THE EVOLUTION OF SPECIFICITY IN EVOLVING AND COEVOLVING ANTAGONISTIC INTERACTIONS BETWEEN A BACTERIA AND ITS PHAGE
Abstract
The evolution of exploitative specificity can be influenced by environmental variability in space and time and the intensity of trade-offs. Coevolution, the process of reciprocal adaptation in two or more species, can produce variability in host exploitation and as such potentially drive patterns in host and parasite specificity. We employed the bacterium Pseudomonas fluorescens SBW25 and its DNA phage Φ2 to investigate the role of coevolution in the evolution of phage infectivity range and its relation with phage growth rate. At the phage population level, coevolution led to the evolution of broader infectivity range, but without an associated decrease in phage growth rate relative to the ancestor, whereas phage evolution in the absence of bacterial evolution led to an increased growth rate but no increase in infectivity range. In contrast, both selection regimes led to phage adaptation (in terms of growth rates) to their respective bacterial hosts. At the level of individual phage genotypes, coevolution resulted in within-population diversification in generalist and specialist infectivity range types. This pattern was consistent with a multilocus gene-for-gene interaction, further confirmed by an observed cost of broad infectivity range for individual phage. Moreover, coevolution led to the emergence of bacterial genotype by phage genotype interactions in the reduction of bacterial growth rate by phage. Our study demonstrates that the strong reciprocal selective pressures underlying the process of coevolution lead to the emergence and coexistence of different strategies within populations and to specialization between selective environments.
Despite the abundance of studies on the evolution of specialization in trophic interactions (Berenbaum 1996), experimental work on the evolution of generalists versus specialists and their coexistence have usually assumed an abiotic heterogeneous (Reboud and Bell 1997; Barrett et al. 2005) or a biotic homogeneous (Crill et al. 2000; Turner and Elena 2000) environment. Biotic heterogeneous environments are less understood in this regard, but potentially important because they are more realistic caricatures of natural systems. Coevolution is a prime example of biotic heterogeneous environments, and is thought to be an important factor governing patterns of diversification and species evolution (Thompson 1994, 2005; Vamosi 2005; Thrall et al. 2007). In antagonistic coevolution, an exploiter evolves to exploit the phenotype of its prey or host, whereas the latter evolves to escape or resist the former (Woolhouse et al. 2002). The resulting interaction may lead to the resource (e.g., susceptible hosts) being highly variable in space and time. Consequently, the resources can be (1) variable in time because of ongoing changes in genotype frequencies (Dybdahl and Lively 1998), (2) fine-grained spatially because of the role of parasites in maintaining host diversity through negative frequency-dependent selection, and (3) coarse-grained at larger spatial scales because of evolutionary divergence among populations (Gandon et al. 1996; Frank 1997; Burdon and Thrall 1999). Thus, even in an abiotic homogeneous environment, coevolution may generate the conditions for the evolution and the coexistence of specialist and generalist strategies in both host and parasite. At the between-population level, ongoing adaptation to local host genotypes may lead to parasite specialists that experience costs to living in alternative environments (Futuyama and Moreno 1988; Sicard et al. 2007), especially when migration is restricted (Holt 1996).
Investigating the evolution of generalists versus specialists in host–parasite interactions requires the characterization of the underlying genetic patterns. The two most employed representations of specificity are the gene-for-gene (GFG) model and the matching allele (MA) model (Flor 1956; Parker 1994; Frank 1994; Agrawal and Lively 2002). Under GFG, parasites can vary in host range where, at the extremes, some are restricted to a single host genotype (specialist parasites) and others able to attack all host genotypes (generalist parasites). GFG also assumes that fitness costs are associated with virulence/infectivity alleles (and resistance alleles), which offset the benefit of a wider host range in the parasite (and broader resistance in the host). These costs are necessary, otherwise a universally infective parasite will evolve (Jayaker 1970; Leonard 1977; Thompson and Burdon 1992; Sasaki 2000; Thrall and Burdon 2003). In contrast with the GFG model, under MA there are no differences among parasites in the number of host genotypes that can be attacked and no differential costs of resistance or virulence/infectivity between alleles. Agrawal and Lively (2002) studied the dynamics of hybrid GFG–MA models and showed that the coevolutionary outcomes depend on the extent to which one genetic system or the other dominates fitness changes. Accurate characterizations of specificity patterns are of importance for predicting the evolution of virulence (Kirchner and Roy 2002; Dybdahl and Storfer 2003), patterns in local adaptation (Gandon 2002; Morgan et al. 2005; Nuismer 2006) and the evolution of recombination (Parker 1994; Otto and Nuismer 2004).
In this article, we investigate the role of coevolution between the bacterium Pseudomonas fluorescens SBW25 and its lytic dbDNA phage Φ2 (Buckling and Rainey 2002) in the evolution of phage infectivity range and its relation with phage growth rate. We consider effects both at population and individual levels after either experimental evolution or coevolution. Many phages, such as the tailed T-even phages and the nontailed ΦX174, exhibit substantial molecular variation in regions involved in host range evolution (Montag et al. 1987; Hashemolhosseini et al. 1994a, b; Crill et al. 2000). We hypothesized that Φ2 could carry similar variation and thus be a good model system to study the evolution of specificities. More specifically, Φ2 has been shown to drive between-population divergence of bacteria in directly selected resistance traits (Buckling and Rainey 2002) and other ecologically important traits (colony morphology) (Brockhurst et al. 2004), thus providing opportunities for phage divergence and specialization between populations. In addition, Φ2 can mediate within-population diversification through a trade-off between both exploiter resistance and competitive ability in the bacteria (Brockhurst et al. 2004). Given these observations, we hypothesize that the phage has the potential to diversify, generalize, or possibly specialize on different bacterial resistance genotypes if increased host range incurs trade-offs.
Our first goal was to study how coevolution may affect the dynamics of phage adaptation and patterns in phage specialization at the population level. We predicted that in the absence of coevolution, there would be no increase in host range, but rather we would observe the evolution of higher growth rates, because of selection on a constant host with low genetic diversity (Ebert 1998; Turner and Elena 2000). In contrast, we predicted that with coevolution, phage would increase its infectivity range to overcome the emergence of bacterial resistance (Buckling and Rainey 2002) and would exhibit decreased growth on ancestral bacteria. The nature of this population cost was further tested by assaying phage reduction in growth rate (RBG) of susceptible bacteria from the alternative treatment. A low performance of the same intensity on both coevolved and ancestral hosts would be indicative of a cost of generalism, whereas a better performance on the coevolved susceptible bacteria compared to the ancestral bacteria would reveal a trade-off of specialization (Kassen 2002; Caley and Munday 2003).
Our second goal was to better describe the emerging pattern of specificity due to coevolution at the level of the individual genotype. We first checked for host range differences in infectivity between evolutionary and coevolutionary treatments within populations, and then investigated whether the effect on host range at the population level was due to a diversity of specialists, a single generalist genotype, or the coexistence of specialists and a generalist. We used a nestedness analysis (Worthen 1996; Wright et al. 1998) to characterize the pattern of genotype by genotype interactions and generate inferences about the underlying model of specificity (e.g., GFG vs. MA). A nested pattern is defined as a departure from a random interaction between host and parasite genotypes. We expect multilocus GFG models to produce nested subset patterns in phage by bacteria interactions, with a hierarchy among host resistance and among parasite infectivity (e.g., increases in infectivity (resistance) range operate through addition of infectivity (resistance) genes). In contrast, in the MAM, we expect an antinested pattern because there is no intrinsic hierarchy among both host resistance and parasite infectivity. Second, we tested for the occurrence of within-population specificity in phage-imposed reductions in bacterial growth by looking for bacteria by phage genotypic interactions when the host and pathogen coevolved. Indeed, once infection has been successful, some phage genotypes are likely to grow better on some bacterial genotypes than on others (Lambrechts et al. 2006).
Finally, validation of a given model of specificity relative to another requires investigation of the individual costs associated with larger host range in the phage. Thus, we measured costs at the genotype level by relating the host range of several coevolved phage genotypes with their performance on susceptible sympatric host genotypes.
Material and Methods
THE SYSTEM
Pseudomononas fluorescens is a gram-negative aerobic bacterium that belongs to the Pseudomonadaceae family. Pseudomononas fluorescens SBW25 was isolated from a sugar beet leaf at the University farm of Wytham (Oxford) in 1989 (Rainey and Travisano 1998). Its genome (6.7 Mpb) has been fully sequenced (Rainey and Bailey 1996). Its naturally associated phage Φ2 is a 40 kb DNA lytic phage (A. Spiers, pers. comm.).
CULTURE CONDITIONS
All populations were selected in batch culture in 6 mL Kings B (KB: 10 g glycerol, 20 g proteose peptone No. 2, 1.5 g magnesium sulphate, 1.5 g potassium phosphate, 1 L Millipore water) aerated microcosms, shaken 1 min every 30 min at 28°C (Brockhurst et al. 2003). Solid medium used in plating consisted of KB agar (liquid KB medium supplemented with 12 g bacteriological agar).
SELECTION LINES PREPARATION
The bacteria used to initiate the replicated populations consisted of six clones isolated from the ancestral SBW25 stock (B1 to B6). Six phage clones (P1 to P6) were isolated from the ancestral Φ2 stock and each was coevolved with one of the bacterial clones (B1 to B6) for two weeks (eight transfers). Diverged phage populations were then isolated from bacteria using 10% chloroform and centrifuged for 4 min at 13,000 rpm. A single phage plaque was isolated per population (D1 to D6), amplified on its respective ancestral bacteria clone (B1 to B6) for 24 h, and then isolated from the bacteria as previously described. Diverged phage (D1 to D6) and bacterial clones (B1 to B6) were frozen in 20% glycerol at −80°C for subsequent experiments. This procedure ensured that our replicated populations were independent from one another. Indeed, Wichman et al. (1999) showed that similar selective pressures applied to two different replicates led to at least seven different mutations in the ΦX174 genome after 10 days.
SELECTION EXPERIMENT
The diverged phage clones (D1 to D6) were added to their respective ancestral bacteria clones (B1 to B6) and then (1) coevolved with it or (2) selected on the ancestral bacterium phenotype (control) for 16 transfers or c 120 bacterial generations. In both treatments, each of the six phage clones was added at a concentration of 104 to 60 μl of the exponentially growing ancestral bacteria culture in KB microcosms. In the coevolution treatment, 60 μl of culture was transferred to fresh microcosms every two days. In the evolution (control) treatment, phage populations were isolated at each transfer from their bacterial hosts and 60 μl of the resulting phage solution was added into fresh microcosms with 60 μl of ancestral bacteria culture (thereby preventing sustained bacterial evolution). For every two transfers, a sample was frozen in 20% glycerol at −80°C. All the subsequent assays involving the replicated phage populations that used ancestral bacteria were performed on that which they had been paired.
POPULATION LEVEL ASSAYS
At the end of the selection experiment, infectivity range and growth rate of phage populations were measured. Coevolved bacterial populations from 4 time points (transfers 4, 8, 12, and 16) were plated on KB agar from frozen stock to isolate 10 single colonies per population per time point. Ancestral, evolved, and coevolved phage populations were streaked against all of these colonies on square KB agar plates (streaking assay: Buckling and Rainey 2002; Brockhurst et al. 2003). Ancestral bacterial clones were used on every plate as a control. A colony was scored as “susceptible” if there was detectable inhibition of growth. For the coevolved phage populations, the measures on their sympatric populations were removed from the analyses. This precaution ensured that the infectivity range of coevolved phages was not overestimated due to a better performance on their sympatric bacteria. Therefore, phages from the different treatments (ancestral, evolved and coevolved) were compared on the same basis (i.e., allopatric hosts from the coevolved treatments).
We measured phage population growth rate as the number of doublings per hour when grown on ancestral bacteria at a low multiplicity of infection (Bull et al. 2000). Phage populations were added to KB microcosms at a concentration of c 104–105 per mL along with c 108 ancestral bacteria in the exponential growth phase. Microcosms were incubated in the same conditions as the selection experiment for 4 h. Phage densities were measured at t= 0 and t= 4 h. The number of doublings per hour (Nd/h) was calculated as Nd/h =[log2 (N4– N0)]/4, where N0 is the concentration at t= 0 and N4 is the concentration after 4 h. Between one and three replicate assays were conducted depending on the population.
SPECIFICITY AND COST OF THE INTERACTION
Twenty-four phage isolates and 24 bacterial colonies were isolated from each single coevolved and single evolved population at the 16th transfer. Cross-infection experiments were performed at the individual clone level using streaking assays to determine the host range of each genotype. Each of the 24 bacterial colonies isolated was streaked against each phage isolate in triplicate. Infectivity was scored as positive when inhibition of bacterial growth was observed in at least two of the three replicates (probability of infection > 66%).
Another measure of the performance of phage isolates was obtained by monitoring phage-imposed reduction in bacterial growth rate (RBG). This assay was performed to obtain phage performance on susceptible bacteria only. Measures were made on 15 arbitrarily selected bacterial colonies of the 24 colonies used in the streaking assay and on the ancestral bacteria. Bacterial colonies were introduced in microtitre plates containing liquid KB medium at 5.8 × 107± 4.47 × 107 per mL, along with 3.44 × 107 phage particles or without phage (control). Absorbance at 630 nm was measured using a spectrophotometer (microplate reader EL 800, Bio-Tek Instruments Inc., Winooski, VT) at t= 0 and then again after 20 h of static incubation at 28°C. The reduction in bacterial absorbance “RBG” induced by phage i on bacterial colony j, was calculated as RBGij=[abs630(t= 20) − abs630(t= 0)]ij/[abs630(t= 20) − abs630(t= 0)]controlj. Each cross-infection was performed in triplicate at the same time. Thus only data corresponding to RBGij < 0.95 were considered in the analyses (positive infection). Data are presented as 1 – RBGi for more clarity. At the end of the assay, we detected a significant edge effect of the microtiter plates (see also Oliver et al. 1989; Johnsen et al. 2002). Therefore, the analysis was performed using 20 central clones instead of 24. Note that starting densities of coevolved phage were assessed on the ancestral bacteria. If a trade-off exists between infectivity range and the probability of infecting the ancestral bacteria, starting phage densities may be underestimated, which in turn will underestimate the cost. This assay was also used to investigate the pattern of specialization in RBG at the population level by averaging the RBGi of evolved and coevolved phage isolates on the coevolved susceptible and ancestral bacteria.
STATISTICAL ANALYSES
The evolution of infectivity range at the population level was analyzed using a general linear model (GLM) on nontransformed infectivity data, testing for treatment (ancestral vs. selected populations) and population effects. Numbers of doublings per hour of phage populations were analyzed using GLMs including treatment (ancestral vs. selected populations, coevolution vs. evolution), population, and replicate effects. Interaction effects were removed when nonsignificant. Specialization in RBG between treatments was tested using one-way anovas for the effect of evolved versus coevolved phage on the ancestral and coevolved susceptible bacteria.
The treatment effect on host range at the individual level was analyzed with a Mann–Whitney test for mean values, and a Kolmogorov–Smirnoff test for unequal variance. Specificity in infectivity was analyzed using cross-infection data at the individual level. Patterns in specificity can be inferred using nestedness analyses (Worthen 1996; Poulin and Guegan 2000). This method was originally employed to infer the structure of species communities, where a group of species assemblages is said to be nested when the species making up less rich biotas are also found in all richer ones (Wright et al. 1998). Indeed, it can readily be used to discriminate among patterns produced by specialist genotypes (as in the MA) or coexistence between specialists and generalists (as in the GFG) in the population. Thus, a nested pattern of specificity could result from a GFG interaction in which host ranges of specialists are increasingly nested subsets of those of generalists. A high diversity of host ranges would also be indicative of a multilocus GFG. In contrast, if the population is composed of genotypes specialized on different host genotypes, then patterns of infection would not overlap. This corresponds to anti-nested patterns (Poulin and Guegan 2000) and would be indicative of MA genetics. A final case would be a random pattern of infection and would indicate no population structure.
We employed Monte Carlo simulations to identify nestedness in the infection of our phage isolates. Nestedness scores, N, were computed as follows. Host clones were ranked by decreasing resistance range to all phage isolates (Fig. 1A, B). For each phage isolate, we counted the number of times it failed to infect a host genotype with a lower range of resistance. We call this the nestedness subscore, ni. The sum of these subscores, over all phage isolates, gave the N value (host resistance ranges, nestedness subscores and scores are indicated in Fig. 1A, B). The higher the N value, the less nested the pattern of infection. For each interaction matrix in which the probability of infection was above 66%, N-values were compared with those of 1000 randomly generated binary matrices (Fig. 1C, D). The number of successful infections in the simulations was set equal to the total number of infections observed in our interaction matrices. When the observed N-values have a probability less than 0.05 of being in the simulated distribution, patterns of infection of the different phage isolates are significantly nested within one another. Observed N-values with a probability greater than 0.95 are antinested. P-values between 0.05 and 0.95 indicate a random structure of infection.
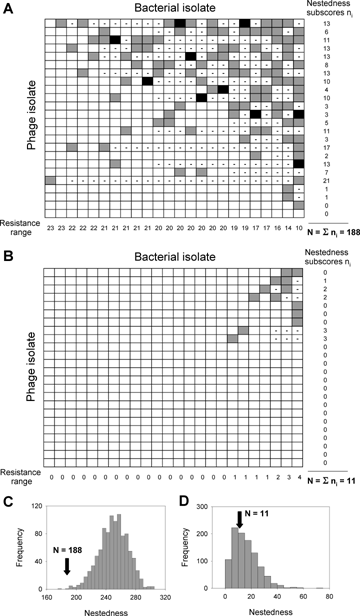
Infection matrix of coevolved phage isolates against sympatric coevolved bacteria isolates (A) and of evolved phage isolates against the same coevolved bacteria isolates (B), for probabilities of infection = 66% (light gray areas) and = 100% (dark gray areas). The resistance range of each bacterial isolate is shown as a margin row. Dashes indicate instances when a phage failed to infect a host with a lower range of resistance. The nestedness subscore, ni, that is the number of times phage isolates failed to infect a host with a lower range of resistance, is included for each phage isolate as a margin column. The sum of the nestesdness subscores gives the nestedness value, N, of the sample. The latter is compared to a random distribution of nestedness, obtained by Monte Carlo simulations, for the coevolution (C) and evolution (D) treatments.
The data for phage-imposed RBG were not normally distributed, so we performed a GLM on ranked data (Sokal and Rolf 1995) to test for a host colony by phage isolate interaction effect. This procedure has the advantage of improving the distribution of residuals. However, because this method lacks power, we also calculated the variance in RBGij of each phage isolate, and averaged across all phage isolates. This was done for the coevolved and the evolved phage isolates and the difference between mean variances was tested using Mann–Whitney test.
The cost of generalist phage isolates was assessed using the regression of RBGi (mean reduction in bacterial absorbance for which phage isolates had RBGij < 0.95) against host range (obtained from streaking assays). Statistical analyses were performed using Minitab 13 (Minitab, State College, PA) or R 1.6.2 (http://cran.r-project.org/).
Results
COEVOLUTION VERSUS EVOLUTION TREATMENTS
Consistent with previous work on this system (Buckling and Rainey 2002), antagonistic coevolution induced an increase in host range in all the phage populations. Coevolved phage populations significantly increased their infectivity range compared to ancestral phage when tested on allopatric bacteria, whereas the evolved phage populations did not (Fig. 2; GLM, ancestral vs. coevolved treatment F1,11= 37.02, P < 0.01; ancestral vs. evolved treatment F1,11= 3.52, P= 0.120, population P > 0.05). The host range of coevolved phage on the sympatric bacteria was not significantly different from that on allopatric bacteria (mean infectivity on sympatric ± SE = 0.318 ± 0.107; GLM host F5,11= 1.05, P > 0.5).
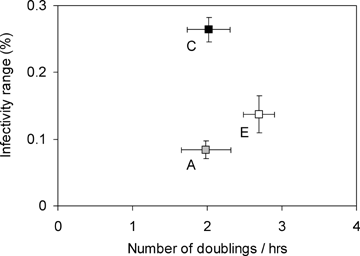
Relationship between mean infectivity range and mean number of doublings per hour of phage populations (±SE). Ancestral phage clones (A, gray square); phage populations from the coevolution (C, black square) and the evolution treatments (E, white square). Infectivity range is the mean proportion of different infected bacterial colonies. The test bacterial colonies are those from allopatric coevolved populations at four time points (transfer 4, 8, 12, and 16) and represent a set of diversified hosts. Mean infectivity (±SE) across all phage populations on their sympatric bacteria is 0.318 ± 0.107. Number of doublings per hour was assayed on the ancestral bacteria.
In contrast, the evolved phage populations exhibited significantly higher growth rates (Nd/h) on ancestral bacteria compared to that of ancestral (Fig. 2; GLM treatment F1,26= 7.19, P= 0.015, population P < 0.05, replicate P > 0.05) and coevolved phage populations (GLM treatment F1,27= 7.26, P= 0.014, population P > 0.05, replicate P > 0.05). Thus, the phage became increasingly adapted to the ancestral bacteria in the evolution treatment. Coevolved phage populations showed no difference in the number of doublings compared to the ancestral phages when measured on the ancestral bacteria (GLM treatment F1,22= 0.09, P= 0.769, population P > 0.05, replicate P > 0.05).
To better explain this unchanged performance of the coevolved phages, the clones isolated from a single coevolved and evolved population were tested for phage-imposed RBG of the ancestral bacteria and a set of coevolved susceptible bacteria, as an alternative to measure phage adaptation. When averaging the performance of the coevolved phage isolates, the RBG was higher on the coevolved bacteria compared to the evolved isolates (Fig. 3; one-way anovaF1,39= 65.09, P < 0.001). Similarly, evolved phage isolates had a larger impact on ancestral bacteria than the coevolved phage isolates (Fig. 3; one-way anovaF1,39= 8.99, P < 0.01). These results indicate that in both treatments (with or without coevolution) the phage evolved adaptations to exploit its host and exhibited costs of specialization on hosts from the alternative treatment.
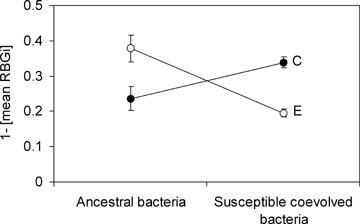
Population-level costs on hosts from the alternative treatment. Mean intensity of reduction of bacterial growth rate (1−[mean RBGi]) (±SE) by the coevolved (C, black symbol) and evolved phage isolates (E, white symbol) on the ancestral and susceptible coevolved bacteria.
SPECIFICITY OF THE INTERACTION
All coevolved and evolved phage isolates were able to infect the ancestral bacteria in both streaking and RBG assays. In streaking assays, phage isolates from a single population of the coevolution treatment at transfer 16 were not able to infect all the coevolved bacterial colonies, confirming the existence of genetic specificity through resistance in the bacteria and/or counter-defenses in the phage. Coevolved phage isolates had a significantly broader host range than those from the evolved population (Fig. 4; mean ± SE: 4.46 ± 0.58 vs. 0.54 ± 0.16, Mann–Whitney W= 832, P < 0.001), confirming the results obtained at the population level (Fig. 2). In addition, coevolved phage had a more diverse host range ranking (from 0 to 10) than did evolved phage (0 to 2) (Fig. 4; Kolmogorov–Smirnoff D= 0.67, P < 0.0001). This suggests that the coevolved population is composed of both generalists and specialists.
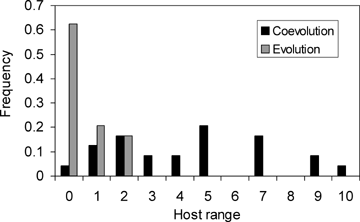
Host range at the individual level. Frequency of phage isolates sampled from one coevolved and one evolved population, with a given infectivity range (number of coevolved bacterial colonies that a phage isolate can infect) obtained from streaking assay. Only probabilities of infection above or equal to 66% are considered.
Using the interaction matrix of infectivity, we found that coevolved phage had a significantly nested pattern of infection (Fig. 1A, C; Monte Carlo test N= 188, P= 0.0017). This pattern is consistent with a multilocus GFG model of interaction (Sasaki 2000; Morgan et al. 2005). By contrast, evolved (i.e., control) phage showed a random pattern of infection (Fig. 2B, D; Monte Carlo test N= 11, P= 0.39).
For RBG assays, the effect of phage isolates on bacterial growth was not significant within both the evolved and the coevolved populations (Table 1). Rather, a higher amount of the variation was due to the effect of the bacterial colony and the replicate. Nevertheless, we found a highly significant interaction effect between phage isolate and bacterial colony in the coevolved treatment (Table 1; GLM F266,899= 1.52, P < 0.001), but not in the evolved one (GLM F266,899= 1.12, P= 0.146). The mean variance in RBGij was significantly higher in the coevolved than in the evolved population (Fig. 5; Mann–Whitney W= 566, P < 0.001). This means that within-population diversity and associated specificity in RBG emerged only when antagonistic coevolution was permitted.
| Treatment | Source of variation | df | SS | MS | F | P |
|---|---|---|---|---|---|---|
| Coevolution | Replicate | 2 | 215,211 | 107,606 | 0.67 | 0.517 |
| Phage isolate | 19 | 2,159,438 | 113,655 | 1.45 | 0.144 | |
| Bacterial colony | 14 | 23,934,138 | 1,709,581 | 11.88 | <0.001 | |
| Phage×Bacteria | 266 | 12,293,285 | 46,215 | 1.52 | <0.001 | |
| Replicate×Phage | 38 | 2,380,895 | 62,655 | 2.06 | <0.001 | |
| Replicate×Bacteria | 28 | 3,587,198 | 128,114 | 4.21 | <0.001 | |
| Error | 532 | 16,179,669 | 30,413 | |||
| Total | 899 | 60,749,835 | ||||
| Evolution | Replicate | 2 | 745,514 | 372,757 | 3.16 | 0.058 |
| Phage isolate | 19 | 1,599,062 | 84,161 | 1.23 | 0.284 | |
| Bacterial colony | 14 | 13,370,925 | 955,066 | 8.78 | <0.001 | |
| Phage×Bacteria | 266 | 14,241,285 | 53,539 | 1.12 | 0.146 | |
| Replicate×Phage | 38 | 2,389,018 | 62,869 | 1.31 | 0.105 | |
| Replicate×Bacteria | 28 | 2,888,544 | 103,162 | 2.15 | 0.001 | |
| Error | 532 | 25,515,459 | 47,961 | |||
| Total | 899 | 60,749,807 | ||||

Mean variance in phage-imposed reduction in bacterial growth (RBGij) across phage isolates (±SE).
COST OF GENERALISM
Phage isolates issued from the same single population in the coevolution treatment at transfer 16 show a cost of adaptation with increasing host range (Fig. 6; linear regression R2= 0.236, F1,17= 4.94, P= 0.041). The higher the host range, the lower the reduction of the bacteria growth rate by the phage isolate. This cost at the genotypic level is consistent with a GFG model of interaction.

Within-population costs associated with higher host range in coevolved phage. Regression between host range obtained from streaking assay for probabilities of infection above or equal to 66% and phage-imposed RBG averaged across coevolved susceptible bacteria isolates for each coevolved phage isolates (1−[RBGi]) (y =−0.019 x + 0.395, R2= 0.236, F1,17= 4.94, P= 0.041). RBG of ancestral phage is indicated by a gray dot and was assessed on the ancestral bacteria from three separate replicated assays.
Discussion
HOW COEVOLUTION AFFECTS THE ADAPTATION AND SPECIFICITY OF PHAGE POPULATIONS
Experiments not permitting host coevolution (i.e., serial passage experiments) were first employed in applied sciences for vaccine development and have since become an important tool in evolutionary biology (Ebert 1998). Parasites are transferred from one host to another under defined conditions and their evolved characters are compared with those of the ancestral parasite. The general result of this approach is an increase in parasite-induced reduction of host fitness (higher virulence or aggressiveness) and a decreased performance on previous hosts. Here, we have shown that phage evolution on a nonevolving host indeed led to adaptation through higher growth rates and a cost on hosts from the alternative treatment.
In contrast to the simple case of no host evolution, the ability of the host to continuously evolve to keep track of phage adaptations and vice versa results in the emergence of different adaptive strategies by the phage. However, direct evidence of reciprocal selective pressures for the evolution of traits is difficult to explicitly demonstrate given the potentially large number of factors that could affect their evolution (Woolhouse et al. 2002). By using an appropriate control, we provide both individual-level and population-level evidence that the evolution of broad infectivity range in the phage can be attributed to selective pressures imposed by the appearance of resistant host genotypes. These results confirm the hypothesis of a coevolutionary arms race, demonstrated in earlier studies on the same system (Buckling and Rainey 2002).
The absence of a detectable cost of broader host range in the coevolved populations compared to their ancestor indicates that a majority of genotypes maintained the ability to infect the ancestral bacteria despite changing selective environments. Because phage were selected for eight transfers under the same conditions prior to the experiment, this is unlikely to be due to adaptation to the experimental conditions. However, the phage sample used for the population assays could not have been representative of the coevolved population. Indeed, phage density assays performed on the ancestral bacteria used to determine the number of doublings did not take into account the proportion of phage genotypes that were unable to grow on this host. It is thus conceivable that these populations actually exhibited a lower growth rate per starting phage density, resulting in a population cost.
We found no pattern of local adaptation in terms of the infectivity of coevolved phage populations against sympatric hosts versus their infectivity against allopatric hosts. This might be due to the lack of phage migration among populations (Morgan et al. 2005). Nevertheless, evolved and coevolved phages were adapted both in terms of infectivity and growth rate on the hosts belonging to their respective treatments. This is supported by previous findings on the role of different local environments (i.e., hosts) on population divergence and host specialization at large scales (Futuyama and Moreno 1988; McCoy et al. 2005).
SPECIFICITY OF THE INTERACTION
Our study shows that coevolution led to diversification in host range and in phage-imposed reductions in growth rate. This is consistent with most experimental and natural host–parasite systems that show substantial genetic variation in interaction traits (Henter 1995; Burdon and Thrall 1999; Carius et al. 2001). For instance, in plants, populations with high genetic diversity, offering a greater variety of niches than low-diversity populations, are positively correlated with arthropod diversity (Wimp et al. 2005).
The infectivity pattern is consistent with a GFG interaction, previously studied in natural host–parasite systems (Thompson and Burdon 1992; Thrall and Burdon 2003). In a GFG system, pathogens that counter-adapt to a host resistance allele retain the ability to attack hosts without that allele (Parker 1994). Moreover, pathogens adapt to each resistance allele through genetic changes at an independent locus. Given the substantial within-population diversity in host range detected (in terms of number of range types), the specificity observed in our system is likely to involve several loci (Frank 1997). Our study could have been subject to a sampling effect, meaning that, depending on the sampled genotypes, any pattern could be generated by the competing models (GFG vs. MA) (Frank 1993b). However, the detection of a genotypic cost of higher host range lends support to the GFG interpretation.
COST OF ADAPTATION
Our experiment did not test for the nature of the fitness cost in the phage. Past study in a range of systems including phage–bacteria associations has reported pleiotropic costs that constrain high phage performance (Ebert 1998; Crill et al. 2000; Duffy et al. 2006). Also, if broader host range results from the addition of genes, then physical constraints on genome size caused by the capsid may impair virus stability (De Paepe and Taddei 2006). Such costs of broader infectivity range have also been revealed at the individual level in several rust pathogen–plant systems (Thrall and Burdon 2003; Montarry et al. 2006) and in insect parasitoids of Drosophila melanogaster (Kraaijeveld et al. 2001).
Although the genetic details of the interaction are unknown for our system, they are likely to involve several genes corresponding to sequential steps in the infective process (Frank 1993a; Hochberg 1997). The mechanisms known to be the most important in host infection are receptor-based recognition by the phage and the restriction-modification system in the bacteria (Frank 1994). Several studies have shown that bacteriophages can extend and change host specificity by the acquisition of new genes and by rearrangement, recombination, and duplication of existing regions (Sandmeier 1994; Scholl et al. 2001). These changes also involve several mutations in hypervariable domains of tail proteins (Drexler et al. 1989). Moreover, phage T7 produces a protein g0.3 that binds and inhibits the E. coli type 1 restriction system (Mark and Studier 1981). In E. coli, 10 genes are involved in the synthesis of the T7 LPS receptors (Qimron et al. 2006). Alteration of each of these genes leads to more resistant bacteria that can be exploited by more infective phages.
A full picture of the specificity of antagonistic interactions would require the investigation of individual costs in bacterial genotypes. However, our design did not allow for an accurate assessment of individual costs in the bacteria. Future study should employ more colonies and continuously shaken microcosms to reduce the confounding effects of niche specialist morphotypes in this bacterium (Brockhurst et al. 2004).
Associate Editor: J. Bergelson
ACKNOWLEDGMENTS
We thank two anonymous reviewers for useful comments on the manuscript. This research was financed by Les Fonds National de la Science, Programme Microbiologique grant to MEH and AB.




