cause of subretinal neovascularization.
Diagnosis
Definition
Active CSC is characterized by detachment of the neurosensory retina caused by accumulation of serous fluid between the photoreceptor outer segments and the RPE in combination with monofocal or multifocal changes in the RPE. The detachment should not be attributable to holes or tears in the retina, neovascularization, inflammation, neoplasia or specific hereditary disease. The retinal detachment usually involves the fovea but exceptions to this rule can be found. Unlike hereditary chorioretinal degenerations, the pattern of RPE anomaly in CSC is geometrically irregular in that it generally lacks symmetry about the facial midline (as seen in retinitis pigmentosa) and rotational symmetry about the centre of the fovea (as seen in bull's eye dystrophies). The diagnosis of chronic CSC requires multifocal or diffuse RPE depigmentation combined with a serous detachment of the retina. Inactive CSC, defined by the retina being fully attached, may be diagnostically challenging because the residual abnormalities of the RPE may resemble those of other conditions, but often a history characteristic of acute CSC dating back years or decades can be elicited. Neovascular CSC is usually a sequel of chronic CSC. Macular haemorrhage in CSC should always lead to the suspicion of subretinal neovascularization (Schatz et al. 1977).
Past medical history
Patients are generally in good health. Some may have experienced previous episodes of transient blurred vision.
Family history
Reports of two or more cases within a family suggest that a hereditary predisposition to CSC may exist (Oosterhuis 1996; Park et al. 1998). A fluorescein angiography study of siblings, uncles and nieces of 27 patients with chronic CSC demonstrated RPE atrophy in 35 of 80 relatives, of which 22 were classified as unrecognized cases of chronic CSC (Weenink et al. 2001). The available studies are not informative as to the mode of inheritance.
Symptoms
CSC of recent onset is associated with blurred vision with a relative central scotoma, metamorphopsia, dyschromatopsia, micropsia, hypermetropization and reduced contrast sensitivity. In some patients, CSC is preceded or accompanied by migraine (Gass 1967).
Symptoms are usually confined to the centre of the visual field and frequently the primary complaint is one of transiently seeing a dark spot in the centre of the visual field in one eye (Fig. 1), with or without accompanying metamorphopsia (Fig. 2). Best-corrected visual acuity ranges from 20/20 to 20/200; a hyperopic shift is a common finding, corresponding to the anterograde displacement of the neurosensory fovea (Klien 1956; Peyman & Bok 1972). Scanning laser ophthalmoscopic microperimetry has demonstrated a 10–100 fold reduction of sensitivity within the affected part of the visual field (Toonen et al. 1995). The dark spot, which is the subjective representation of a relative scotoma in the centre of the visual field (Faschinger & Brunner 1982), is usually most prominent in the morning immediately after awakening. Patients often report seeing it most clearly when opening their eyes and looking at the ceiling of their bedroom, presumably because the typical ceiling is bright white and unstructured. The spot can often be made visible later in the day by blinking. These characteristics are typical of a relative scotoma, and like the relative scotoma produced by light (i.e. an after-image) it fades within a few seconds, presumably because of the Troxler effect, a retinal function that subtracts any stationary background stimulus (Fig. 3). The entopic spot produced by a serous retinal detachment can be made visible to the patient by using the pinhole flicker test, as when the retinal vessels are visualized (Iwami 1995). The patient looks at a brightly lit uniform surface through a pinhole held 0.5–1.0 cm in front of the eye. The pinhole plate is then moved sideways a few millimetres back and forth at a frequency of about 4 Hz. In contrast to acute CSC, patients with sequels of CSC or chronic CSC may have blurred vision, but rarely (if ever) complain of seeing a dark spot.
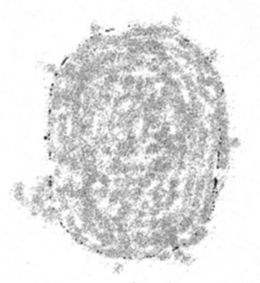
Subjective impression of relative central scotoma illustrated by a patient during acute CSC. The image subtends 4 degrees visual angle vertically and 3 degrees horizontally. The patient described a grey spot that was most easily recognizable within the first 10 seconds of opening his eyes in the morning. The scotoma then disappeared, but could be made to reappear briefly by blinking. The appearance was variable from day to day, but did not exceed the dimensions shown above.

Subjective impression of relative paracentral scotoma in active acute central serous chorioretinopathy, as illustrated by the patient (different from the patient who produced Fig. 1). Features of abnormal vision include metamorphopsia (A), dyschromatopsia (all), reduced contrast (B, C), subjective luminosity increase (B) and decrease in luminosity (C). The patient used blinking to elicit and/or enhance the symptoms.
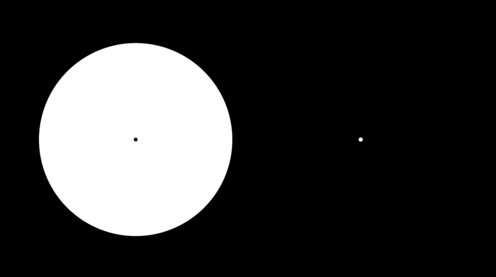
After-image effects similar to those seen by patients with a relative central scotoma in central serous chorioretinopathy can be elicited by gazing at the black dot in the left side of the image for half a minute and then shifting one's gaze directly to the white dot in the right side of the image. Four phenomena will be observed: (1) immediate appearance of a dark after-image corresponding to the shape and diameter of the white area; (2) gradual fading of the after-image within a few seconds; (3) appearance of a transient bright after-image seen for only a fraction of a second immediately after closing the eyelids; and (4) transient reappearance of the after-image after blinking, when the eyelids are opened. The fading of the after-image is attributable to the Troxler effect, i.e. the subtraction of entoptic images that are stationary in relation to the retinal photoreceptor matrix, in this case the entoptic image of the serous detachment.
Visual function and psychophysical tests
Functional testing of eyes with serous retinal detachment has demonstrated that a minimal relative afferent pupillary defect may be present, as may reduced critical flicker-fusion thresholds, prolonged visual evoked potential (VEP) latencies, dyschromatopsia – most often with a tritan axis (Williams 1976) – and depression of central visual field sensitivity. After resolution, the afferent pupillary defect and critical flicker-fusion thresholds are first to improve, followed by visual acuity, VEP latency and colour discrimination. The threshold differential light sensitivity in the central visual field is slowest to improve (Folk et al. 1984).
Biomicroscopy
The subretinal fluid in CSC is commonly clear, but granular or fibrinous deposits may be present in the subretinal space (Ie et al. 1993; Wang et al. 2002). The accumulation of granular material between the RPE and the neurosensory retina increases in relation to the duration of symptoms (Wang et al. 2005). Other characteristics include the absence of the normal foveal light reflex and a distinct visibility of the yellow foveal xanthophyll, presumably caused by increased scatter in the thickened perifoveal tissue of the detached retina (Spaide et al. 1996; Gass 1991; Ie et al. 1993).
Despite a marked propensity of the serous detachment to involve the fovea, to be centred in the fovea and to reach its maximum height in the fovea, the primary lesion in the RPE is rarely subfoveal. Indeed, it can be located anywhere in the posterior pole within or immediately outside the temporal vascular arcades (Gomez-Ulla et al. 1993). Large bullous detachments often show the phenomenon of subretinal fluid shifting position with changes in posture.
Abnormalities of the RPE are present, by definition, in CSC. When most prominent, these abnormalities are seen as one or more yellow spots or a small pigment epithelial detachment. The RPE detachment can occasionally be seen to be ruptured, the rupture being the site of profuse angiographic leakage (Fig. 5). Recent optical coherence tomography (OCT) studies suggest that retinal pigment epithelial detachment may be present in most cases of CSC (Saito et al. 2005; van Velthoven et al. 2005; Mitarai et al. 2006). It is unknown whether small sources of leakage are indeed small pigment epithelial detachment beyond the resolution of current imaging techniques. Less prominent abnormalities at the level of the RPE can be made visible during biomicroscopy using choroidal retroillumination. These appear as minute refractile granules deep in the retina. Unlike degenerative conditions such as retinitis pigmentosa or photocoagulation scarring, geographic RPE atrophy does not occur in CSC, whereas hypopigmented RPE is a frequent finding, characterized biomicroscopically by translucency without transparency. It is not often that true hyperpigmentation occurs, but dense subretinal deposits can look like hyperpigmentation when seen in choroidal retroillumination. Hypopigmentation of the RPE is more prominent on fluorescein angiography than on biomicroscopy. The diagnosis of CSC is supported by CSC-compatible RPE changes in the fellow eye.
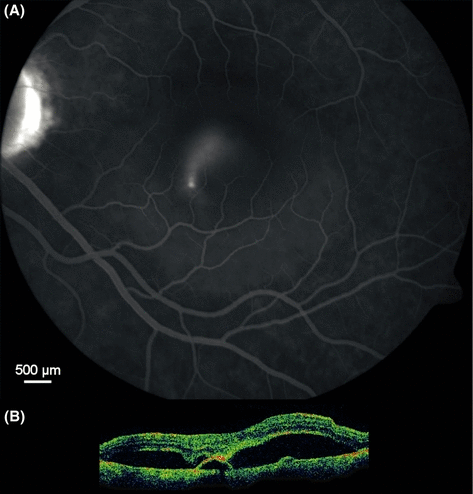
Smokestack fluorescein angiographic type of central serous chorioretinopathy recorded 15 min after intravenous fluorescein injection (A). From the site of leakage, approximately 50 µm in diameter, located 700 µm inferior of the foveola, fluorescein-stained fluid is flowing upward within a 3000 µm diameter cavity filled by colourless serous fluid, the hyperfluorescent outline of which is faintly visible. Optical coherence tomography (B), approximately to scale, is showing focal attachment of the neurosensory retina to the apex of a pigment epithelial detachment.
Less frequent manifestations of CSC include retinal capillary dilation, choriocapillaris atrophy, atrophic tracts of the RPE and inferior peripheral dependent serous retinal detachment (Yannuzzi et al. 1984). When telangiectasia, leakage, retinal lipid deposition and/or cystoid macular oedema are seen (Yannuzzi et al. 1984), these features likely attest to the presence of choroidal neovascularization (CNV).
The subretinal fluid in CSC is usually clear, but the subretinal cavity may contain granular or cloudy material of a pale yellowish hue (Spaide et al. 1996). Non-granular subretinal material is often called fibrin. The subretinal material may disappear but it often leaves an imprint of permanent RPE hypopigmentation (Fig. 8) with associated fluorescein angiographic transmission hyperfluorescence. Small granular subretinal deposits are associated with a favourable prognosis (Perkins et al. 2002), probably because they signify a short duration of detachment (Wang et al. 2005). Notably, chronic detachments with subretinal deposits leave highly visible imprints (Gismero et al. 2003). Long-standing serous detachment of any type may be followed by attenuation of the RPE corresponding to the detachment, and it may persist after resolution (Wang et al. 2002). Subretinal fibrosis can arise as a consequence of CNV (Fig. 11) or independently of neovascularization, as a result of severe fibrinous deposition (Fig. 12). The fibrinous material may play a role in anchoring a partly detached retina to an underlying pigment epithelial detachment (Hussain et al. 2006) (Fig. 5).
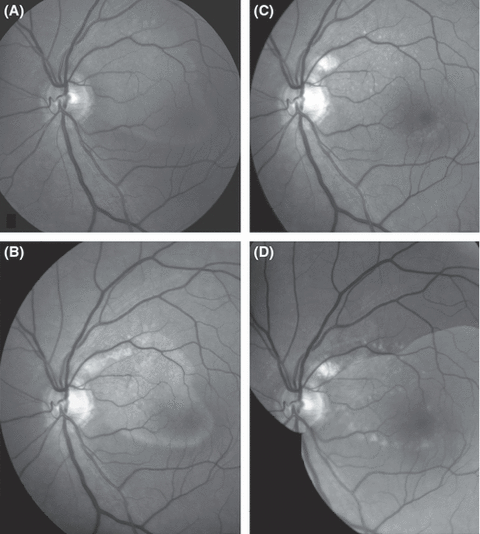
Fundus photographic montage (red-free, greyscale) demonstrating evolution of subretinal deposits over time in an otherwise healthy male, who presented at age 47 years with unilateral complaints of seeing a dark spot overlaid his central visual field in the left eye. The spot was described as having the shape of the footprint of Donald Duck (A). Best-corrected visual acuity in the left eye was 0.9. A yellowish patch of confluent subretinal material having said shape was seen to extend from the superonasal macula to the temporal aspect of the fovea. Photocoagulation treatment applied 0.5 disk diameter above and temporal of the optic disk was followed by gradual resolution of the subretinal fluid, the residual substance presenting a mixed diffuse and granular appearance 1.5 months after treatment (B), the appearance having changed to a granular one at 6 months (C) and to multifocal patches of RPE hypopigmentation after 6 years (D). At this time, automated perimetry of the left eye (Humphrey 24–2, full threshold) was entirely normal. Nevertheless, the patient described a distinct grey pattern overlaid his central visual field, the drawing of which closely matched the pattern of hypopigmentation.
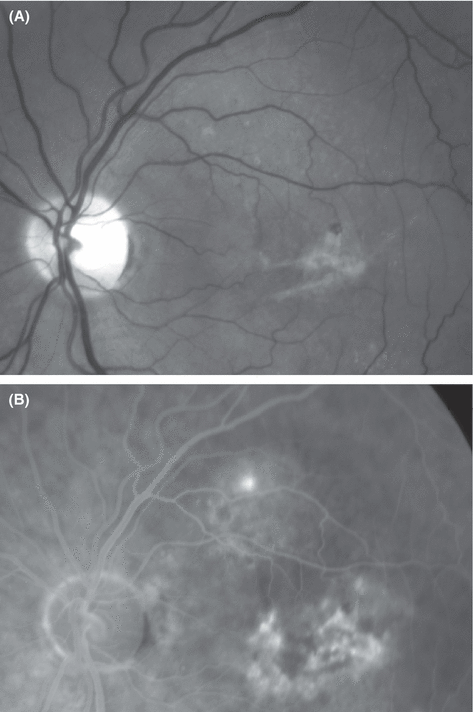
Fundus photography (red-free, A) and mid-phase fluorescein angiography (B) from the left eye of a 61-year-old man with a 20 year history of chronic recurrent central serous chorioretinopathy. A lesion near the upper temporal vascular arcade shows expanding leakage from a subretinal point source and a surrounding serous detachment that did not reach the fovea. The subfoveal lesions demonstrated staining without leakage. Best-corrected visual acuity in the left eye was 0.3. Punctate subretinal deposits were seen in the upper macula, and fibrinous deposits without haemorrhage or hard exudate were found under the fovea. Three months later, the patient presented with visual acuity 0.1 in the left eye and subretinal haemorrhage, hard exudate and late angiographic leakage from a subfoveal occult-only choroidal neovascularization (CNV). The history suggests that conversion from inactive occult subfoveal CNV to active neovascularization occurred between the two visits.
Development of subretinal fibrosis in direct transition from acute subfoveal accumulation of fibrinous material at presentation (red-free fundus photography, A; early phase fluorescein angiography, B; late phase, C) and follow-up 2.5 years later (D). Shortly after presentation, the right eye underwent extrafoveal photocoagulation treatment to the hyperfluorescent area above the subfoveal serous detachment (C). This was followed by resolution of the serous subretinal fluid within 2 weeks and precipitation of subretinal material that was resorbed slowly over about 1 year, leaving a small area of subretinal fibrosis under the fovea at follow-up 2.5 years after treatment. No evidence of subretinal neovascularization was seen at any time.
Fluorescein angiography
The diagnosis of classic CSC can often be made without fluorescein angiography, but angiography is a critical element in the differential diagnosis, notably of subretinal neovascularization, and in the planning of treatment. The diagnosis of CSC should not be made in the absence of fluorescein angiographic RPE abnormalities. These can range from a single clearly demarcated focus of RPE translucency and leakage to extensive areas of irregular hypopigmentation and fluorescence-blocking elements with or without true pigmentation. Non-pigmented elements that block choroidal fluorescence often display marked autofluorescence (Framme et al. 2005).
A prominent angiographic characteristic of CSC is that of an expanding point of fluorescein leakage under a serous detachment of the sensory retina, without signs of subretinal neovascularization (Fig. 4) (Robertson 1986). Usually, one or two leakage points are seen. The site of leakage is often hypopigmented, showing transmission of choroidal fluorescence. The hypopigmentation of the RPE is not associated with choriocapillaris atrophy or geographic atrophy of the RPE. Most leakage points are located within 0.5–1.5 mm of the centre of the fovea (Spitznas & Huke 1987). Less than 10% of cases demonstrate leakage in the foveal area. The most common location of leakage points is the superonasal quadrant of the posterior pole. The serous detachment may involve the site of leakage in CSC but often the two are not visibly connected. In a minor fraction of cases – 7% in one study (Spitznas & Huke 1987) and 25% in a study of acute cases only (Yamada et al. 1992) – the leaking dye is seen to stream upwards from the site of hypopigmentation in a ‘smokestack’ pattern (Fig. 5). It rises vertically to expand laterally at the top of the subretinal cavity in a mushroom- or umbrella-like fashion. It is unknown whether the flow is caused by a temperature gradient, by a density gradient existing between the newly secreted fluid and surrounding fluid that has been in the subretinal cavity long enough to have cooled or to have come hyperdense because of preferential resorption of water and small solutes. In other cases of CSC, multiple distinct foci of hyperfluorescence are present, yet none of them demonstrate expanding fluorescence (minimally enlarging spot configuration) whence it is difficult or impossible to attribute the serous detachment to a specific subset of leakage points. Finally, some cases show partial confluence of multiple irregularly distributed patches of hypopigmentation (multifocal ink-blot-like leakage) (Fig. 6). This order of listing is believed to reflect the development of fundus changes over time in non-resolving CSC. The number of RPE lesions and the configuration of lesion clusters are highly variable, ranging from a single focal lesion in one eye only to multiple clusters of lesions in both eyes. Pigment epithelial detachments are occasionally seen in CSC, as isolated lesions or adjacent to areas of mottled RPE, suggesting the presence of occult CNV (Fig. 7).
Acute central serous chorioretinopathy with multifocal RPE hyperfluorescence during early phase fluorescein angiography (A). A cluster of point sources of leakage 1.5 disk diameters temporal of the optic are visible in the late phase angiogram (B). Optical coherence tomography demonstrated detachment of the entire macula.
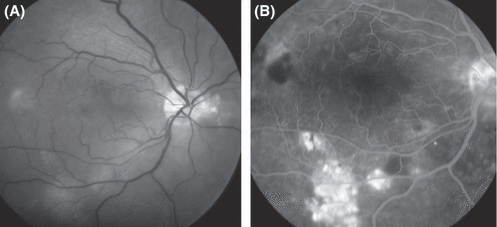
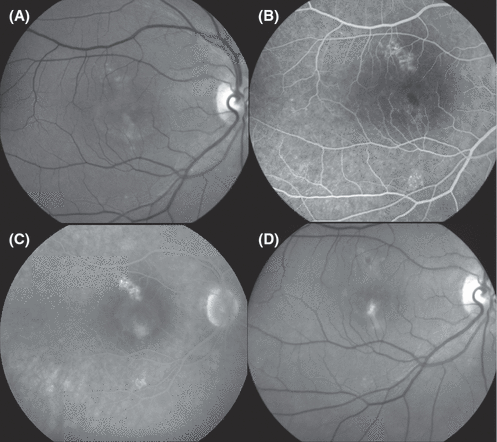
Fundus photography (A) and fluorescein angiography (B) in the right eye of a 27-year-old woman with systemic lupus erythematosus, lupus nephropathy, iatrogenic hypercortisolism and well-controlled arterial hypertension. The patient had suffered, to variable degrees, from painless blurred vision, dyschromatopsia and micropsia for more than 2½ years. Best-corrected visual acuity in the right eye was 0.3. Fundus abnormalities included an irregular pattern of hypopigmentation of the retinal pigment epithelium, amorphous subretinal deposits and a shallow serous detachment involving large parts of the posterior pole. These findings are highly suggestive of chronic central serous chorioretinopathy.
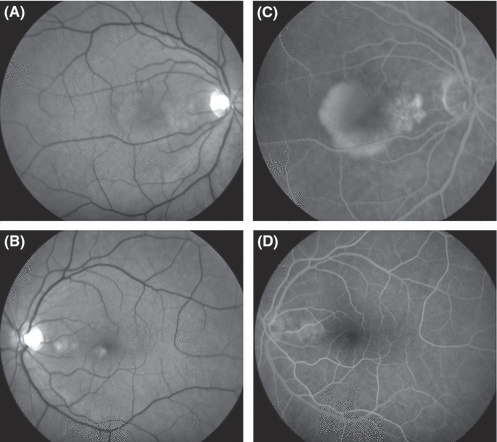
Fundus photographs (A, B) and fluorescein angiograms (C, D) from a 54-year-old woman who presented with pigment epitheliopathy between the fovea and the optic disk in both eyes. In the right eye (A, red-free fundus photograph; C, fluorescein angiogram), the circumscribed area of mottled pigment epitheliopathy was associated with a pigment epithelium detachment (PED) that extended under the fovea as well as a shallow serous detachment of the fovea. Best-corrected visual acuity in the right eye was 0.7. The angiographic characteristics did not enable a clear distinction between chronic central serous chorioretinopathy and occult subretinal neovascularization. Following confluent photocoagulation treatment of the area of mottled pigment epitheliopathy nasal of the fovea, all subretinal fluid resolved, as did the patient's symptoms. Best-corrected visual acuity achieved 1.0 within 1.5 months and the condition has remained stable for 8 years. Equally favourable outcomes have been reported following photodynamic therapy of the primary RPE lesion. The left eye presented a PED between the optic disk and the fovea and subretinal deposits, notably under the inferonasal rim of the fovea. Confluent photocoagulation treatment of moderate intensity over the PED resulted in flattening of the retina. The left eye has remained stable during 8 years of follow-up.
Hyperpigmentation is seen more rarely in CSC; it should be distinguished from subretinal deposits associated with angiographic choroidal shadowing (Figs. 6 and 8) (Iida et al. 2002). Such deposits appear to be prominent in CSC arising during pregnancy (Sunness et al. 1993).
The area of detachment in relation to the area of leakage is higher in smokestack cases than in pin-point leak cases without the smokestack phenomenon (Friberg & Campagna 1989). Assuming that the extent of the detachment is proportional to the rate of leakage, this is evidence that smokestack cases leak faster than other lesions. Particularly high rates of leakage appear to be associated with RPE detachments with a tear at the edge, the fluid leaking through this opening into the subretinal cavity. In one third of the cases, the point of leakage is in the papillomacular bundle, most commonly above the horizontal raphe (Wessing & Meyer-Schwickerath 1971).
Autofluorescence fundus photography
The autofluorescence characteristics of the fundus in CSC are clearly different from healthy eyes. In acute CSC, hypoflourescence has been demonstrated at the very point of leakage (Eandi et al. 2005). Acute CSC that has persisted for some time often shows granular or semi-confluent hyperfluorescence throughout the area of detachment (Fig. 9). In chronic CSC, irregular patterns of mixed hyper- and hypofluorescence can be seen (Framme et al. 2005; von Ruckmann et al. 2002). After reattachment, the autofluorescent subretinal deposits disappear slowly over a period of several months (Fig. 9).
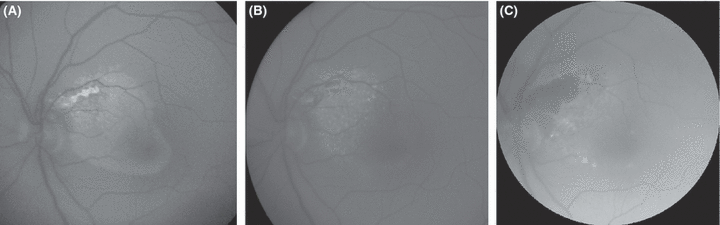
Fundus autofluorescence photographs taken using fluorescein angiographic filters and a greyscale charge-coupled device fundus camera in the left eye of the same patient as in Fig. 5. After resolution of the serous retinal detachment following photocoagulation treatment 1.5 months earlier (A), moderately hyperfluorescent subretinal deposits were found throughout the area where the detachment had previously been located; a more prominently hyperfluorescent area was seen where treatment had been applied near the rim of the optic disk. The hyperfluorescence of the subretinal material faded with its gradual resorption and it was of granular appearance 6 months after treatment (B). A weak pattern of hyperfluorescence remained 6 years after treatment (C), except for a the dark area corresponding to the photocoagulation scar.
OCT
OCT can demonstrate shallow serous detachments that are difficult to diagnose using slit-lamp biomicroscopy (Wang et al. 1999). OCT is also useful in determining whether reattachment has occurred after treatment. Especially in chronic cases, OCT is useful because morphological and functional restitution may be incomplete, thus providing little clinical guidance (Hee et al. 1995). Recent use of three-dimensional OCT has revealed small pigment epithelium detachments (PEDs) in 13 out of 29 patients with active CSC; two of the 29 patients having RPE detachments larger than 0.5 disc diameter (van Velthoven et al. 2005). Multiple PEDs can be found in some cases, usually located superior to the neurosensory detachment.
Electrophysiology
Focal electroretinography (ERG) has demonstrated reduced photoreceptor function of the detached retina in CSC (decreased amplitude and increased latencies of the b-wave, decreased oscillatory potentials) (Papakostopoulos et al. 1984; Miyake et al. 1988; Shiroyama & Miyake 1990). Multifocal ERG (mfERG) has demonstrated variable degrees of dysfunction outside the area of detachment (Marmor & Tan 1999) but normal function outside the area of visible fundus involvement (Vajaranant et al. 2002). Obviously, the delineation of past detachment is often uncertain. Subclinical fellow-eye involvement is suggested by the finding of abnormally low mfERG amplitudes (Dohrmann et al. 2001), but the patient having had subclinical transient detachment in the fellow eye cannot be excluded. Electro-oculography and ERG have not shown signs of RPE dysfunction in CSC (Gupta & Marmor 1995).
Course and complications, staging
Prospective studies of the natural history of CSC are incomplete. The classification in Table 1 is based on prevalent expert opinion about the stages of progression of the disease and fragments of evidence from a large number of reports.
| Duration | Biomicroscopy, IVFA, OCT | Detachment | Stress, hypercortisolism | Treatment |
|---|---|---|---|---|
| Acute CSC | Fine granular subretinal deposits if duration more than a few weeks;monofocal leakage | High, bullous;OCT > 100 µm subretinal fluid | Common, recent | Conservative, counselling;if no resolution within3 months of onset ofsymptoms consider focalphotocoagulation or, incases where photocoagulationis considered unsafe, PDT |
| Recurrent | Paucifocal (1–5) | Moderate,51–100 µm | Common | Photocoagulation ifdeemed safe, PDTotherwise |
| Chronic | Multiple, semi-confluent hypopigmented RPE lesions;confluent subretinalmaterial | Shallow, often< 50 µm | Past, current, inconclusiveor none | consider PDT |
| Sequelae | RPE depigmentation without RPE atrophy | None | Past, inconclusiveor none | None |
| Neovascular | CNV plus CSCsequelae; subretinal fibrosis | Variable, mainlyaround CNV | History of CSC andassociated RPE changes | PDT and/or intravitreal VEGF-inhibitor for active CNV(blood, lipid, serous, detachment,recent visual loss) |
- IVFA, intravenous fluorescein angiography; OCT, optical coherence tomography; CSC, central serous chorioretinopathy; PDT, photodynamic therapy; RPE, retinal pigment epithelium; CNV, choroidal neovascularization.
- * The table summarizes a large number of publications, of varying evidence levels. For a full discussion of each item, consult the text.
Acute CSC
Typical acute CSC is characterized by a duration of symptoms and/or retinal detachment of less than 6 months and monofocal or paucifocal fluorescein angiographic RPE leakage (Eandi et al. 2005). Large pigment epithelial detachments can be seen in a small fraction of patients with CSC – probably less than 5% (Castro-Correia et al. 1992; van Velthoven et al. 2005). Occasionally, the PED may rupture (Shanmugam & Bhende 2000), in which case pronounced angiographic ‘blow-out’ leakage through the rip can be observed.
Chronic CSC
Chronic CSC, which is also known as diffuse retinal pigment epitheliopathy, is generally characterized by multifocal, irregularly distributed and often widespread RPE changes associated with varying degrees of low-grade leakage (Cohen et al. 1983; Beuchat et al. 1988; Yannuzzi et al. 1992; Otsuka et al. 2002). The relation to acute CSC is documented by published cases of long-term follow-up (Castro-Correia et al. 1992; Bujarborua 2001; Katsimpris et al. 2006). Chronic CSC is more often bilateral and may occasionally present with gravitational tracts (Brancato & Bandello 1991; Bujarborua 2001; Gismero et al. 2003), a term used for oblong, vertical patches of RPE hypopigmentation that extend inferiorly from the macula. Presumably, gravitational tracts are produced by subretinal fluid of high specific gravity sinking toward the inferior fundus and dissecting its way through the subretinal space. CSC with inferior retinal detachment and/or tracts of attenuated RPE pigmentation is also called atypical CSC. In a study that recalled 50 out of 150 patients 3 years after their first visit, diffuse epitheliopathy had developed in eight patients (Castro-Correia et al. 1992). The extent of RPE attenuation on fluorescein angiography has been found to be predictive of subsequent visual outcome (Bandello et al. 2000, 2001). Retinal abnormalities also described in CSC include pigment migration, capillary teleangiectasia and capillary non-perfusion in the detached retina.
Sequels of CSC, inactive CSC and CNV
In the absence of retinal detachment, the diagnosis of CSC – or rather sequels of CSC – relies upon the history (which may or may not be informative) and on finding RPE lesions characteristic of CSC. The diagnosis should not be made in the absence of fluorescein angiographic RPE changes. Lesions are often found in both eyes, but their distribution is not usually the same. A shallow relative scotoma may remain after resolution of CSC, occasionally with metamorphopsia (Natsikos & Hart 1980), mild dyschromatopsia, and reduced contrast sensitivity.
As with other lesions of the RPE and/or Bruch's membrane, subretinal neovascularization of choroidal origin may appear as a complication of CSC (10, 11) (Faurschou et al. 1977). CNV can arise at any time after CSC (Gomolin 1989) and usually does so long after the resolution of the acute disease. In older patients, CNV following CSC is often assumed falsely to be a manifestation of age-related macular degeneration (AMD), but it is worth recalling that the diagnosis of AMD cannot be made if the other eye is healthy and does not demonstrate drusen. If photocoagulation treatment has been used to treat active CSC, it will be difficult or impossible to determine whether the CNV arose as a complication of CSC or as a complication of photocoagulation (Schatz et al. 1977). However, the risk of CNV in CSC is low: one study reported a single case in 39 patients followed for an average 9.6 years (0.3% per patient per year) (Dickhoff et al. 1989); another study found that CNV developed in four eyes in three out of 51 patients followed over a mean of 34.7 months (2% per patient per year) (Bandello et al. 2001).
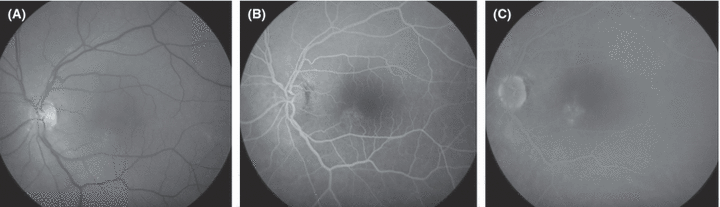
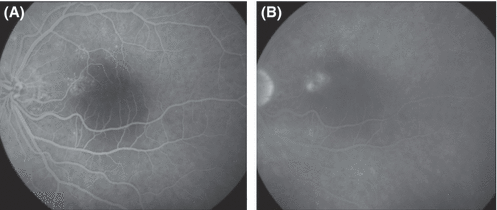
Fundus photograph (A) and fluorescein angiograms (B, C) from the left eye of a 62-year-old man with a history of recurrent central serous chorioretinopathy who presented with 2 months' duration of blurred vision, best-corrected visual acuity 0.5, a serous foveal detachment and a circumscribed area of mottled RPE at the inferonasal rim of the fovea. Confluent moderately bleaching photocoagulation treatment of a small area of pigment epitheliopathy was followed within 2 weeks by complete resolution of the serous detachment as well as disappearance of the symptoms. The patient presented four months later, with a subfoveal choroidal neovascular membrane. Retrospectively, it may be argued that the patient had occult choroidal neovascularization at presentation, although the late-phase hyperfluorescence was too weak to fulfil the conventional criteria for such a diagnosis. When overt neovascularization was eventually diagnosed, it could have been a consequence either of photocoagulation unmasking the existence of a membrane that was already present or of photocoagulation actually eliciting the development of a subretinal neovascular membrane.
In severe CSC with subretinal fibrinous deposits, it appears that instead of resorption of the subretinal material a subretinal fibrotic scar may form directly without the involvement of subretinal new vessels (Fig. 12) (Schatz et al. 1995; Hooymans 1998).
Subretinal deposits in CSC
Subretinal material is frequently present in CSC, mostly as fine granules (Figs 8C and 11A) (Wang 2005), more rarely as confluent patches (Fig. 8B) with a more or less fibrinous appearance (Gass 1991). The amount of subretinal debris increases with the duration of symptoms (Wang 2005). The isolation of photoreceptor outer segment fragments (Matsuo et al. 1986) from the aqueous humor of patients with the Schwartz syndrome suggests that such fragments may be responsible for the granular subretinal deposits in CSC (Larsen et al. 2004; Wang 2005; Schwartz 1973). Schwartz syndrome is an open-angle intraocular hypertension syndrome with aqueous corpuscles that is unresponsive to glucocorticoid therapy occurring in patients with anterior retinal tears or ora serrata dialysis. Aqueous cells and ocular hypertension resolve shortly after repair of the retinal detachment.
Visual prognosis
Acute CSC that resolves spontaneously or following treatment has a good long-term prognosis for visual function (Wong et al. 2004). Chronic CSC frequently results in considerable irreversible visual acuity loss, despite some functional improvement early after reapposition of the retina and a subsequent phase of slow visual recovery (Dohrmann et al. 2001). Among 47 US Air Force aviators with CSC, 51% had recurrent episodes, 17% had bilateral disease and 13% underwent laser photocoagulation (Green et al. 1988). Eighty-six per cent attained a final visual acuity of 20/20 or better.
Recurrences develop in one third to half of cases after the first acute episode, 10% having three or more recurrences. About half of the patients experience recurrence within 1 year of the primary episode (Gass 1967; Ficker et al. 1988). Eyes with recurrent disease tend to have reduced final visual acuity, stereopsis, colour vision and central visual field function. Examples of persistent loss of acuity and attenuation of the retina have mainly been reported in chronically recurrent cases (Newman 2002; Wang et al. 2002).
Extraocular conditions associated with CSC
Early reports on CSC described the frequent association of CSC with psychosocial stress, particularly during transient life crises (Yannuzzi 1987). Conflicts typically relate to dependency versus autonomy, submission versus control, providing versus autarchy and self-esteem (Spahn et al. 2004) or, in lay terms, personally challenging events such as impending divorce, bankruptcy, or critical illness of close relatives. The patients are generally in good health and perfectly sane, but frequently have coping strategies in critical life events that bring them to accept great burdens while obtaining little relief of tension (Conrad et al. 2000). These characteristics are typical of the Type A personality. A comparison of behaviour in patients with CSC versus patients with other ocular disorders demonstrated that Type A behaviour was significantly more frequent in CSC patients (Yannuzzi 1986, 1987). Increased levels of circulating catecholamines may be an important link between the tense, aggression-suppressing behaviour displayed by the Type A personality when subjected to environmental stress and the lesion of the RPE in CSC. Abnormally increased sympathetic nervous activity is supported by cardiac studies (Bernasconi et al. 1998). Elevated circulating epinephrine and norepinephrine levels have been measured in patients with CSC (Sun et al. 2003). Catecholamine levels have been shown to return to normal during the convalescent stage of the disease. Endogenous serum cortisol levels are on average more than 50% elevated in patients with CSC, also supporting that a stress response is involved (Garg et al. 1997).
Systemic glucocorticoid treatment is associated with increased risk of developing CSC (Carvalho-Recchia et al. 2002), possibly with a particular risk of developing chronic CSC (Polak et al. 1995). The disease can be seen in patients who use intranasal or inhalation glucocorticoids (Haimovici et al. 1997) but because CSC is a common disease, it remains uncertain whether the association is incidental or causal. The same is true for the frequent elevation of urinary cortisol excretion (Haimovici et al. 2003). A case–control study of 312 patients identified the following risk factors: systemic glucocorticoid use [odds ratio (OR) 37.1], pregnancy (OR 7.1), antibiotic use (OR 6.2), alcohol use (OR 4.9), untreated hypertension (OR 3.3) and allergic respiratory disease (OR 2.5) (Haimovici et al. 2004). The latter may be related to the therapeutic use of inhalation glucocorticoids. Subretinal fibrin may be more likely to form in CSC associated with systemic glucocorticoid treatment (Quillen et al. 1996).
CSC has been described in relation to a large number of other systemic conditions, the majority of which are associated with glucocorticoid treatment of conditions such as systemic lupus erythematosus and in relation to endogenous hypercortisolism (Eckstein et al. 1993; Bouzas et al. 2002). Fifteen out of 16 patients with CSC among organ transplant recipients also suffered from systemic hypertension (Fawzi & Cunningham 2001; Fawzi et al. 2006). The latency between onset of glucocorticoid treatment and disease is generally in the range of months, and there may be no lower dose limit (Song et al. 1997). CSC has also been described in relation to the intake of methylenedioxymethamfetamine (ecstasy) (Hassan et al. 2001).
Pregnancy has been reported to be associated with CSC, perhaps because of the increase in circulating glucocorticoids (Chumbley & Frank 1974). The condition is most often present in the third trimester and resolves within 1–2 months of delivery (Quillen et al. 1996).
Three reports from France have described elevated prevalence of Helicobacter pylori infection in patients with CSC compared to the background population (Mauget-Faysse et al. 2002; Ahnoux-Zabsonre et al. 2004; Cotticelli et al. 2006). One quarter of the population of France has a positive response to the C13-urea breath test for H. pylori, which is much higher than the prevalence of CSC, indicating that the vast majority of patients with H. pylori do not develop CSC.
A racial and hence genetic predisposition to CSC is suggested by the prevalence of the disease being high in Caucasians and Hispanics and higher in Asians but extremely low in African Americans (Spaide et al. 1996; Fukunaga 1969; Desai et al. 2003). Patients with CSC achieved higher than normal scores for hypochondria and hysteria in a personality test and were more likely to use psychomimetic medication (Werry & Arends 1978; Tittl et al. 1999).
Pathogenesis of RPE dysfunction and serous detachment
In acute cases, there is visible leakage of fluorescein-stained fluid from one or more small lesions in the RPE. The mechanism whereby subretinal fluid is produced in CSC is poorly understood. The constant inflow of fluid in a non-expanding subretinal cavity, which is seen to advantage in smokestack cases, is a vivid demonstration that the detachment is maintained by a balance between constant inflow and outflow, the latter presumably taking place across intact RPE and the retinal vessels.
The detachment is associated with leakage or secretion of fluid through dysfunctional RPE or RPE defects. The possibility that simple focal loss of RPE cells could be responsible for the leakage is excluded by the observation that a defect of the RPE promotes resorption, rather than leakage of subretinal fluid (Negi & Marmor 1984). Indeed, the rate of leakage in CSC is so high that active pumping of fluid across the RPE in the inward direction has been postulated. Alternatively, the leakage may emanate from a localized injury of the choriocapillaris with associated abnormally elevated leakage from these vessels (Prunte 1995). This model is difficult to accept unless rupture of the RPE is postulated; indeed, severe cases of focal leakage are often associated with ruptured RPE detachments.
In CSC, serous detachment of the retina is almost invariably foveal, despite the source of leakage most commonly being extrafoveal. Theoretically, this could be a consequence of the strength of the attachment of the retina being weaker in the fovea than elsewhere in the retina because the suction of the RPE exerts a weaker attraction on the thin foveal retina than on the thicker extrafoveal retina, the latter presumably having the lower hydraulic conductivity.
Experimental injection of epinephrine in monkeys can produce posterior pole serous retinal detachment (Yoshioka et al. 1982). Fluorescein angiography demonstrates choroidal vascular leakage associated with histologically irregular, narrow choroidal arterioles and choriocapillaries, hypertrophy of Bruch's membrane and loss or degeneration of the overlying RPE (Yoshioka & Katsume 1982). This response to adrenalin injection was abolished by ganglionic blockade and by systemic pretreatment using an alpha-adrenergic blocker (Yoshioka 1991). These observations suggest that adrenergic stimulation from the circulation and/or the sympathetic nerves may lead to CSC by inducing choroidal vasoconstriction and alteration of blood flow.
Indocyanine green angiography (ICGA) in patients with CSC has demonstrated evidence of choroidal lobular ischaemia and choroidal venous congestion (Hayashi et al. 1986; Prunte 1995; Prunte & Flammer 1996) (Fig. 13). Segments with late choroidal hyperpermeability also show a delay in filling. Focal areas of ICG hyperfluorescence can also be seen in clinically unaffected fellow eyes. The cause of the venous dilation has not been determined. Theoretically, it may be a response to ischaemia and delayed arterial filling or a consequence of outflow obstruction. ICGA hypofluorescence induced by the shadowing effect of a detached retina or subretinal deposits should not be mistaken for delayed lobular filling.
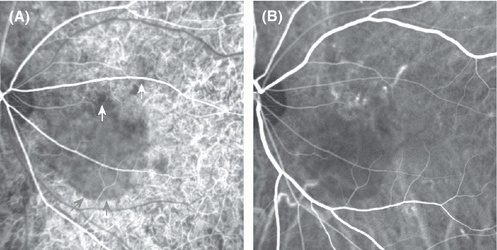
Indocyanine green angiography recorded using a scanning laser ophthalmoscope, from the left eye of a patient with acute central serous chorioretinopathy. The early frame (A) shows choroidal hypofluorescence corresponding to a two-disk-diameter-wide area of serous detachment (inferior margin indicated by grey arrows). Smaller areas of selective choroidal hypofluorescence near its upper border are marked using white arrows. During the angiographic mid-phase (B), these hypofluorescent areas demonstrate choroidal venous dilation and leakage at the locations in (B) that correspond to the white arrows in (A). An animated overlay of the two images can be found at http://www.oculus.suite.dk/CSC_ICGA_Animation1.gif.
ICG angiography is of value in diagnosing occult CNV in CSC, especially if performed using a confocal scanning laser camera. In the absence of CNV, ICGA is of no certain value in the management of CSC (Stanga et al. 2003). Choroidal vascular hyperpermeability at foci of subretinal fluorescein leakage is a frequent finding (Piccolino & Borgia 1994; Menchini et al. 1997; Uyama et al. 1999; Constantinides 2000; Yannuzzi et al. 2003); however, choroidal hyperpermeability can also be found without associated fluorescein leakage, as can subclinical RPE detachment (Guyer et al. 1994; Iida et al. 1996).
Laser interferometry has demonstrated increased choroidal pulsation in CSC, supporting the presence of a perfusion anomaly (Tittl et al. 2003).
Pathogenesis of visual dysfunction
Reduced colour and contrast sensitivity are present when the retina is detached, regardless of the cause. In some patients with CSC these sensory modalities are also affected after resolution of the detachment (Bartos et al. 1998). Several mechanisms may contribute to this functional defect: the lack of apposition of photoreceptors and RPE cells; moderate oedema of the detached retina; the absence of the stray light-absorbing RPE right next to the photoreceptors; photoreceptor disorientation interfering with the Stiles–Crawford effect (Bek & Kandi 2000); and impaired regeneration of visual pigment.
When the retina is detached in CSC, a relative central scotoma can be documented by psychophysical measurements (Burns et al. 1987). In theory, this scotoma should be deeper under scotopic conditions because the RPE visual cycle subserves exclusively or predominantly the rods, whereas a separate visual cycle in the neurosensory retina supports cone function (Mata et al. 2005). Indeed, rod dysfunction is more pronounced than cone dysfunction in CSC (Chuang et al. 1987). Patients often relate that their scotoma is most distinct when they leave their bed at night without turning the light on. Nevertheless, substantial photopigment regeneration appears to take place in the rod photoreceptors of the detached retina (Mori et al. 1990). Physiologically, the increased length of diffusion between the RPE and the photoreceptors may contribute to the reduced sensitivity and/or delayed adaptation of the detached retina. Photostress testing in areas of serous detachment shows a lower sensitivity change immediately after bleaching than in healthy subjects and a slower recovery (Verma & Sahai 1990; Horiguchi et al. 1998). Delayed adaptation of the detached retina is also supported by the demonstration of elevated fatigability of the double-flash ERG (Elenius 1968). In agreement with this, deeper scotomata are found with a flickering stimulus than with a steady-state stimulus (Vingrys & Pesudovs 1999). It is unknown whether this is because cone function is not entirely independent of the RPE or whether it demonstrates the loss of a contribution of rods to photopic vision.
Persistent visual loss despite reattachment of the retina has been shown to be associated with atrophy of the outer retina (Wang et al. 2002; Piccolino et al. 2005). This may be taken as evidence that direct contact between the (rod) photoreceptor outer segment and the RPE is essential for the survival of the photoreceptor and, possibly, for the cells of the RPE.
Differential diagnosis
The differential diagnosis of CSC requires exclusion of other causes of retinal detachment or RPE detachment, including rhegmatogenous detachment (which is very rarely confined to the macula) and serous detachment of other causes (including choroidal tumour, choroiditis, hypertensive choroidopathy, diabetic macular oedema, retinal vein occlusion, optic pit or other malformation and specific retinal degeneration or dystrophy). Additionally, the presence of CNV, independent of CSC or as a complication of CSC, must always be suspected.
Choroidal neovascularization
The presence of CNV is suggested by the presence of subretinal haemorrhage, hard exudate and visible new vessels on biomicroscopy or fluorescein angiography (Schatz et al. 1977). Some cases of CNV, especially occult-only CNV, may lack such signs of activity. A classic fluorescein angiographic criterion for defining occult CNV is circumscribed late-phase fluorescence that is more prominent than that of the optic disk. Cases of chronic CSC with RPE hypopigmentation and, possibly, choroidal vascular congestion come close to fulfilling this criterion and may be difficult or impossible to distinguish from occult CNV, although ICG angiography may be of some value.
AMD
The diagnosis of AMD requires the presence of drusen or the assumption that drusen were present but disappeared with the development of CNV or geographic atrophy. Unilateral CNV and a healthy fellow eye without drusen exclude the diagnosis of AMD (Schatz et al. 1992). Confluent drusen with RPE detachment, pseudovitelliform degeneration and pattern dystrophy are rarely associated with significant serous detachment of the neurosensory retina.
Hypertensive choroidopathy
Arterial hypertension can be accompanied not only by intraretinal vascular changes and optic disk oedema but also by serous retinal detachment and RPE lesions known as Elschnig spots. These are focal RPE hyperpigmentations 50–200 µm in diameter with a hypopigmented halo. The lesions are believed to be caused by choroidal ischaemia secondary to extreme vasoconstriction during arterial hypertension. The purported mechanism is very similar to that proposed for CSC. In this context, it is remarkable that CSC is associated only weakly with arterial hypertension (Haimovici et al. 2004) and that in CSC secondary to systemic glucocorticoid therapy, arterial hypertension is conspicuously absent (Chaine et al. 2001). Elschnig spots only develop in a fraction of patients with arterial hypertension and in a highly variable pattern, but with a propensity for being located above major choroidal vessels. These observations suggest that an idiosyncratic reaction in susceptible subjects is involved in producing focal choroidal ischaemia in both conditions.
Pigment epitheliitis
Acute retinal pigment epitheliitis is an entity that has been observed in healthy young adults who present with reduced visual acuity and a central scotoma. The characteristic macular lesions, discrete pigment clumps with surrounding hypopigmented halos that show hyperfluorescence without leakage on fluorescein angiography (Chittum & Kalina 1987), are indistinguishable from Elschnig spots. Acute retinal pigment epitheliitis was described before the advent of OCT.
Idiopathic polypoidal choroidal vasculopathy
Idiopathic polypoidal choroidal vasculopathy is characterized by polypoidal dilations of choroidal vessels, notably in the peripapillary area, with associated serosanguinous detachment of the RPE and the neurosensory retina. If fluorescein angiography is not diagnostic, indocyanine green angiography may be helpful in displaying the vascular polyps (Schneider et al. 2001; Stanga et al. 2003; Yannuzzi et al. 2000).
Isolated RPE detachment
Although not a common ophthalmoscopically visible component of CSC, RPE detachment may occur in CSC in the absence of CNV. This suggests that isolated RPE detachment, in the absence of serous neurosensory retinal detachment or other RPE lesions typical of CSC, could be a manifestation of CSC. Indeed, cases have been reported where such solitary RPE detachment converted spontaneously to monofocal classic CSC (Bandello et al. 2000; Gomez-Ulla et al. 2000). Additionally, ICG angiographic findings have been interpreted as suggesting that a pattern of choroidal venous dilation and leakage underlies both CSC and idiopathic RPE detachment (Giovannini et al. 1997; Gomez-Ulla et al. 2000). A few patients with idiopathic, multiple serous RPE detachments have been described in a clinicopathological study (Gass et al. 2005). Sporadic reports of successful elimination of RPE detachments following photocoagulation treatment may represent cases of CSC (Fig. 7).
Vascular disorders
Systemic inflammatory disease may be associated with CSC, which has been described in patients with systemic lupus erythematosus, polyarteritis nodosa, sclerodermia, dermatomyositis and relapsing polychondritis (Eckstein et al. 1993; Taga et al. 2001). Usually, it is impossible to determine whether the serous detachment is caused by inflammatory disease of the choroidal vessels or by systemic glucocorticoid treatment. Of particular interest is that severe arterial hypertension (Venkatesh et al. 2006), toxemia of pregnancy (Somfai et al. 2006) and disseminated intravascular coagulation (DIC) can present with a neurosensory retinal detachment. Endothelin-1 is a candidate mediator for the vasoconstriction that is an essential feature of these conditions (Asakura et al. 1992). These observations fit the concept that idiopathic CSC is caused by choroidal vasoconstriction, as described earlier, suggesting that serous retinal detachment in arterial hypertension, toxemia, etc. is a type of secondary CSC, as opposed to idiopathic or primary CSC.
Vogt–Koyanagi–Harada's disease
This posterior uveitis or panuveitis with exudative retinal detachment and disc hyperemia may have only posterior pole manifestations at presentation and the patient may fail to report prodromal central nervous system (CNS) symptoms. Vogt–Koyanagi–Harada's disease (VKH) is characterized by acute multifocal exudative posterior pole pigment epitheliopathy with serous detachment of the neurosensory retina (Read et al. 2000), often more extensive than in CSC. The multifocal RPE lesions are typically more numerous and more leaky than in CSC, the leakage coalescing over time to fill the subretinal space. Because early, aggressive systemic glucocorticoid therapy is believed to reduce the risk of complications and visual loss, early diagnosis is mandatory. The diagnosis is based on the ocular characteristics and the association with Asian, Middle Eastern or Native American ethnicity, concomitant or closely associated aseptic meningitis, inner ear symptoms, anterior uveitis, alopecia, poliosis, and vitiligo. A favourable response to systemic glucocorticoid therapy will support the theory that the condition is not CSC, whereas lack of response should prompt renewed diagnostic efforts. If serous retinal detachment develops in a patient with VKH during glucocorticoid treatment, the possibility of glucocorticoid-induced CSC should be considered (Schalenbourg et al. 2002).
Optic nerve pit with serous macular detachment
All patients who present with a serous detachment of the macula or maculoschisis should be examined for the presence of an optic nerve pit. This abnormality of the optic nerve is not associated with fluorescein angiographic leakage and may be more or less conspicuous, with or without a visible communication between the pit and the subretinal fluid.
Optic neuritis
Optic neuritis can result in complaints comparable to those of patients with CSC, but in optic neuritis there is often more prominent loss of colour saturation, brightness and contrast, including a sensation of greyness without the Troxler effect seen in CSC. Meta-morphopsia is absent in optic neuritis. An afferent pupillary defect is prominent in optic neuritis and often demonstrable with the Pulfrich test, whereas it is minimal or absent in CSC (Sadun 1990). During the acute phase, optic neuritis is associated with pain and tenderness, which are never seen in CSC. After the resolution of optic neuritis, visual dysfunction comparable to that seen in CSC may be found, with uncharacteristic dyschromatopsia and reduction of acuity and contrast. In contrast to CSC, there is often a residual relative afferent pupillary defect after optic neuritis, a visual evoked potential delay, and a reduced critical flicker frequency (Han et al. 1985).
Posterior scleritis
This painful condition may be associated with serous retinal detachment. Ultrasonography reveals posterior scleral thickening and fluid in the subtenonal space.
Inflammatory choroiditis
Multifocal choroiditis (Gass & Little 1995), uveal effusion syndrome, sympathetic ophthalmia (Gomez-Valcarcel et al. 2004) and other posterior pole inflammatory or infectious processes may be associated with serous retinal detachment. This is a characteristic of the active inflammatory stages of such diseases. Serous detachment is highly unlikely over a postinflammatory chorioretinal scar, unless CNV has developed. It is important to distinguish fluffy, elevated lesions and granulomatous choroidal lesions that leak fluorescein from the non-expanding hyperfluorescent lesions of chronic CSC, which are flat, except for the occasional PED. Systemic diseases associated with choroiditis include systemic lupus erythematosus and sarcoidosis (Eckstein et al. 1993; Cunningham et al. 1996). When considering the diagnosis of choroiditis, it should be remembered that recurrent or chronic CSC often leaves multifocal irregular changes in fundus pigmentation that may resemble sequels of inflammatory choroidopathy (Cunningham et al. 1996). When systemic glucocorticoid treatment has been used in the treatment of systemic inflammatory disease, it is our experience that the treatment is more likely than the disease to be responsible for the retinopathy (Fig. 6) (Bouzas & Mastorakos 1994; Gass & Little 1995). Discontinuation of glucocorticoid treatment may be followed by resolution of the retinal detachment in CSC, but this response is less certain if chronic CSC has developed. Although the association between hypercortisolism and CSC is clearly accepted, a survey of patients with Cushing's syndrome found evidence of past or present CSC in only 5% of cases (Bouzas et al. 1993).
When related to the use of exogenous glucocorticoids, CSC has a less prominent male predilection, presents more often a chronic or atypical form and is more frequently bilateral (Quillen et al. 1996; Bouzas et al. 2002). Glucocorticoids should not be used in the treatment of CSC.
Choroidal tumours
Inferior detachments of the neurosensory retina are a common feature in eyes with choroidal haemangioma, melanoma, or metastatic tumour (Haut et al. 1984). Serous detachment of the macula can also be seen in choroidal osteoma and leukaemic choroidal infiltration.
Treatment
The treatment of CSC is based largely on uncontrolled observations. The high spontaneous remission rate favours conservative management, lifestyle counselling and discontinuation of glucocorticoid medication as first-line therapeutic options. Such a strategy can be expected to be followed by a resolution of detachment in nearly 90% of cases within 1.5 months (Sharma et al. 2004). In most cases, visual acuity returns to 20/25 or better (Klein et al. 1974; Watzke et al. 1974). Only 5% experience severe vision loss (Gass 1967). If detachment persists for more than 3 months, photocoagulation or photodynamic therapy should be considered.
Counselling
Counselling should suit the condition and attitude of the individual patient. The interview should use neutral terms to prompt a general account of the patients well-being, rather than leading questions that incorporate terms such as ‘stress’. Sufficient counselling to reassure the patient and enable relevant action may follow from a simple explanation of the association between CSC and stress. Controlled trials or case observations on the effect of psychosocial therapy have not been published.
Adrenergic receptor inhibition
The relation to stress and adrenergic hyperactivity has prompted attempts at systemic treatment of CSC by antiadrenergic medication. An uncontrolled case series of patients treated using the betaadrenergic inhibitor metoprolol described a promising course in some patients (Avci & Deutman 2005), but the treatment has not been adopted widely. In an uncontrolled case series of 13 eyes with subtype-unspecified CSC treated with the beta-blocker metipranolol, two eyes had persistent detachment after 4 months of treatment (Chrapek et al. 2002). Another uncontrolled trial found no difference in the outcome between the non-selective beta-blocker metipranolol and the beta 1-selective blocker metoprolol; all patients (21 and 30, respectively) experienced remission within 3 months (Fabianova et al. 1998). In experimental adrenalin-induced CSC in animals, alpha-adrenergic blockade is more effective than beta-blockade (Yoshioka 1991), but sporadic therapeutic tests in humans have not led to any significant clinical use of this principle (Heinrich 1974).
Acetazolamide
Systemic acetazolamide treatment promotes the resorption of subretinal fluid and case reports suggest that it may reduce subretinal fluid in CSC (Gonzalez 1992). However, there is no evidence that treatment promotes healing of the RPE lesion, long-term preservation of visual function, or a reduced rate of recurrence.
Photocoagulation
Retinal photocoagulation treatment has been used for decades in the treatment of CSC (Kanagawa & Matsubara 1970; Watzke et al. 1974). Photocoagulation accelerates resolution of the detachment (Burumcek et al. 1997). A prospective study compared eyes with leaks smaller than 250 µm in diameter treated by argon laser photocoagulation, with sham photocoagulation or photocoagulation away from the site of leakage (Robertson & Ilstrup 1983). Treatment appeared to shorten the duration of detachment by approximately 2 months and to reduce recurrences within a follow-up period of 18 months from 34% to none. Other studies have found no evidence of effect on long-term visual acuity or the prevalence of chronic disease (Ficker et al. 1988; Gilbert et al. 1984). The most benefit of treatment appears to be earlier resolution of disease. A retrospective comparison of treated versus untreated cases over 11 or more years of follow-up found that six out of six patients who were treated had no recurrences whereas 32 untreated patients had 17 recurrences (Yap & Robertson 1996). Photocoagulation treatment is often successful in achieving retinal reattachment, but visual acuity fails to recover in about 10% of eyes (Yannuzzi et al. 1992).
A potential benefit of early resolution may be mediated by a lower rate of RPE degeneration in treated eyes (Fuhrmeister 1983). The demonstration that long-standing serous detachment is associated with retinal atrophy supports the theory that photocoagulation may benefit visual outcome, given that a low rate of complications can be achieved (Wang et al. 2002). The available data do not enable assessment of the long-term risk of CNV.
The photocoagulation treatment strategy is to apply laser energy so as to obtain a confluent coagulation of moderate intensity covering the site of leakage responsible for the foveal detachment. Argon laser photocoagulation or photocoagulation using another source of green light should be directed to the site of leakage, using spot sizes of 200 µm diameter, exposure times of 0.2 seconds and a light intensity that enables bleaching without whitening of the outer retina (Robertson 1986; Greite & Birngruber 1975). When multiple candidate lesions are present, the leakiest one should be treated first, provided that it is not under or very nearly under the fovea. Subfoveal lesions should probably not be photocoagulated because it entails a risk of damaging foveal photoreceptors and of inducing secondary subretinal neovascularization.
Unlike treatment of CNV, it does not appear that CSC treatment that is too mild to induce resolution cannot be corrected by a second treatment. Eyes that have undergone photocoagulation treatment demonstrate a risk of later development of subretinal neovascularization, an event that can also occur without photocoagulation (Kanyange & De Laey 2002). The more intense the photocoagulation, the higher is the risk of secondary CNV. The strategy described above is easily applicable in acute CSC, whereas identification of the site or sites of leakage responsible for detachment may be problematic in chronic CSC.
The available evidence suggests that retinal atrophy can be avoided if resolution of detachment can be obtained within 4 months of the onset of symptoms (Wang et al. 2002). In long-standing serous detachments, the retina may demonstrate pigment migration, capillary dilation and capillary non-perfusion, to the extent that atypical CSC has been described as pseudo-retinitis pigmentosa (Gass 1977). Because the relation between the onset of detachment and the onset of symptoms is often uncertain, earlier treatment may be warranted – especially if the presence of subretinal granular deposits suggests that the detachment has lasted longer than the duration of symptoms (Wang 2005). Further support for early treatment includes best-corrected visual acuity 20/40 or less and multiple recurrences (Robertson 1986). Complications of photocoagulation treatment are rare (Robertson & Ilstrup 1983), but the treatment should be used with caution. Early photocoagulation may be warranted by special occupational demands for binocular visual function.
The RPE lesion responsible for subretinal fluid accumulation may be located at a considerable distance from the fovea and even along the temporal vascular arcades (Fig. 8) (Bonamour et al. 1977). A subfoveal location is seen in 4% of cases (Bonamour et al. 1977). This is usually considered a contraindication for photocoagulation, although an anecdotal report suggests that subfoveal photocoagulation can be made without adverse effects if the fovea is detached when treatment is applied (Gartner 1987).
Lack of fluid resorption after photocoagulation should lead to suspicion that the serous detachment is caused by CNV rather than by CSC. Photocoagulation in itself may cause damage to Bruch's membrane and lead to the induction of subretinal neovascularization or the development of a classic CNV component by extension from occult CNV.
A single paper has reported better visual outcome in 15 patients treated by infrared 810 nm photocoagulation than in 15 patients treated by argon 514 nm laser photocoagulation (Verma et al. 2004). However, conclusive results have not been produced.
Transpupillary thermotherapy
A small non-randomized study of transpupillary thermotherapy (TTT) suggests that this treatment may accelerate the resolution of CSC, but long-term safety and efficacy are not known (Shukla et al. 2006).
Photodynamic therapy
Verteporfin photodynamic treatment (PDT) of chronic CSC has been described in uncontrolled case series. Resolution of serous detachment followed treatment in the majority of patients, as did visual improvement. No complications have been reported (Canakis et al. 2003, 2004; Cardillo et al. 2003; Valmaggia & Niederberger 2006; Chan et al. 2003a; Yannuzzi et al. 2003). PDT being a more recent therapeutic option, it has been used mainly in cases where it was thought wise to avoid photocoagulation because of a juxtafoveal or subfoveal location of the RPE lesion, lack of a clearly defined leakage hotspot, concern about the potential induction of CNV or suspicion that CNV was already present. Under these circumstances, PDT has also been followed by a favourable course in nine cases of acute CSC (Ober et al. 2005). In 18 patients with chronic CSC, use of half-dose PDT also appeared to be effective (Lai et al. 2006); similar findings have been reported for the mechanistically comparable ICG photothrombosis technique (Costa et al. 2002). The use of half-dose verteporfin or 50% reduced light fluence PDT is suggested as a precaution against permanent RPE or choriocapillaris damage.
The treatment of subfoveal CNV secondary to CSC has never been the objective of a controlled clinical trial. PDT is widely favoured, by extension of the experience with AMD-related CNV and numerous case reports of a favourable outcome with lasting resolution of fluid and visual improvement often following only a single treatment (Canakis et al. 2003; Chan et al. 2003b; Ergun et al. 2004).
A controlled trial of PDT in chronic CSC would involve the problem of differentiating chronic CSC alone from chronic CSC with occult CNV. ICG angiography may demonstrate CNV in some otherwise questionable cases, but the false-negative diagnostic rate is unknown. The issue is complicated because all major studies of occult CNV have applied treatment only to occult CNV with activity in the form of subretinal haemorrhage, hard exudate or recent visual loss, the rationale being that occult CNV of lesser activity grades cannot reliably be distinguished from other causes of sub-RPE hyperfluorescence on fluorescein angiography. Resolution of intraretinal and subretinal fluid after PDT may be attributable, theoretically, to one or more of the known consequences of PDT: choriocapillaris photothrombosis, RPE damage or CNV closure.
Comments
CSC is the prototype cause of serous retinal detachment involving the fovea. The symptoms of CSC reflect the loss of contact between the photoreceptors and the RPE (a relative scotoma) and the bullous distension of the foveal retina (metamorphopsia and micropsia). The serous detachment is secondary to focal lesions and pathological leakage through the RPE. The consequent impairment of binocular vision is often the predominant symptom.
The observation of foveal photoreceptor atrophy despite successful reattachment after a duration of symptoms of approximately 4 months suggests that active treatment should commence when the duration exceeds 3 months, given that it will take some time to bring about reattachment. Although the level of evidence for a role of stress is modest, it seems reasonable to counsel the patient about the possible association with stress from the first visit. The history must include current and past use of glucocorticoid medication.
If the patient presents evidence of chronic CSC, despite a short reported duration of symptoms, it suggests that asymptomatic detachment may have been present for longer than the patient has been aware. Patients frequently overlook symptoms if only one eye is involved.
In CSC of recent onset, retinal photocoagulation is usually successful in reducing or eliminating RPE leakage and hence in inducing resolution of the serous detachment. The immediate benefit of treatment is early resolution of subretinal fluid and, in the long term, possibly a reduction of recurrences and RPE degeneration. Photocoagulation should be used with caution because it can induce subretinal neovascularization, often many years after the primary incident.
Chronic CSC, also called diffuse retinal pigment epitheliopathy, presents a more complex pattern of RPE abnormalities. Although severe widespread affection of the RPE may be seen early in the course of the disease, it appears to be associated primarily with unresolving or chronically recurring serous retinal detachment. Indeed, irregular RPE hypo- and hyperpigmentation may be the corollary of photoreceptor atrophy in chronic detachment.
The effect of photocoagulation on chronic CSC is less convincing than in acute CSC. The risk of inducing CNV or, if already present, of inducing conversion to classic CNV of occult CNV merits consideration of photodynamic therapy. PDT can be repeated in CNV, but repeated PDT is rarely if ever needed in chronic CSC without CNV. The long-term efficacy and safety of PDT in CSC have not been described. Systemic medications have no clear role in the treatment of CSC.
Although improved visual acuity and reduction of symptoms, spontaneously or following treatment, supports the theory that subretinal fluid has been resorbed, functional recovery may be incomplete even in the best of cases if the detachment was longstanding. Therefore, OCT is an indispensable tool for the diagnosis and management of all but the simplest cases of CSC.
The mechanism behind the preferential detachment of the fovea by subretinal fluid is unknown. Because the fovea is thinner than the rest of the macula (Fig. 14), higher hydraulic conductivity could render the foveal retina less susceptible to the suction force of the RPE and hence more loosely attached than the surrounding retina.
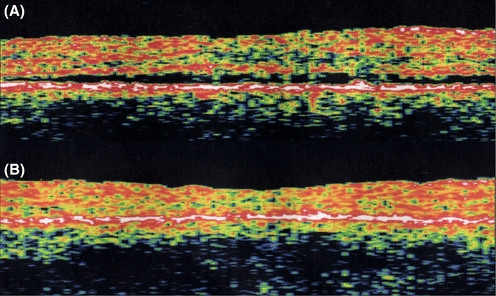
Shallow serous detachment of the fovea (A) demonstrated using optical coherence tomography. An identically located section through the centre of the fovea of the same eye after resolution of the subretinal fluid following extrafoveal photocoagulation (B) shows that the neurosensory retina has not only reattached, but it has also become thinner and the foveal depression has been reformed. Apparently, the relative underhydration that is induced by RPE suction is more pronounced closer to the centre of the fovea. The magnitude of change in hydrostatic pressure in the subretinal space between the two conditions is unknown. The reason for the fovea being underhydrated more effectively may be that its hydraulic conductivity is higher than that of the surrounding retina. This characteristic may also underlie the propensity of the fovea to detach before the surrounding retina in CSC, despite the source of leakage rarely being located under the fovea.
When detached by CSC, the foveal depression is often abolished and general thickening of the detached retina may be seen (Iida et al. 2000). This indicates that the healthy retina is maintained in a physiologically underhydrated state by the suction force generated with RPE pumping of fluid away from the subretinal space. The effect is likely to increase the transparency of the retina, in the same manner that the corneal endothelium keeps the cornea clear (Fig. 14). The loss of RPE suction in the presence of subfoveal RPE or CNV may explain why these conditions can be accompanied by cystoid foveal oedema, in the absence of intraretinal vascular leakage (Iida et al. 2003). Loss of foveal underhydration has optical effects, as shown by the increased brightness of the oedematous retina, presumably a consequence of increased light scatter (Figs 7, 8, 10). This phenomenon must be considered when evaluating psychophysical studies of retinal function in CSC because it will reduce performance for purely optical reasons, even in the absence of any compromise of neuronal function (Springer et al. 2006).
Acknowledgements
This study was supported by the Værn om Synet, Copenhagen, Denmark and by a Patient-Oriented Diabetes Research Career Award from the Juvenile Diabetes Research Foundation (grant number 8-2002-130).
Financial/proprietary interest
The authors or the authors' institutions have been reimbursed for consultancy work for manufacturers of photocoagulation and photodynamic therapy equipment and medications.