RIBOSOMAL RNA GENE DIVERSITY, EFFECTIVE POPULATION SIZE, AND EVOLUTIONARY LONGEVITY IN ASEXUAL GLOMEROMYCOTA
Abstract
Arbuscular mycorrhizal fungi (phylum Glomeromycota) are among the oldest and most successful symbionts of land plants. With no evidence of sexual reproduction, their evolutionary success is inconsistent with the prediction that asexual taxa are vulnerable to extinction due to accumulation of deleterious mutations. To explore why Glomeromycota defy this prediction, we studied ribosomal RNA (rRNA) gene evolution in the Claroideoglomus lineage and estimated effective population size, Ne, in C. etunicatum. We found that rRNA genes of these fungi exhibit unusual and complex patterns of molecular evolution. In C. etunicatum, these patterns can be collectively explained by an unexpectedly large Ne combined with imperfect genome-wide and population-level rRNA gene repeat homogenization. The mutations accumulated in rRNA gene sequences indicate that natural selection is effective at purging deleterious mutations in the Claroideoglomus lineage, which is also consistent with the large Ne of C. etunicatum. We propose that in the near absence of recombination, asexual reproduction involving massively multinucleate spores typical for Glomeromycota is responsible for the improved efficacy of selection relative to drift. We postulate that large effective population sizes contribute to the evolutionary longevity of Glomeromycota.
Sexual reproduction is crucial for keeping the mean fitness of a population high through evolutionary time (Muller 1964). Small asexual populations are thought to be subject to Muller's ratchet, the progressive random loss of genotypes that carry the lowest burden of deleterious mutations and cannot be recreated in the absence of recombination (Muller 1964; Felsenstein 1974). Each loss irreversibly advances the ratchet and reduces mean population fitness (Lynch and Gabriel 1990). The advance of the ratchet is accompanied by rapid fixation of slightly (Ohta 1972) and moderately (Charlesworth and Charlesworth 1997) deleterious mutations as genetic drift is magnified and natural selection is impaired in small populations. The loss of the most fit genotypes together with relentless accumulation of deleterious mutations are expected to result in mutational meltdown and population extinction (Gabriel et al. 1993). Effective population size, Ne, which reflects the efficacy of natural selection relative to genetic drift (Charlesworth 2009), is considered to be a good predictor of population's vulnerability to extinction (Whitlock 2000). Each population is expected to have a critical Ne below which it will, on average, decline in fitness, and above which beneficial and compensatory mutations will keep mean population fitness high (Whitlock 2000; Whitlock et al. 2003). Among the many factors that influence Ne, reproductive mode plays a large role; sexual reproduction tends to increase Ne whereas asexuality decreases it (Orive 1993).
With no evidence of sexual reproduction (Smith and Read 2008) and predominantly clonal population structure (Rosendahl and Taylor 1997; Vandenkoornhuyse et al. 2001; Stukenbrock and Rosendahl 2005; Rosendahl and Matzen 2008; Croll and Sanders 2009; Rosendahl et al. 2009; den Bakker et al. 2010), arbuscular mycorrhizal fungi (AM fungi, phylum Glomeromycota) could be expected to have populations of small Ne, which should put them in danger of Muller's ratchet and extinction. Yet, Glomeromycota have persisted for more than 460 million years (Redecker et al. 2000; Heckman et al. 2001) and are among the most successful symbiotic organisms on the planet. Modern Glomeromycota colonize roots of the majority of land plants, forming arbuscular mycorrhizae in which they trade mineral nutrients from the soil for plant-assimilated carbon (Smith and Read 2008).
Glomeromycota have several unusual features that could be related to their asexual lifestyle, including a puzzling pattern of intraindividual and intraspecific ribosomal RNA (rRNA) gene polymorphism (Stockinger et al. 2010). Eukaryotic genomes typically contain several hundred rRNA gene repeats arranged in one or several tandem arrays (Hillis and Dixon 1991). Despite the large number of rRNA gene repeats, most eukaryotes exhibit very little intraindividual and intraspecific rRNA gene variation (Hillis and Dixon 1991). The homogeneity of the rRNA gene repeats is explained by concerted evolution (Arnheim et al. 1980), in which genome-level processes of unequal crossing over and gene conversion, together with population-level processes of interindividual gene exchange and genetic drift, cause the spread of new sequence variants through the rRNA gene arrays in individual genomes and in the population. By increasing the number of mutant gene copies, concerted evolution exposes each new mutation to selection and thus contributes to its ultimate fixation or elimination from the population (Slatkin 1986; Ohta 1989). Concerted evolution therefore modulates the interplay between mutation accumulation within gene repeats and selection acting on functional regions of each repeat. The amount of neutral polymorphism among rRNA genes depends directly on Ne, the number of gene repeats as well as the relative rates of mutation and sequence homogenization (Ohta 1982; Ohta 2000). Concerted evolution typically generates gene families whose members are more closely related within a species than between species.
In the functional ribosome, rRNAs acquire specific secondary and tertiary structures (Peculis and Greer 1998). Deleterious mutations are expected to compromise these structures and reduce their stability (Lambert and Moran 1998). Thus, the strength of selection experienced by each mutant molecule type is reflected in its structure and stability. From the combination of rRNA structural models and phylogenetic comparisons of the sequence data, the substitutions that accumulate in rRNA gene sequences can be readily identified as mismatch, compensatory, and complementary mutations. Mismatch mutations have detrimental effects on molecule functionality because they disrupt base pairing in double-stranded stem regions, altering the structure of the molecule and lowering its stability (Lambert and Moran 1998). Base pairing can be restored by compensatory mutations in the opposing strand. Complementary mutations convert Watson–Crick base pairs (G–C, A–U) to noncanonical base pairs (e.g., G•U, A•G) or vice versa (Gutell et al. 1994). These mutations may affect functionality by slightly decreasing or increasing the molecule stability, while maintaining its secondary structure.
To explore factors underlying the evolutionary success of Glomeromycota and understand forces responsible for rRNA gene polymorphism in these organisms, we examined patterns of mutation accumulation in rRNA genes in three species of the Claroideoglomus lineage and estimated Ne of the model fungus C. etunicatum by assessing diversity at a collection of noncoding anonymous loci.
Materials and Methods
rRNA GENE SAMPLING, DIVERSITY, AND EVOLUTIONARY HISTORY
We characterized rRNA gene diversity in individual spores (isolates) collected from nine fungal populations representing the Claroideoglomus lineage (Table 1) according to Daniels and Skipper (1982). Total DNA was amplified from individual spores with the illustraTM GenomiPhiTM Version 2 whole genome amplification kit (GE LifeSciences, Piscataway, NJ). GenomiPhiTM products were diluted 1:20 in ddH2O, and 1 μL of this diluted product was used as template in 25 μL polymerase chain reactions (PCRs) consisting of 1.25 U PfuTurbo® HotStart polymerase (Agilent Technologies, Santa Clara, CA), the supplied buffer, 0.4 mM total dNTPs, and 0.2 μM each primer, ITS3 5′-GCATCGATGAAGAACGCAGC-3′ (White et al. 1990) and NDL22 5′-TGGTCCGTGTTTCAAGACG-3′ (van Tuinen et al. 1998) (Fig. 1). Cycling conditions were 95°C for 2 min followed by 13 cycles of 30 s at 95°C, 30 s at 58°C, and 1.5 min at 72°C. Primer and dNTP concentrations as well as cycling conditions (13 cycles) were designed to maximize recovery of low-frequency rRNA gene variants and minimize PCR artifacts (Cline et al. 1996). PCR products were cloned using the TOPO® TA kit for sequencing (Invitrogen, Carlsbad, CA). Clones were randomly picked for plasmid amplification with the illustraTM TempliPhiTM kit (GE LifeSciences), and 1.5 μL of the products used directly in 12 μL sequencing reactions using the BigDye® v3.1 cycle sequencing kit (Applied Biosystems, Foster City, CA). Sequencing reactions were analyzed at the Cornell University Life Sciences Core Laboratory Center on an ABI 3730xl sequencer. We sequenced at least 15 clones from each of 25 spores, resulting in 533 sequences (GenBank accessions HQ856918-HQ857097).
| Species | Populationa | Geographic origin | Number of spores sampled | rRNA gene variantsb | Nucleotide diversity π (SDc) | Indel diversity π(i) |
|---|---|---|---|---|---|---|
| C. etunicatum | AU401Ad | Australia | 3 | 13 | 0.07059 (0.01061) | 0.01487 |
| C. etunicatum | CA-OT135e | USA | 2 | 10 | 0.07289 (0.01521) | 0.01449 |
| C. etunicatum | CL372d | Colombia | 2 | 14 | 0.07813 (0.00650) | 0.01596 |
| C. etunicatum | MR202Ad | Morocco | 3 | 14 | 0.07274 (0.00875) | 0.01447 |
| C. claroideum | BR147Ad | Brazil | 3 | 22 | 0.07096 (0.01097) | 0.01352 |
| C. claroideum | SF119Ad | South Africa | 3 | 13 | 0.06159 (0.01575) | 0.00909 |
| C. claroideum | SW201d | Switzerland | 3 | 19 | 0.07324 (0.01254) | 0.01112 |
| C. luteum | SA112d | Canada | 3 | 22 | 0.06001 (0.01411) | 0.01057 |
| C. luteum | SW202d | Switzerland | 3 | 14 | 0.07933 (0.00929) | 0.01309 |
- aEach population is derived from a specific sampling point and maintained as a live culture.
- bThe number of unique sequences within the population after clone correction. Identical sequences were found in multiple populations.
- cSD, standard deviation.
- dMaintained at the International Culture Collection of Arbuscular Mycorrhizal Fungi (INVAM), West Virginia University, USA.
- eMaintained at the Pawlowska Lab, Cornell University, USA.
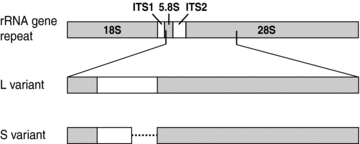
Ribosomal RNA gene repeat organization in Glomeromycota. In eukaryotes, rRNA genes consist of three functional RNA regions (18S, 5.8S, and 28S; gray boxes) separated by internal transcribed spacers 1 and 2 (ITS1, ITS2). PCR amplification of a portion of the rRNA genes in Claroideoglomus revealed two distinct variants: L (long, ∼1100 bp) and S (short, ∼1000 bp) differentiated primarily by a deletion in the 3′ end of the ITS2.
rRNA gene sequences were edited in Sequencher 4.9 (GeneCodes, Ann Arbor, MI) and aligned using MUSCLE (Edgar 2004). All polymorphisms were verified in the original chromatograms. We applied clone correction to sequences recovered from a single spore. Only sequences that differed from others by more than one change, which was attributed to PCR error, were retained in the final alignment. Nucleotide diversity, that is, the average number of nucleotide differences per site between two randomly chosen sequences, π, and insertion/deletion (indel) diversity, π(i), in rRNA gene sequences were estimated using DnaSPv5 (Librado and Rozas 2009). To retain phylogenetic information from indels in the rRNA genes, gaps in the alignment were coded using simple indel coding (Simmons and Ochoterena 2000) implemented by the IndelCoder module of SeqState (Müller 2005; Müller 2006). To determine the relationships of the rRNA gene sequences recovered from Claroideoglomus isolates, we reconstructed their phylogeny using both Bayesian and maximum likelihood (ML) approaches. Bayesian phylogeny reconstructions were carried out in MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003) on the CIPRES portal (Miller et al. 2010) using a general time-reversible model with Γ-distributed rate heterogeneity across sites (GTR+Γ) (Tavaré 1986) for the nucleotide partition. We allowed MrBayes to estimate nucleotide frequencies and substitution rate parameters (Table S1). Rate variation across sites was set to equal and coding was set to variable for the binary (coded gap) partition (Ronquist and Huelsenbeck 2003). All likelihood settings were unlinked between the two partitions (Ronquist and Huelsenbeck 2003). The analysis included two runs of 10,000,000 generations with the burn-in of 2,500,000 generations. We used jModelTest 0.1 (Posada 2008) to determine the appropriate nucleotide substitution model for ML searches (GTR+Γ; Table S1). ML searches and 1000 bootstrap replicates were executed in RAxML 7.2.6 (Stamatakis 2006; Stamatakis et al. 2008).
We reconstructed phylogenetic relationship between the Claroideoglomus rRNA genes and those in other major clades of Glomeromycota using the 5′ end of the 28S rRNA gene (Fig. 1). All characters in the alignment that contained gaps in multiple sequences were removed. Reference taxa were from Stockinger et al. (2009) and Stockinger et al. (2010) (Table S2). Phylogenies were inferred using MrBayes 3.1.2 (Huelsenbeck and Ronquist 2001; Ronquist and Huelsenbeck 2003) in two runs of 6,000,000 generations with the burn-in of 1,500,000 generations, and RAxML 7.2.6 with 1000 bootstrap replicates (Stamatakis 2006; Stamatakis et al. 2008).
We examined the evolution rates of Claroideoglomus rRNA gene variants using the likelihood ratio (LR) relative rates test implemented in r8s version 1.71, rrlike (Sanderson 2003; O’Meara et al. 2006), and Tajima's 1D relative rates tests (Tajima 1993) in MEGA version 4.0 (Tamura et al. 2007). The rrlike algorithm tests the hypothesis that a model with one constant rate of sequence evolution for a clade, which descended from a given most recent common ancestor (MRCA), fits the data as well as a model in which each of the subclades (two or more) that descended from the MRCA have different but constant rates (Sanderson 2003). Tajima's 1D test explores the differences in the rates of evolution of two ingroup sequences by evaluating the number of substitutions at segregating sites between a reference outgroup sequence and the two ingroup sequences (Tajima 1993).
MUTATION ACCUMULATION AND rRNA THERMODYNAMIC STABILITY
Because rRNA secondary and tertiary structure is crucial for rRNA function, we assessed how mutations affected the thermodynamic stability of the structures formed by internal transcribed spacer 2 (ITS2; Fig. 1) and the 5.8S/5′-end of the 28S rRNA regions. We estimated Gibb's free energy change, ΔG, of structures using mfold 2.7 (Walter et al. 1994; Zuker 2003). ΔG is the change in free energy of a folded rRNA molecule compared with the free energy of an unfolded rRNA strand; more negative ΔG indicates a more stable structure. For a given RNA sequence, mfold uses thermodynamic models to identify the secondary structure(s) that minimize ΔG. To analyze folding conformations of the ITS2, sequences were trimmed at the base of the ITS2-proximal stem (van Nues et al. 1995) (Fig. S1) before folding. To analyze the 5.8S and 5′-end of the 28S regions, the 5.8S-ITS2–28S alignment was trimmed to contain only the 5.8S and 28S regions (Fig. S2). We aligned the sequences to Saccharomyces cerevisiae U53879, which allowed us to use the published yeast thermodynamic model (Fields and Gutell 1996) to guide the folding of representative Claroideoglomus sequences. Claroideoglomus sequences and the yeast sequence showed an average divergence, K, (Nei 1987) of 0.22 nucleotide substitutions per site. This level of nucleotide divergence enabled us to finely guide folding of much of the 5.8S and 5′∼340 bp of the 28S regions by dividing the regions predicted to fold into stem loops, folding individual sections, and adding sections back together in VARNA version 3–4 (Darty et al. 2009).
To determine the pattern of accumulation of specific types of mutations and evaluate the selection pressure against deleterious mutations, we identified mismatch, compensatory, and complementary mutations occurring in the stem (double-stranded) regions of rRNA secondary structures from the combination of rRNA secondary structure models and phylogenetic comparisons of the sequence data. The substitutions were mapped onto the secondary structure predictions to determine if they occurred in a stem region or within a loop. For substitutions that occurred in a stem, we compared the effect that the mutation had on the base pairing with its partner and determined whether there had been a mismatch mutation, a compensatory mutation, or a complementary mutation by comparisons with the ancestral Claroideoglomus sequence, which was inferred using the ML method of Yang et al. (1995) implemented in PAML 4.4 (Yang 2007).
Because rRNA gene sequences in the Claroideoglomus lineages existed in the form of two major variants, S and L (Fig. 1), we used a generalized linear mixed model approach to assess the effect of molecule type (S or L) on 28S rRNA secondary structure stability and accumulation of mutations. This allowed us to take into account any random effects on the characteristics of a sequence due to its presence in a particular spore (isolate). We used a normal distribution to model the effect of molecule type on secondary structure stability and a Poisson distribution to model the effect of molecule type on mutation accumulation; a Poisson distribution more accurately describes patterns of mutation accumulation in DNA sequences (Luria and Delbrück 1943). All statistical analyses were done in R 2.12.1 (http://www.r-project.org).
EXPRESSION OF rRNA GENE VARIANTS
Total RNA was extracted from C. etunicatum CA-OT135–4-2 using the RNaqueous Micro Kit (Ambion, Austin, TX). Claroideoglomus etunicatum CA-OT135–4-2 was a single spore in vitro culture established and maintained in a root organ culture according to the protocol of Pawlowska et al. (1999), but with a layer of cellophane (LabSource, Romeoville, IL) separating the roots and the fungus from the medium. This system generates large amounts of genetically homogeneous material (Pawlowska and Taylor 2004). Roots were removed before harvesting the fungus for total RNA extractions. Pre-rRNA transcripts were amplified using the MasterAmpTM RT-PCR Kit for High Sensitivity (Epicentre Biotechnologies, Madison, WI) using either primers ITS3 and NDL22 or ITS3 and 862r 5′-GACACATCGCCACATCGC-3′, a primer specific to S variant rRNA genes.
DISTRIBUTION OF rRNA GENE VARIANTS IN NUCLEI
Glomeromycota spores contain hundreds of nuclei. To assess the distribution of rRNA gene variants within and between nuclei we conducted fluorescence in situ hybridization (FISH) experiments in C. etunicatum CA-OT135–4-2. Details of these experimental procedures are presented in Text S1.
EFFECTIVE POPULATION SIZE IN C. ETUNICATUM
We measured nucleotide diversity, π, and the diversity from the number of segregating sites, θ, at seven anonymous noncoding loci, a1, a2, a3, a7, 4a, 4j, 5f, sampled from 20 isolates representing the Euro-American population of C. etunicatum (AZ201C-1, BR220–1, CA301–1, CA-GT24–1, CA-OT140–31, CL372–1, CU127–41, FL705A-4, IA205–1, KS887–1, MA104–1, MD127–2, MX916B-2, NE102–1, PL118B-1, SP108C-1, TN101B-1, TX104–1, VZ102–2, and WV579A-1). The loci and the isolates were characterized in detail in our earlier study (den Bakker et al. 2010). Briefly, to ascertain that markers are not variable within spore individuals, an important consideration given multinucleate structure of Glomeromycota spores, we cloned their PCR amplicons, sequenced, and analyzed clones from isolates representing several geographic locations (Table S1 in den Bakker et al. 2010). We found no evidence of intraindividual polymorphism. The Euro-American population was defined through an exact test for population differentiation (Raymond and Rousset 1995) involving pairwise comparisons between populations representing different continents (den Bakker et al. 2010). We rejected the null hypothesis that alleles at different loci in this population are in linkage equilibrium by calculating the classical index of association IA (Maynard Smith et al. 1993) and the standardized index of association ISA (Haubold and Hudson 2000). Moreover, using the genetic algorithm recombination detection method (Kosakovsky Pond et al. 2006), which accounts for nucleotide substitution rate heterogeneity that could confound recombination signal, we found no evidence of recombination breakpoints in the alignment of concatenated marker sequences (den Bakker et al. 2010). Each of the Euro-American isolates sampled in the present study represented a subpopulation from a different geographic location (Table 1 in den Bakker et al. 2010). This scattered sampling approach was chosen to best approximate the assumptions of the coalescent model (Lessard and Wakeley 2004; Cutter 2006). DnaSPv5 (Librado and Rozas 2009) was used for diversity estimates, π and θ, and to conduct tests of neutrality, including Tajima's D (Tajima 1989), and Fu and Li's D* and F* tests (Fu and Li 1993) as well as the analysis of the distribution of pairwise genetic differences for concatenated sequence data (Rogers and Harpending 1992). The neutrality tests, in addition to departures from neutrality caused by selection, are expected to detect departures from demographic equilibrium caused by recent population expansion or decline (Simonsen et al. 1995). Episodes of population expansion or decline are also predicted to generate characteristic unimodal distribution of pairwise genetic differences (Rogers and Harpending 1992).
To measure the mutation rate per site per year in C. etunicatum, we estimated: (1) nucleotide divergence K (Nei 1987) at four anonymous noncoding loci (a1, a2, a3, 5f) shared between C. etunicatum and its sister C. claroideum/luteum, and (2) divergence times between species of Glomeromycota. (1) To measure K, we used DnaSPv5 (Librado and Rozas 2009), and included the Euro-American isolates of C. etunicatum together with isolates AU401A-1, KE118–13, MR102A-1, and NB119–17, characterized in den Bakker et al. (2010) as well as C. claroideum/luteum isolates MT109–2, SA112–1, and SW202–1 (GenBank accessions EF627536-EF627538, EF627634-EF627636, EF627722-EF627724, EF641304, and EF641307). (2) Divergence times were determined using BEAST version 1.6.1 (Drummond and Rambaut 2007) from published 18S rRNA gene sequences after aligning them in MUSCLE (Edgar 2004). We used LogCombiner version 1.6.1 to merge four independent MCMC runs of 10,000,000 steps with the burn-in of 1,000,000 steps each under the uncorrelated lognormal relaxed clock model (Drummond et al. 2006), the GTR+I+Γ nucleotide substitution model (Tavaré 1986), and with two calibration points.
As synonymous substitution rates at protein-coding loci are expected to approximate neutral mutation rates (Kasuga et al. 2002), we compared the mutation rate at noncoding anonymous loci of C. etunicatum with synonymous mutation rates among other species of Glomeromycota. We computed the numbers of synonymous and nonsynonymous sites in four published protein-coding gene sequences (alpha-tubulin, beta-tubulin, elongation factor 1-alpha, RNA polymerase II subunit RPB1) using the F3×4 model, and we estimated the synonymous and nonsynonymous substitution rates using the ML method (Goldman and Yang 1994) implemented in the codonml module of PAML version 4.4 (Yang 2007).
To estimate Ne, we sampled the posterior distribution of the coalescent parameter Neτ in a demographic model of the Euro-American population, where τ is the generation length in units of time, using BEAST version 1.6.1 (Drummond and Rambaut 2007). With LogCombiner version 1.6.1 we merged four independent MCMC analyses of 10,000,000 steps with the burn-in of 1,000,000 steps each run under the GTR+I+Γ nucleotide substitution model (Tavaré 1986) and the strict molecular clock. We also obtained point estimates of Ne from π=θ= 2Neμ, where μ is the mutation rate per site per generation.
Results
PATTERNS OF rRNA GENE POLYMORPHISM AND DIVERGENCE IN CLAROIDEOGLOMUS
Although intraindividual variation in rRNA gene sequences has been reported in virtually all species of Glomeromycota, its extent has not been explored or catalogued. To understand the patterns of rRNA gene variation in Glomeromycota, we examined the rRNA gene repeat segment comprising the 5.8S rRNA gene, ITS2, and 5′-end of the 28S rRNA gene in the Claroideoglomus lineage of Glomeromycota (Fig. 1, Table 1). This fragment was PCR amplified from individual spores, cloned, and sequenced. We found 130 unique 5.8S-ITS2–28S rRNA gene sequences in 25 spores from nine globally distributed populations of C. etunicatum, C. claroideum, and C. luteum (Table 1, Fig. 2). Between 4 and 12 unique sequences were present in each individual spore. Each spore contained two distinct rRNA gene types, a long (L) type (∼1100 bp) and a short (S) type (∼1000 bp), which were primarily differentiated by a ∼100 bp indel at the 3′ end of ITS2 (Figs. 1 and 2). Phylogenetic reconstructions showed that the L and S sequences formed two distinct clades; each of these two clades contained sequences from all three species (Fig. 2B, C, Table S1). In both, the L and S clades, rRNA genes of C. etunicatum clustered away from C. claroideum and C. luteum. Sequences from C. claroideum and C. luteum could not be separated from each other with confidence and were therefore examined jointly.
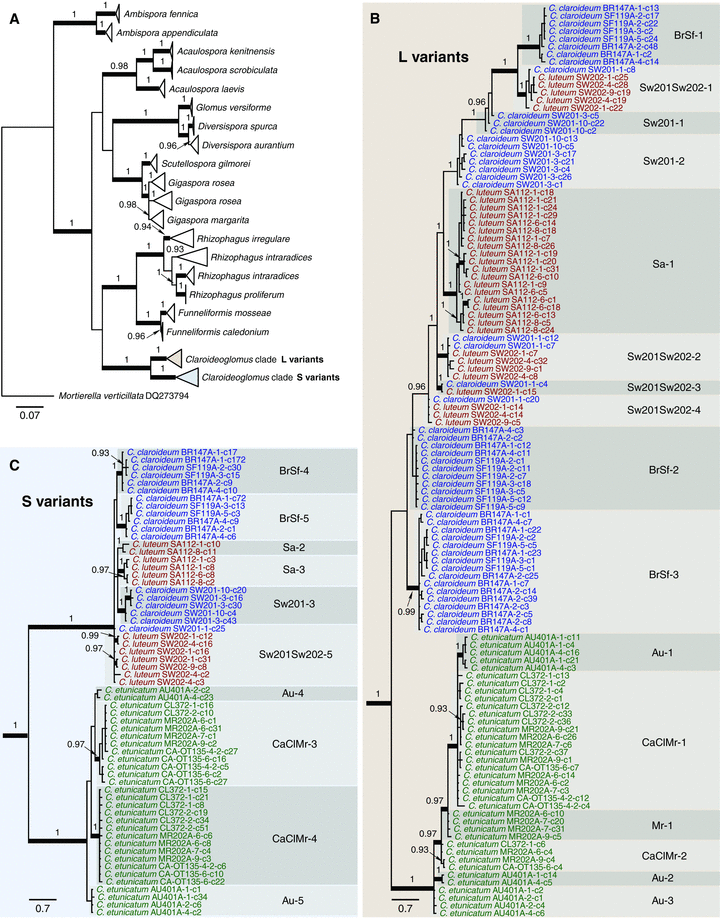
Evolutionary history of rRNA gene variants in Glomeromycota. (A) Relationships among rRNA genes in the Claroideoglomus clade relative to other major clades of Glomeromycota (Table S2) were reconstructed using the 5′-fragment of 28S rRNA gene and are represented by a maximum likelihood tree. Branches with bootstrap support over 70% are thickened, Bayesian posterior probabilities are shown above branches. Terminal branches are collapsed for clarity. (B and C) Phylogenetic relationships of the unique 5.8S-ITS2–28S rRNA gene sequences recovered from individual spores of the Claroideoglomus lineage. A Bayesian tree was bisected into the L variant clade (B) and the S variant clade (C). Posterior probabilities are shown above branches; branches with over 70% maximum likelihood bootstrap support are thickened. Sequence identifiers comprise the isolate name, spore number, and clone number (e.g., AU401A-2-c1 is clone 1 from spore number 2 extracted from experimental population AU401A). Sequences representing C. etunicatum are shown in green, C. claroideum in blue, and C. luteum in red. Nucleotide substitution model parameters are presented in Table S1. Identical rRNA gene sequences were found in multiple spores, both from the same and different populations.
Claroideoglomus etunicatum and C. claroideum/luteum rRNA genes show further differentiation and clustering within the L and S clades (Fig. 2B, C). For example, in C. etunicatum, the rRNA genes from the Australian population (AU401A), which is highly divergent from the remaining globally distributed populations (den Bakker et al. 2010), group away from all other C. etunicatum rRNA genes and are differentiated into five distinct subclades, Au-1, Au-2, Au-3, Au-4, and Au-5 (Fig. 2B, C). In contrast, the rRNA genes from the California (CA-OT135), Colombia (CL372), and Morocco (MR202A) populations cluster together while still differentiating into several subclades, including CaClMr-1, CaClMr-2, CaClMr-3, and CaClMr-4, with some of the Moroccan sequences (Mr-1) separating from the Californian and Colombian sequences (Fig. 2B, C). Clustering of sequences from the American and African isolates is unexpected because the American population is significantly differentiated from the African population (den Bakker et al. 2010). A similar pattern of multiple rRNA gene polymorphisms present in individual isolates and often shared by geographically distinct populations is also noticeable in the C. claroideum/luteum clade (Fig. 2B, C). Such sharing of polymorphisms by geographically distinct populations suggests that the divergent sequence variants are genetically linked.
To infer the history of rRNA genes in the Claroideoglomus lineage in the context of evolution of all Glomeromycota, we included in our phylogeny GenBank sequences representing intraisolate rRNA gene variation in other major Glomeromycota lineages (Stockinger et al. 2009; Stockinger et al. 2010). This reconstruction revealed that rRNA genes sampled from the Claroideoglomus lineage are specific to this lineage (Fig. 2A, Table S1). Such pattern of separation is consistent with the birth of the 5.8S-ITS2–28S rRNA gene length polymorphism in the MRCA of the Claroideoglomus lineage. We next tested whether the L and S 28S rRNA genes have evolved at similar rates since their divergence using LR (Sanderson 2003; O’Meara et al. 2006) and Tajima's 1D (Tajima 1993) relative rates tests. The LR test showed a significant difference in the evolution rate of the L and S clades (LR = 407.02, P < 0.001, df = 3). Representative Tajima's 1D tests corroborated this result (Table 2). In contrast, tests between sequences from the same clade were not significant (Table 2). Altogether, these results indicate that L and S gene clusters experienced distinct evolutionary histories characterized by different rates of nucleotide substitutions.
| Outgroup sequence | Ingroup sequencesa | χ2 | P-value | |
|---|---|---|---|---|
| R. irregulare FM865586 | AU401A-1-c11 (L) | AU401A-1-c1 (S) | 10.67 | 0.00109 |
| R. irregulare FM865586 | AU401A-1-c14 (L) | CL372-2-c34 (S) | 13.75 | 0.00021 |
| R. irregulare FM865586 | CL372-1-c13 (L) | CL372-1-c8 (S) | 12.07 | 0.00051 |
| R. irregulare FM865586 | BR147A-1-c1 (L) | BR147A-1-c17 (S) | 9.92 | 0.00163 |
| R. irregulare FM865586 | BR147A-1-c13 (L) | BR147A-1-c72 (S) | 10.90 | 0.00096 |
| R. irregulare FM865586 | SW201-10-c2 (L) | SW201-10-c20 (S) | 8.97 | 0.00275 |
| R. irregulare FM865586 | SW201-1-c8 (L) | SW201-1-c25 (S) | 10.96 | 0.00093 |
| R. irregulare FM865586 | SA112-1-c18 (L) | SA112-1-c8 (S) | 11.27 | 0.00079 |
| F. mosseae FN547474 | AU401A-1-c11 (L) | AU401A-1-c1 (S) | 13.00 | 0.00031 |
| F. mosseae FN547474 | AU401A-1-c14 (L) | CL372-2-c34 (S) | 14.29 | 0.00016 |
| F. mosseae FN547474 | CL372-1-c13 (L) | CL372-1-c8 (S) | 14.52 | 0.00014 |
| F. mosseae FN547474 | BR147A-1-c1 (L) | BR147A-1-c17 (S) | 9.28 | 0.00232 |
| F. mosseae FN547474 | BR147A-1-c13 (L) | BR147A-1-c72 (S) | 12.07 | 0.00051 |
| F. mosseae FN547474 | SW201-10-c2 (L) | SW201-10-c20 (S) | 9.62 | 0.00193 |
| F. mosseae FN547474 | SW201-1-c8 (L) | SW201-1-c25 (S) | 11.08 | 0.00087 |
| F. mosseae FN547474 | SA112-1-c18 (L) | SA112-1-c8 (S) | 8.64 | 0.00328 |
| F. mosseae FN547474 | AU401A-1-c11 (L) | AU401A-4-c6 (L) | 0.33 | 0.56370 |
| F. mosseae FN547474 | BR147A-1-c13 (L) | BR147A-1-c1 (L) | 1.80 | 0.17971 |
| F. mosseae FN547474 | SW201–10-c2 (L) | SW201–1-c8 (L) | 0.20 | 0.65472 |
| F. mosseae FN547474 | SA112–1-c10 (S) | SA112–1-c8 (S) | 0.20 | 0.65472 |
| F. mosseae FN547474 | CL372–1-c16 (S) | CL372–1-c15 (S) | 2.00 | 0.15730 |
| F. mosseae FN547474 | OT135–6-c10 (S) | OT135–4-2–6-c2 (S) | 2.00 | 0.15730 |
- aSequence type is indicated in parentheses.
MUTATION ACCUMULATION PATTERNS IN rRNA GENES
To understand how mutations differentiating the L and S gene variants affect function of the rRNA molecules, we explored the patterns of mutation accumulation in different functional regions of these two sequence types using several approaches.
Folding conformations of ITS2
The secondary structure of ITS2 is important for pre-rRNA processing and maturation (Peculis and Greer 1998) and, in contrast to the nucleotide sequence, is well conserved in all eukaryotes (Schultz et al. 2005). To investigate the functional consequences of the large indel at the 3′-end of ITS2 (Fig. 1) in L and S rRNA gene variant, we modeled secondary structures of the ITS2 region using mfold 2.7 (Zuker 2003). The L variant ITS2 were predicted to assume conformations typical of eukaryotic molecules and show all pre-RNA processing hallmarks that have been identified in Saccharomyces cerevisiae (Côté and Peculis 2001) (Fig. S1). In contrast, the S variant ITS2 were found to acquire conformations of largely reduced complexity (Fig. S1, Table S3). Nevertheless, despite their altered conformations, the S structures retain the ITS2 proximal stem, which in yeast is known to be critical for transcript processing (Peculis and Greer 1998; Côté and Peculis 2001).
Levels of nucleotide conservation along the 28S rRNA gene sequence
Comparative studies of eukaryotic 28S rRNA gene sequences revealed that the level of sequence conservation varies along the molecule (Schnare et al. 1996). Regions that map to the ribosome functional sites display high degree of primary sequence and secondary structure conservation whereas regions that map to the surface of the ribosome show variability in both size and structure. In addition to divergent ITS2 regions, Claroideoglomus L and S variants are differentiated by multiple nucleotide polymorphisms and small indels along the 28S rRNA gene. To assess whether these polymorphisms are contained in specific variable regions or evenly distributed along the molecules, we computed nucleotide diversity, π, separately in L and S variants of C. etunicatum and C. claroideum/luteum using a sliding window approach (Fig. 3). We found that both L and S variants exhibit low π values in the same sequence regions. These regions are also among the most highly conserved regions across eukaryotes (Schnare et al. 1996). The π data suggest that functional constraints modulate mutation accumulation in both L and S variants, which, in turn, indicates that the S variants are not pseudogenes that accrue mutations in a random fashion.
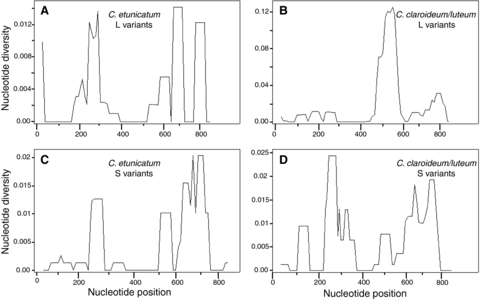
Distribution of sequence polymorphism across the 28S rRNA gene fragments in C. etunicatum and C. claroideum/luteum. The alignment of 180 sequences (Table 1) used to generate Figure 2B, C was trimmed to contain only the 28S region. Nucleotide diversity was calculated using a sliding window 50 bp wide and a 10 bp step length. “Nucleotide position” is the position of that nucleotide in the alignment of all 180 sequences, allowing the comparison of homologous regions of the 28S rRNA gene while ignoring indels. Regions of high π indicate highly variable regions. (A) and (B) show π estimates using all C. etunicatum and C. claroideum/luteum L variant sequences, respectively. (C) and (D) show π estimates using all C. etunicatum and C. claroideum/luteum S variant sequences, respectively.
Structural stability of the 5.8S/28S rRNAs
Most deleterious mutations in rRNA genes are expected to destabilize structures of rRNA molecules (Lambert and Moran 1998). To determine the effects of polymorphisms in the L and S rRNA genes on rRNA molecule stability, we modeled the secondary structure of the 5.8S and 5′∼340 bp of the 28S rRNA gene products using mfold 2.7 (Zuker 2003). We found that these 5.8S/28S structures were highly similar between the L and S variants in terms of their topology, including the number and position of hairpin structures (Fig. S2). The region that differs most between the L and S 28S rRNA structures is one of the molecule regions that is most variable across eukaryotes (Schnare et al. 1996) (Fig. S2). The S variants contain in this region one or two small insertions (4 or 11 bp) relative to the L variants (Fig. S2). Despite their overall structural similarity, the S variant 5.8S/28S structures are significantly more stable than the L variant structures in both C. etunicatum (two-sample t-test, t60= 24.1, P < 0.0001) and C. claroideum/luteum (two-sample t-test, t35= 5.92, P < 0.0001) (Table S4). This improved stability of S variant structures cannot be attributed to indels that form base pairing interactions absent from the L variant structures, because the S variants are significantly more stable than the L variants even after removal of these additional base pairs (Fig. S2).
Types of mutations in the 5.8S/28S rRNA stem regions
Selection pressure against deleterious mutations can be evaluated by assessing the patterns of mutation accumulation in the stem regions of rRNA molecules (Lambert and Moran 1998). To understand selective forces acting on the L and S molecules, we examined whether nucleotide substitutions in the 5.8S/28S stem structures resulted in mismatch, compensatory, or complementary changes relative to the ancestral sequence of both variant types (Fig. S2, Table S4). Nearly all of the mutations accumulated by S variant rRNAs occurred at exactly the same positions within the predicted S rRNA structures, indicating that they arose early in S variant evolution. In C. etunicatum, the molecule type (L or S) was a significant predictor of the number of mismatch (z= 5.543, P < 0.0001), compensatory (z= 2.000, P= 0.0455), and complementary mutations (z= 31.024, P < 0.0001). In C. claroideum/luteum, the molecule type was only a predictor of the number of mismatch (z= 5.545, P < 0.0001) and complementary mutations (z= 38.52, P < 0.0001) but not of compensatory mutations (z= 0.883, P= 0.377), a result likely caused by the very small number of compensatory mutations in both S and L variant sequences (Table S4). In both taxa, S variants accumulated significantly more mismatch (C. etunicatum t58.5= 15.3, P < 0.0001; C. claroideum/luteum t31= 22.3, P < 0.0001), complementary (C. etunicatum t64= 17.6, P < 0.0001; C. claroideum/luteum t33.3= 28.4, P < 0.0001), and compensatory (C. etunicatum t41= 12.0, P < 0.0001; C. claroideum/luteum t31= 14.6, P < 0.0001) mutations than L variants.
Overall, the patterns of mutation accumulation in S variant genes of the Claroideoglomus lineage indicate that deleterious mutation buildup is effectively counteracted. In spite of the aberrant folding of the S variant ITS2, the accumulation of complementary and compensatory mutations in the 28S rRNA gene sequences suggests that selection has a relatively strong effect on S variant evolution. The resulting S variant 28S rRNA secondary structure is significantly more stable than the inferred ancestral sequence or the L variant structure.
EXPRESSION OF rRNA GENE VARIANTS
The patterns of mutation accumulation in the L and S variant rRNA genes throughout the Claroideoglomus lineage suggested that both variants are functional. To provide more evidence for this claim, we examined expression of the rRNA genes in the C. etunicatum isolate CA-OT135–4-2. Reverse transcriptase-PCR of both L and S variant pre-rRNA transcripts followed by cloning and sequencing of the products showed that both variants are transcribed and their transcripts are stable. We confirmed this result by targeted amplification of the S type transcripts using primers specific to S variant pre-rRNAs (Fig. S3).
DISTRIBUTION OF rRNA GENE VARIANTS IN NUCLEI
Our phylogenetic reconstructions and transcript analyses suggested that both the L and S variants existed in the genome in high copy number. To examine how these multiple copies are organized among and within nuclei, we performed FISH experiments using probes designed to differentiate between the L and the S 28S rRNA genes in C. etunicatum CA-OT135–4-2. Spores, which were crushed to release nuclei, and intact hyphal fragments were embedded in polyacrylamide pads to preserve three-dimensional structures of the nuclei. Both probes hybridized to chromatin in every examined nucleus (n = 310; Fig. 4). In spores, which contain spherical nuclei, the probes localized to a centrally located area of the nucleus (Fig. 4D–F). The probes also showed a pattern of distinct co-localization in hyphae nuclei, which assume various shapes (Fig. 4G–I). Three-dimensional image reconstructions revealed that the L and S variant signals were close to each other but were spatially separated (Movie S1). Overall, the FISH data suggest that the L and S rRNA gene arrays, while associated with the same nucleoli, have a disjunct organization. Such organization is likely to impair unequal crossing over and gene conversion, which are normally responsible for rRNA gene homogenization (Seperack et al. 1988).

L and S variant rRNA genes co-occur within Claroideoglomus etunicatum nuclei. FISH specifically targeting L and S variant rRNA genes was carried out with ∼300 bp long probes targeting a region of the 28S rRNA gene exhibiting ∼30% sequence divergence between the variants. (A–C) S and L variant probe localization in spore nuclei surrounded by cytoplasm residue. Signal from both probes is visible above background levels without image correction. Scale bar 5 μm. (D–F) Three-dimensional reconstructions show that S and L variant probes target distinct domains of the nucleus (Movie S1). Scale bar 1 μm. (G–I) Nuclei within hyphae display a pattern of L and S probe hybridization similar to the pattern apparent in spore nuclei despite strikingly different nuclear morphologies. Scale bar 5 μm. Green, L variant probe; red, S variant probe; blue, DAPI.
EFFECTIVE POPULATION SIZE IN C. ETUNICATUM
Effective population size, Ne, influences the degree of identity among the rRNA gene copies (Ohta 2000) and reflects the efficacy of selection relative to drift (Charlesworth 2009). To understand forces that shape rRNA gene diversity in Glomeromycota, we estimated Ne in the Euro-American population of C. etunicatum (den Bakker et al. 2010). We measured π= 0.00192 and θ= 0.00235 in seven randomly selected anonymous noncoding single-copy genomic fragments of a total length of 2538 bp sampled from 20 isolates (Fig. 5, Table S1) characterized in detail in our earlier study (den Bakker et al. 2010). Tests of neutrality, including Tajima's D (Tajima 1989) as well as the Fu and Li's D* and F* (Fu and Li 1993), did not reveal any significant departures from the neutral equilibrium model (Table 3). Similarly, the distribution of observed pairwise differences did not show a signature wave pattern expected in a population with a history of expansion or decline (Rogers and Harpending 1992; Rosendahl et al. 2009). Instead, the empirical mismatch distribution was characterized by multiple peaks expected in equilibrium populations (Fig. 6).
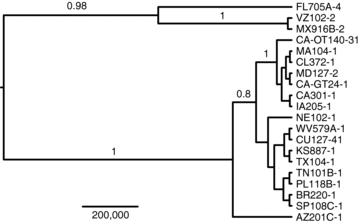
Relationships among the Euro-American isolates of C. etunicatum reconstructed using seven anonymous noncoding loci characterized by den Bakker et al. (2010). Bayesian posterior probabilities are shown above branches. Scale bar represents time in years. Nucleotide substitution model parameters are presented in Table S1.
| Locus | S | h | H d | π (SD) | θ (SD) | Tajima's D | Fu and Li's D* | Fu and Li's F* |
|---|---|---|---|---|---|---|---|---|
| a1 | 2 | 3 | 0.637 | 0.00194 (0.00030) | 0.00146 (0.00109) | 0.76374 | 0.86615 | 0.96211 |
| (P>0.1) | (P>0.1) | (P>0.1) | ||||||
| a2 | 1 | 2 | 0.268 | 0.00097 (0.00041) | 0.00101 (0.00101) | −0.08610 | 0.64952 | 0.52031 |
| (P>0.1) | (P>0.1) | (P>0.1) | ||||||
| a3 | 6 | 4 | 0.363 | 0.00320 (0.00127) | 0.00425 (0.00217) | −0.78678 | −0.15415 | −0.38373 |
| (P>0.1) | (P>0.1) | (P>0.1) | ||||||
| a7 | 1 | 2 | 0.337 | 0.00102 (0.00033) | 0.00085 (0.00085) | 0.35195 | 0.64952 | 0.65294 |
| (P>0.1) | (P>0.1) | (P>0.1) | ||||||
| 4a | 8 | 4 | 0.363 | 0.00358 (0.00173) | 0.00605 (0.00286) | −1.37225 | 0.21232 | −0.27741 |
| (P>0.1) | (P>0.1) | (P>0.1) | ||||||
| 4j | 1 | 2 | 0.268 | 0.00070 (0.00029) | 0.00073 (0.00073) | −0.08610 | 0.64952 | 0.52031 |
| (P>0.1) | (P>0.1) | (P>0.1) | ||||||
| 5f | 2 | 2 | 0.268 | 0.00201 (0.00085) | 0.00211 (0.00157) | −0.11187 | 0.86615 | 0.69109 |
| (P>0.1) | (P>0.1) | (P>0.1) |
- S, segregating sites; h, haplotypes; Hd, haplotype diversity; π, nucleotide diversity; θ, diversity from the number of segregating sites; SD, standard deviation.
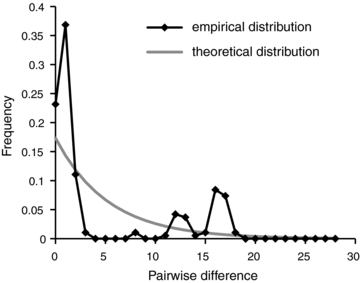
Distribution of pairwise genetic differences in the Euro-American population of C. etunicatum is characterized by many peaks expected in an equilibrium population.
Because the mutation rate in C. etunicatum was not known, we determined the neutral mutation rate by measuring the nucleotide divergence K between C. etunicatum and C. claroideum/luteum at four anonymous noncoding genomic loci that could be sampled from both taxa (Table 4), and by estimating the between-species divergence time from the 18S rRNA gene phylogeny (Fig. 7, Table S1). The 18S rRNA gene phylogeny was reconstructed under the uncorrelated lognormal clock model (Drummond et al. 2006) with two calibration points: the 396 ± 12 million year old fossils marking the Scutellospora radiation (Dotzler et al. 2006), and the divergence of moss and vascular plants 700 ± 35 million years ago marking the upper bound of the Glomeromycota radiation (Heckman et al. 2001). Given the average divergence K= 0.12351 (Table 4) and the divergence time of 25.9 million years between C. etunicatum and C. claroideum (Fig. 7), the average neutral mutation rate is 2.38 × 10−9± standard error (SE) of 0.85 × 10−9 per site per year. This value is comparable to the synonymous substitution rates estimated at four independent protein-coding loci (alpha-tubulin, beta-tubulin, elongation factor 1-alpha, RNA polymerase II subunit RPB1) sampled across Glomeromycota (Table S5). The synonymous substitution rates in Glomeromycota ranged from 0.24 × 10−9 to 2.34 × 10−9 per site per year and were similar to the ones measured in other fungi (0.9 × 10−9–16.7 × 10−9; Kasuga et al. 2002).
| Locus | K |
|---|---|
| a1 | 0.06667 |
| a2 | 0.22712 |
| a3 | 0.13486 |
| 5f | 0.06538 |
| Average | 0.12351 |
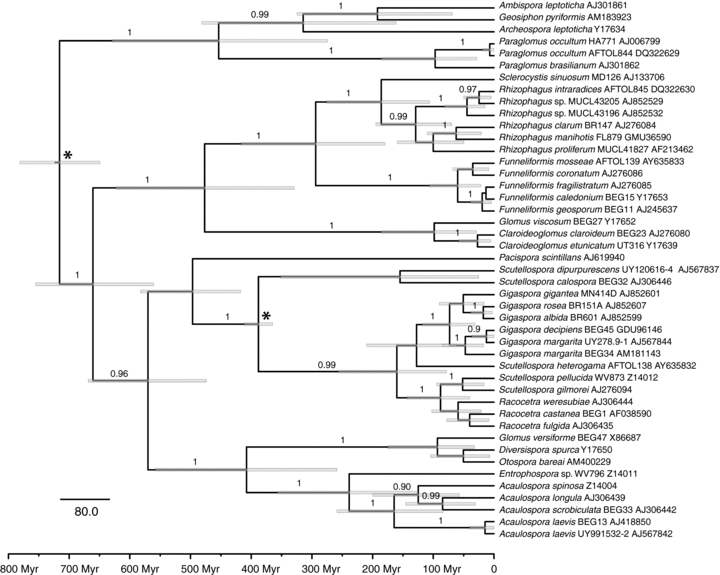
The 18S rRNA gene phylogeny of Glomeromycota with the combined posterior distribution of the divergence times estimated under the uncorrelated lognormal relaxed clock model. Bayesian posterior probabilities are shown above branches; the gray bars represent the mean 95% highest posterior densities; the nodes with normally distributed calibration priors are marked by asterisks. Nucleotide substitution model parameters are presented in Table S1.
Given the neutral mutation rate of 2.38 × 10−9 per site per year and a generation time of 1 year, the coalescent demographic model of the Euro-American population of C. etunicatum constructed under the assumption of constant population size (Fig. 5) generated the mean value of Ne posterior distribution equal to 428,000 with the lower bound of the 95% highest posterior density (HPD) interval at 167,000 and the upper bound of the 95% HPD interval at 750,000. The diversity estimates π and θ (Table 3) yielded Ne(π) = 402,000 with a confidence interval of 296,000–625,000 based on SE of the neutral mutation rate, and Ne(θ) = 493,000 (363,000–767,000).
The Ne estimates suggest that, while smaller than populations of many fungi (Fisher 2007; Ellison et al. 2011), the C. etunicatum population is relatively large and similar in size to populations of Daphnia magna, a partially asexual crustacean with Ne of ∼500,000 (Haag et al. 2009), Mus musculus castaneus, a wild mouse with Ne of ∼580,000 (Halligan et al. 2010), and Capsella grandiflora, an obligately outcrossing cruciferous plant with Ne of ∼500,000 (Slotte et al. 2010). In contrast, populations of many sexual fungi can be considerably larger, reaching Ne of ∼107 (Fisher 2007).
Discussion
PATTERNS OF rRNA GENE EVOLUTION IN GLOMEROMYCOTA
Most eukaryotic species, other than interspecific hybrids (Hillis et al. 1991), harbor uniform sets of rRNA genes (Hillis and Dixon 1991). Glomeromycota are unique in that they exhibit extensive intraindividual rRNA gene sequence variation (Stockinger et al. 2010). Species in the Claroideoglomus lineage show three distinct patterns of rRNA gene variation: (1) ancestral polymorphism, (2) concerted evolution, and (3) imperfect homogenization of minor intraspecific polymorphisms. Our data indicate that several different processes are collectively responsible for generation of these patterns.
Ancestral polymorphism
Based on phylogeny reconstructions and the mutations that differentiate the extant S and L rRNA genes, we hypothesize that the ancestral S gene was generated by a deletion in the ancestor of the Claroideoglomus lineage and proliferated in the whole lineage in the absence of homogenization with the ancestral L gene copies. The lack of homogenization between the two variant types can be attributed to their disjunct organization in interphase nuclei. Based on this observation, we speculate that the event that led to the origin of the S gene involved a transposition or a translocation of a part of the ancestral L array, or perhaps even a whole-genome duplication event. Spatial separation of the rRNA gene arrays during interphase would be expected to impair unequal crossing over and gene conversions, the processes driving concerted evolution (Seperack et al. 1988). In the absence of homogenization with the L variants, the S variants evolved along their own evolutionary trajectory at a rate that is significantly accelerated relative to the L variants, yet constant within the S variant group. The large number of shared mutations among the S variants and the long branch separating the S and L variant clades suggest that the different rates of evolution of the L and S variants are due to an initial burst of mutation accumulation at the time of the S variant origin or soon thereafter. The mutations that spread in the S variants do not seem to compromise their 28S rRNA secondary structure stabilities. These mutations include compensatory and complementary nucleotide substitutions, which are expected to preserve the functionality of the S molecules. Such mutation pattern indicates that selection acting on the S variants is rather effective against deleterious mutations, which is consistent with the large Ne of C. etunicatum. Consequently, we speculate that the S gene copies have been retained throughout the evolutionary history of the lineage because of selective pressure to maintain the S molecules; they may satisfy the basic need for a minimal number of ribosomes required for metabolism (Ohno 1970).
Concerted evolution
Although there is no rRNA gene homogenization between the L and S gene clusters, patterns consistent with concerted evolution are evident within each of these two clusters in C. etunicatum and C. claroideum/luteum. A similar pattern of largely concerted evolution is also apparent in many other species of Glomeromycota (Stockinger et al. 2010). We believe that DNA repair mechanisms of gene conversion and unequal crossing over explain this pattern (Tsang and Carr 2008; Eckert-Boulet and Lisby 2009). DNA break repair favors local regions of sister chromatids as templates for repair (Eckert-Boulet and Lisby 2009). In C. etunicatum, the lack of interactions between the L and S gene clusters combined with imperfect within-cluster homogenization is consistent with DNA repair on a local scale.
Imperfect homogenization
The existence of minor intraindividual/intraspecific rRNA gene polymorphisms suggests that rRNA gene homogenization is highly localized and limited to neighboring gene copies. This pattern can be best explained by the combination of a large Ne and a low rate of homogenization relative to the mutation rate (Ohta 2000) (Fig. 8). An alternative scenario of minor polymorphisms being distributed among different nuclei (Kuhn et al. 2001; Hijri and Sanders 2005) is unlikely because these polymorphisms are often shared by geographically differentiated populations (e.g., C. etunicatum CaClMr-1 and CaClMr-2 variants; Fig. 2), which is consistent with genetic linkage that can occur between gene copies but would not be expected between different nuclei. In the clonal population of C. etunicatum (den Bakker et al. 2010), the apparent absence of meiotic crossing over and chromosome reassortment eliminates the potential for population-level homogenization (Nagylaki 1984). If the rate of homogenization is not sufficient to outpace the rate of mutation, a pattern of neutral rRNA gene sequence polymorphisms is expected to emerge in a population due to genetic drift (Nagylaki 1984; Seperack et al. 1988), which is precisely what we observe in C. etunicatum (Fig. 8).
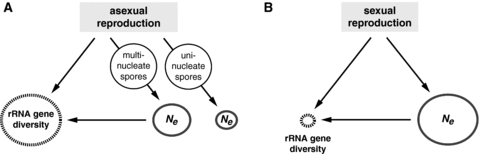
Magnitudes of Ne and rRNA gene diversity expected in populations with different reproductive modes. Under asexual reproduction (A), Ne is predicted to be lower than under sexual reproduction (B). Ribosomal RNA gene diversity is expected to be higher in asexual populations with a moderate Ne (A) than in sexual populations with a large Ne (B). In asexual populations (A), the sporogenesis mode, combined with multilevel selection, is thought to affect the efficacy of selection against deleterious mutations with multinucleate spores being more effective than the uninucleate ones.
EFFECTIVE POPULATION SIZE IN C. ETUNICATUM IS A PRODUCT OF MULTILEVEL SELECTION
Our Ne estimates suggest that the C. etunicatum population is relatively large and similar in size to obligately outcrossing populations of the wild mouse M. m. castaneus (Halligan et al. 2010) and the crucifer C. grandiflora (Slotte et al. 2010). Both, M. m. castaneus and C. grandiflora show evidence of efficient positive and purifying selection (Halligan et al. 2010; Slotte et al. 2010). Given the clonal structure of the C. etunicatum population (den Bakker et al. 2010), Ne of such magnitude suggests that, in the absence of sexual recombination, this organism must have other mechanisms that reduce accumulation of slightly and moderately deleterious mutations (Fig. 8). For example, Glomeromycota possess a unique mode of asexual sporogenesis that could be responsible for the improved efficacy of selection against deleterious mutations in their asexual populations (Jany and Pawlowska 2010). Glomeromycota spores, unlike propagules of most other eukaryotes, harbor hundreds of nuclei. In other eukaryotes, propagules are uninucleate, which is believed to facilitate resolution of within-organismal conflicts generated by mutations that accumulate during a lifetime of a multicellular organism (Bell and Koufopanou 1991; Roze and Michod 2001). In Glomeromycota, live microscopy observations revealed that spore primorida are populated by influx of a stream of random nuclei from the surrounding aseptate mycelium (Jany and Pawlowska 2010). Moreover, the nuclei with damaged DNA are selectively eliminated from the mycelium by a regulated process consistent with programmed death in response to deleterious mutations. Quantification of the collective effects of these nuclear dynamics on the population mutation load revealed that for uniformly deleterious mutations, that is, mutations that reduce both the mutant nucleus replication rate and the organismal fitness, multinucleate propagules are expected to surpass uninucleate propagules in the ability to eliminate mutations (Jany and Pawlowska 2010). Consequently, we believe that the mode of sporogenesis combined with selection acting at the level of individual nuclei (Jany and Pawlowska 2010) may be a key factor increasing the efficacy of selection against deleterious mutations and contributing to the relatively large Ne of C. etunicatum (Fig. 8).
IS MULLER'S RATCHET A THREAT TO GLOMEROMYCOTA?
There are no known sexual taxa in Glomeromycota (Smith and Read 2008). Fossil records indicate that Glomeromycota existed as a diversified clade as early as 400 million years ago (Redecker et al. 2000; Dotzler et al. 2006; Dotzler et al. 2009). Therefore, it is unlikely that these organisms lost sexual reproduction during their recent evolutionary history and Muller's ratchet is only starting to operate in their populations. Instead, we believe that Glomeromycota have been asexual for an extended evolutionary time and their vulnerability to extinction is reduced thanks to relatively large effective population sizes.
Muller's ratchet is believed to be a problem even in moderately sized populations such as self-fertilizing hermaphrodite worms Caenorhabditis elegans, which have Ne of ∼80,000 and experience impaired selection against mildly deleterious mutations (Loewe and Cutter 2008). The magnitude of Muller's ratchet threat in the relatively large population of C. etunicatum is uncertain. Evidence of functional constrains in mutation accumulation in rRNA gene sequences suggests that C. etunicatum experiences rather effective elimination of deleterious mutations. Moreover, compensatory mutations, which restore fitness losses inflicted by past mutations, are apparent in C. etunicatum's rRNA gene sequences. This evidence of compensatory evolution is important for understanding the fate of AM fungal populations because both theoretical (Wagner and Gabriel 1990; Poon and Otto 2000) and empirical studies (Burch and Chao 1999; Estes and Lynch 2003; Estes et al. 2011) indicate that compensatory mutations can decelerate the advance of Muller's ratchet.
Like other filamentous fungi, Glomeromycota occasionally engage in vegetative hyphal fusions that can mediate gene exchanges (Croll et al. 2009). It is also possible that a yet undiscovered sexual process operates in Glomeromycota and is controlled by the meiotic genes present in these fungi (Halary et al. 2011). Nevertheless, with few exceptions (Vandenkoornhuyse et al. 2001; Croll and Sanders 2009), populations of Glomeromycota surveyed to date display significant linkage disequilibrium (Rosendahl and Taylor 1997; Stukenbrock and Rosendahl 2005; Rosendahl and Matzen 2008; Rosendahl et al. 2009; den Bakker et al. 2010). Evidence of linkage disequilibrium is not inconsistent with occurrence of rare recombination events in the population history (Maynard Smith et al. 1993). For example, the global population of C. etunicatum is overwhelmingly clonal although a pattern of homoplasy consistent with history of rare recombination is discernible in the phylogeny of the C. etunicatum lineage (den Bakker et al. 2010). Theory predicts that Muller's ratchet can be arrested by recreating mutation-free genotypes through recombination if the product of the recombination rate and population size achieves a numerical value of about 106 (Bell 1988). Consequently, small populations require recombination rates associated with nearly obligate sexual reproduction to ensure their long-term survival whereas in large populations, rare recombination events may be sufficient to halt the ratchet (Bell 1988; Charlesworth et al. 1993). Given this prediction, rare cryptic recombination may contribute to arresting the ratchet in the sizable population of C. etunicatum.
Our model species, C. etunicatum, is likely not the only AM fungus with a relatively large Ne. A recent study in Funneliformis mosseae, another globally distributed AM fungus with a clonal population structure, revealed levels of diversity (Rosendahl et al. 2009) that are also consistent with a sizeable Ne.
Conclusion
To explore whether asexual Glomeromycota can avoid mutational meltdowns, we studied evolution of rRNA genes in the Claroideoglomus lineage and estimated the effective population size in C. etunicatum. We found that natural selection plays a significant role in the C. etunicatum population, which is consistent with C. etunicatum effective population size being relatively large for an asexual organism. The intraindividual and intraspecific rRNA gene polymorphisms typical for Glomeromycota can be attributed to inability of concerted evolution to keep up with mutation in a population of a large effective size and in the apparent absence of meiotic population-level homogenization. We postulate that a large effective size of Glomeromycota populations minimizes the threat of Muller's ratchet and contributes to their long-term evolutionary persistence under conditions of infrequent gene exchanges.
Associate Editor: A. Cutter
ACKNOWLEDGMENTS
We thank M. Milgroom, S. Mondo, and J. Taylor for comments on the earlier version of this manuscript. We are grateful to A. Cutter and two anonymous reviewers for their insightful suggestions. We also thank D. Douds for the gift of Ri T-DNA-transformed carrot roots developed by G. Bécard. This project was supported by National Science Foundation grant MCB-0538363 (to TEP) and by S. Ann & Robert R. Morley Student Research Grant (to NWV).




