SOURCES OF GENETIC AND PHENOTYPIC VARIANCE IN FERTILIZATION RATES AND LARVAL TRAITS IN A SEA URCHIN
Abstract
In nonresource based mating systems females are thought to derive indirect genetic benefits by mating with high-quality males. Such benefits can be due either to the intrinsic genetic quality of sires or to beneficial interactions between maternal and paternal haplotypes. Animals with external fertilization and no parental care offer unrivaled opportunities to address these hypotheses. With these systems, cross-classified breeding designs and in vitro fertilization can be used to disentangle sources of genetic and environmental variance in offspring fitness. Here, we employ these approaches in the Australian sea urchin Heliocidaris erythrogramma and explore how sire–dam identities influence fertilization rates, embryo viability (survival to hatching), and metamorphosis, as well as the interrelationships between these potential fitness traits. We show that fertilization is influenced by a combination of strong maternal effects and intrinsic male effects. Our subsequent analysis of embryo viability, however, revealed a highly significant interaction between parental genotypes, indicating that partial incompatibilities can severely limit offspring survival at this life-history stage. Importantly, we detected no significant relationship between fertilization rates and embryo viability. This finding suggests that fertilization rates should not be inferred from hatching rates, which is commonly practiced in species in which it is not possible to estimate fertilization at conception. Finally, we detected significant additive genetic variance due to sires in rates of juvenile metamorphosis, and a positive correlation between fertilization rates and metamorphosis. This latter finding indicates that the performance of a male's ejaculate in noncompetitive IVF trials predicts heritable offspring traits, although the fitness implications of variance in rates of spontaneous juvenile metamorphosis have yet to be determined.
Sexual selection, in the form of mate choice and mating competition (Andersson 1994), and their postmating equivalents, cryptic choice, and sperm competition (Birkhead and Møller 1998), may facilitate the selection of genetically compatible or intrinsically high-quality genes (Neff and Pitcher 2005). In the former case, incompatibilities result in nonadditive genetic variance in fertilization or offspring fitness, which can be due to a diverse array of genetic mechanisms all involving interactions between paternal and maternal genetic elements (Zeh and Zeh 1996; Tregenza and Wedell 2000). Thus, according to the genetic compatibility hypothesis, offspring fitness is determined by specific interactions between maternal and paternal haplotypes. By contrast, selection for good genes results in additive genetic variance in fitness, so that a male with good genes will produce offspring with higher fitness irrespective of the female's genetic background (Andersson 1994; Møller and Alatalo 1999).
Identifying and disentangling genetic and environmental sources of variance in sexual selection remains a key challenge in many animal systems, especially those with internal fertilization where (nongenetic) maternal effects may amplify or mask subtle genetic processes (e.g., Kotiaho et al. 2003). Animals and plants with external fertilization and sexual reproduction offer excellent opportunities to address this problem by providing tractable systems for attributing phenotypic variance in fitness-related traits to genetic and environmental causes. In these systems, gametes from two or more members of each sex can be crossed in all combinations to generate multiple sibling relationships (full siblings, paternal, and maternal half siblings), without recourse to female multiple mating or crosses involving inbred lines (see Lynch and Walsh 1998). Such cross-classified designs therefore expand on alternative approaches (e.g., parent-offspring regression, nested sibling analyses) to detect genetic effects that contribute toward familial resemblance (Lynch and Walsh 1998). A second important advantage of using external fertilizers is that they offer more tractable systems for controlling nongenetic (environmental) sources of variance, thus providing more precise estimates of genetic variance components.
In this article we identify sources of genetic and phenotypic variance in fertilization rates and two early phases of offspring development in Heliocidaris erythrogramma, a common sea urchin inhabiting coastal waters in southern Australia (Keesing 2001), and a model system for developmental studies (Williams and Anderson 1975). We used the North Carolina II (NCII) block breeding design (Lynch and Walsh 1998) to partition genetic variance in these traits following replicated factorial crosses between two males and two females within each block. The NCII design has previously been applied to externally fertilizing animals and plants, where split-clutch and in vitro fertilization (IVF) techniques make it possible to partition gametes from both sexes and cross them in all combinations (Wedekind et al. 2001; Evans and Marshall 2005; Marshall and Evans 2005; Rudolfsen et al. 2005; Trippel et al. 2005; Pitcher and Neff 2006; Steven et al. 2007). Indeed, IVF techniques are well developed in H. erythrogramma and offer unparalleled experimental control over staged artificial fertilizations (Marshall et al. 2004; Evans and Marshall 2005).
We analyzed sources of variance due to male and female effects (and their interaction) using a split-clutch IVF assay (see Evans and Marshall 2005). In common with other broadcast spawning marine invertebrates, we anticipated strong maternal effects at fertilization due to variation among females in the size distribution of eggs (Marshall et al. 2004). Such effects strongly influence fertilization kinetics in broadcast spawning marine invertebrates because larger eggs are more likely to be contacted by sperm than smaller eggs (Levitan 1996; Styan 1998; Marshall et al. 2002). Our analysis of offspring performance focused on two phases of early larval development: (1) premetamorphic survival (hereafter “embryo viability”), defined as the proportion of initially fertilized eggs that successfully hatched from their fertilization membranes and progressed to the free-swimming larval stage, and (2) metamorphosis, the proportion of hatched larvae that metamorphosed by six days into the juvenile form in the absence of settlement cues. Our ability to assess fertilization rates directly and then measure offspring traits during two stages of development enabled us to assess the interrelationships between these potential fitness traits. In particular, we were interested in the relationship between fertilization rates and embryo viability, because the latter is often used to estimate fertilization success in species in which it is not possible to estimate fertilization rates directly (i.e., most internally fertilizing species). This distinction is especially important in studies that estimate paternity (as a proxy for relative fertilization success) from newly hatched or adult offspring genotypes. As emphasized elsewhere, paternity patterns may not reflect fertilization patterns at conception (Gilchrist and Partridge 1997; García-González and Simmons 2007), especially where embryo viability is influenced by differential mortality, maternal and paternal effects or other sources of environmental variance (Olsson et al. 1999; Simmons 2005; García-González and Simmons 2007).
Materials and Methods
STUDY SYSTEM
Heliocidaris erythrogramma is a common sea urchin inhabiting coastal waters (maximum depth 35 m) in southern Australia (Keesing 2001). During the spawning season eggs and sperm are shed freely into the water column where they combine at fertilization and develop into free-swimming lecithotrophic larvae. Larvae enter this free-swimming phase after successfully emerging from a fertilization membrane and egg jelly coat and eventually metamorphose and settle on suitable substrates to become benthic juveniles. Reproductive mature sea urchins were collected from South Mole Jetty in Fremantle, Western Australia (coordinates: 32°03.355′S 115°44.075′E) and used immediately for the artificial fertilization trials. Trials took place during the breeding season (end of March and early April 2006).
BREEDING DESIGN
Our experimental design involved nine blocks of 2 × 2 factorial crosses. In each block, two sires (SA and SB) and two dams (D1 and D2) were mated in all four combinations, with replicate crosses performed for each pair (i.e., a total of eight crosses per block). This design therefore yielded 72 families for our genetic analysis and generated maternal and paternal half siblings within each block. For the IVF trials, males and females were induced to spawn in individual containers (at 22°C) with a nonlethal intracoelomic injection of 3% KCl. Prior to IVF, sperm concentrations were estimated for each sire using an improved Neubauer haemocytometer and adjusted to 7.0 × 105 sperm/mL using seawater. This concentration, as used previously to partition variance in fertilization rates in H. erythrogramma (Evans and Marshall 2005), was used throughout the IVF trials and resulted in fertilization rates ranging from 37.5% to 97.8% (mean = 76.5%). These values fall within the natural range for this species (Styan 1997). This procedure ensured that sperm concentrations were the same within and among the blocks. To obtain eggs, we partitioned the ripe ova from each of the two females in each block into four separate vials and adjusted the volume of each sample so that concentrations were the same (50 eggs/mL). We then took 35 mL (containing approximately 1750 eggs) from each egg sample and exposed two samples from D1 to the same volume of sperm from SA and the remaining two samples to the sperm from SB. This procedure was repeated for D2 so that in each block we prepared two replicate fertilizations for each of the (n= 4) sire–dam crosses (SA:D1; SA:D2; SB:D1; SB:D2). Each urchin was used just once and all were released at the point of capture at the end of the experiment.
FERTILIZATION ASSAYS
The proportion of eggs fertilized in each sample was estimated 2 h after the exposure of eggs to sperm by examining approximately 100 randomly selected eggs from each cross; eggs were classed as fertilized if regular cell division had occurred at this stage (Evans and Marshall 2005). After estimating fertilization rates, each sample was left for 2 h to allow further embryonic development (to the 32–64 cell stage). At this stage we selected 50 developing embryos from each of the eight crosses in each block and placed each sample in a 10-cm diameter dish containing 150-mL seawater and an air-supply. Samples were therefore maintained at identical densities within and among blocks and were kept at 22°C until required for offspring trait assays.
OFFSPRING TRAITS
Embryo viability was estimated for each sample by calculating the proportion of the 50 developing embryos (see above) that reached the free-swimming larval stage of development. The transition from fertilized eggs to free-swimming larvae commences at approximately 15-h postfertilization (when the gastrula emerges from the fertilization membrane and jelly coat) and is complete at 40 h, after which the larvae can commence metamorphosis into the juvenile stage (Williams and Anderson 1975). Embryo viability was therefore estimated for each sample 40 h after fertilization (i.e., when all viable offspring would have emerged but prior to metamorphosis) by counting the number of larvae in each sample that successfully emerged from their fertilization membrane and progressed to the free-swimming stage. We then estimated metamorphosis six days after fertilization by counting the proportion of free-swimming larvae (as estimated above) that metamorphosed into juveniles (see Williams and Anderson 1975).
STATISTICAL ANALYSIS
For each 2 × 2 factorial we used two-way ANOVA to estimate the effect of sires, dams, and their interaction for each of the response variables (proportion of eggs fertilized, embryo viability, and larval metamorphosis). In these analyses, terms for parents (sire and dam) were treated as random effects. Each block was defined simply by the individuals contained therein, rather than temporal or spatial factors that may potentially influence variation among blocks. Unlike fully crossed designs involving n sires crossed with n dams in all (n × n) combinations (e.g., Wedekind et al. 2001; Pitcher and Neff 2006), our design involved several sets of independent crosses (see above). As such, the sums of squares and degrees of freedom were computed individually for each block and summed before calculating the mean squares and degrees of freedom for all blocks (see Lynch and Walsh 1998; pp. 601–602 for a fuller description of this procedure). From these analyses, variance components and their standard errors were calculated following the methods outlined by Lynch and Walsh (1998; p. 600). The total phenotypic variance for each trait was then partitioned into genotypic and environmental variance components. The sire variance component (equivalent to the covariance among paternal half siblings) provides an estimate of additive genetic effects, whereas the dam component (the covariance between maternal half siblings) represents both genetic and environmental maternal effects. Finally, the sire-by-dam interaction variance provides an estimate of the genetic variance due to nonadditive nuclear gene action (dominance, epistatic, and extranuclear interactions).
We also examined the interrelationships between fertilization rates and offspring traits (embryo viability and metamorphosis). To do this, we extracted mean fertilization rates and offspring trait scores from selected cells (see below) within each block and calculated the correlation among the traits. Each block comprised replicate factorial crosses between two males and two females. Thus, within each block there were two possible pairs of independent crosses (i.e., pairings lacking individuals in common), which were either SA:D1 and SB:D2, or SA:D2 and SB:D1. With nine experimental blocks, a total of 512 (29) different combinations, each involving n= 18 independent male–female crosses, were possible. To assess the interrelationships between fertilization rates, embryo viability, and metamorphosis we calculated the mean and 95% confidence limits for the distribution of correlation coefficients generated by the 512 possible combinations (n= 18 for each combination).
Results
Embryo viability (the proportion of initially fertilized eggs that reached the free-swimming larval stage of development) ranged from 65% to 100% (mean ± SD = 76.5 ± 18.8%). From these successful hatchings, a mean of 29.7% (±20.1 SD; range: 1–73.5%) successfully metamorphosed into the juvenile form by the six days census point.
The results from the NCII analysis are summarized in Table 1. The analysis of fertilization rates revealed highly significant male and female variance components and no significant interaction between these main effects. By contrast, embryo viability was strongly influenced by the interaction between males and females, indicative of nonadditive genetic variance in this trait. Finally, our analysis of the number of juveniles that subsequently underwent metamorphosis revealed that the component of variance associated with sires was significant, accounting for 31% of the observed phenotypic variance for this trait (Table 1).
| Source of variation | df | SS | MS | F | P | Var | SE |
|---|---|---|---|---|---|---|---|
| Fertilization | |||||||
| Male | 9 | 1225.0 | 136.1 | 4.36 | 0.002 | 26.2 | 14.9 |
| Female | 9 | 9571.0 | 1063.0 | 34.0 | <0.00001 | 258.1 | 113.4 |
| Interaction | 9 | 281.3 | 31.3 | 1.35 | 0.25 | 4.1 | 7.2 |
| Error | 36 | 833.3 | 23.2 | 23.2 | 5.3 | ||
| Embryo viability | |||||||
| Male | 9 | 574.5 | 63.8 | 0.63 | 0.75 | 0† | 0 |
| Female | 9 | 2341.0 | 260.1 | 2.55 | 0.09 | 39.6 | 29.8 |
| Interaction | 9 | 916.5 | 101.8 | 5.57 | 0.0001 | 41.8 | 21.8 |
| Error | 36 | 658.0 | 18.3 | 18.3 | 4.2 | ||
| Metamorphosis | |||||||
| Male | 9 | 6310.0 | 701.1 | 3.38 | 0.042 | 123.4 | 77.9 |
| Female | 9 | 4305.0 | 478.3 | 2.30 | 0.11 | 67.7 | 55.6 |
| Interaction | 9 | 1869.0 | 207.6 | 1.03 | 0.44 | 2.8 | 50.0 |
| Error | 36 | 7272.0 | 202.0 | 202.0 | 46.3 | ||
- Sums of squares (SS) and degrees of freedom (df) were computed individually for each of the nine blocks and summed before estimating the mean squares (MS) for the entire experiment. Variance components (Var) were obtained by equating the observed MS to their expectations. Standard errors (SE) are square roots of the large sample variances, computed following Lynch and Walsh (1998).
- †The negative variance component and associated SE estimate were converted to zero (Quinn and Keough 2002).
We also examined the interrelationships between fertilization rates, embryo viability, and metamorphosis. The distribution of correlation coefficients for all possible (512) combinations of independent data arising from the blocks is shown in Figure 1 (A–C) for the three relationships. The 95% confidence limits for these distributions inform us about the significance of the association between the pairs of variables (Fig. 1). These analyses revealed that the relationship between fertilization rates and embryo viability is not significant (confidence interval at α= 0.05 included zero; see Fig. 1A). By contrast, we found a positive and significant relationship between fertilization rates and metamorphosis (Fig. 1B), and a negative and significant relationship between embryo viability and metamorphosis (Fig. 1C). In the former case we found that all possible correlation coefficients always yielded r-values that were above zero (range r= 0.197/0.667; see Figure 1B), whereas in the latter all possible correlation coefficients yielded r-values that were below zero (range r=−0.83/−0.08; Fig. 1C).
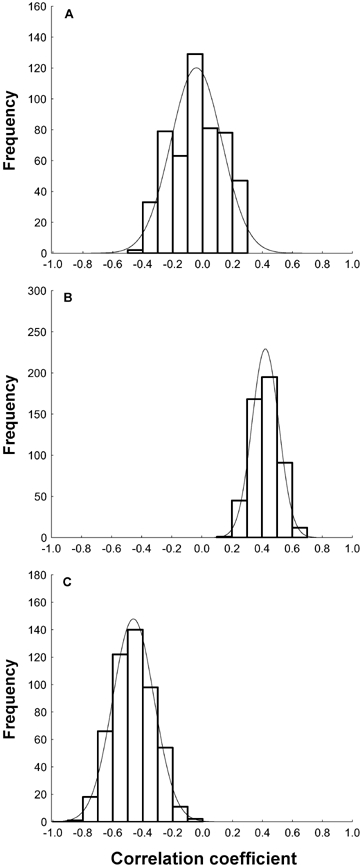
Distribution of correlation coefficients for relationships between fertilization and larval traits. Distributions are for all 512 independent combinations of independent data from the NCII block design (see Material and Methods). Correlations are for (A) fertilization rates and embryo viability (mean r=−0.04, 95% CL =−0.34/0.24), (B) fertilization rates and metamorphosis (mean r= 0.42, 95% CL = 0.25/0.60), and (C) embryo viability and metamorphosis (mean r=−0.46, 95% CL =−0.73/−0.20). Each correlation coefficient has a sample size of 18, the number of independent values that can be extracted from the nine 2 × 2 factorial units in the experimental design. Thin lines indicate normal curves.
Discussion
Our analysis focused on three potential fitness traits in the sea urchin H. erythrogramma: noncompetitive fertilization rates, pre-metamorphic larval survival (embryo viability), and metamorphosis. The relative influence of additive and nonadditive sources of variance depended on which of these traits was measured, mirroring recent findings for decorated field crickets (Ivy 2007) and earlier work on alpine whitefish (Wedekind et al. 2001). Taken together, these studies emphasize the importance of considering multiple components of offspring fitness when considering the genetic basis of mate choice and other forms of selection (see Hunt et al. 2004).
Our analysis of fertilization rates revealed highly significant male and female variance components and no significant interaction between these main effects. In the absence of extrinsic environmental effects (which were rigorously controlled during the assays), this finding implies that individuals of both sexes exhibit consistent variation in gamete traits. The difference between the variance components associated with females and males at fertilization provides an estimate of the variance due to maternal effects (Lynch and Walsh 1998), which in our analysis would have been substantial (see Table 1). This strong maternal effect at fertilization is consistent with a large body of work on other marine broadcast spawners (e.g., Levitan 1993; 2000; Marshall et al. 2000) and may be due to consistent differences in the quality, size, or maturity of eggs among females, all of which constitute important sources of variation at fertilization in H. erythrogramma (Marshall et al. 2004). The lack of interaction at fertilization reported here contrasts with the findings from a different (eastern Australian) population of H. erythrogramma, where in addition to significant main effects, interacting parental effects were also important (Evans and Marshall 2005). This difference may reflect the different population origins of study animals, different study environments, or differences in selection pressures that operate on gamete recognition traits in the two populations (see Zigler et al. 2005).
Although we did not specifically examine sperm performance traits in this study, our finding that variance in fertilization was partially attributable to general male effects is consistent with an increasing body of work showing that consistent variation among males in sperm traits (e.g., motility, swimming speeds, and viability) can influence fertilization (Birkhead et al. 1999; Gage et al. 2004; García-González and Simmons 2005b). Our ability to carefully control sperm densities (and sperm-to-egg ratios) during the IVF trials leads us to speculate that individual males differ consistently in sperm or ejaculate traits (other than sperm number) that influence fertilization (see review by Snook 2005), a possibility that we are currently pursuing using sperm viability assays and computer-assisted sperm analyses. Nevertheless, to rule out possible environmental (i.e., nongenetic) effects on sperm performance we advocate future quantitative genetic experiments that specifically focus on the genetic basis of traits known to regulate fertilization (see forthcoming reviews by Evans and Simmons 2007).
Our analysis of embryo viability yielded a highly significant male-by-female interaction, indicative of nonadditive genetic variance in this trait. This finding is consistent with the genetic compatibility hypothesis because it suggests that during this life-history phase sexual selection will target compatible male–female crosses rather than intrinsically high-quality mating partners (cf. García-González and Simmons 2005a). This result is consistent with findings from several insect studies in which it has been argued that genetic incompatibility can limit hatching rates and offspring viability (Tregenza and Wedell 1998; Newcomer et al. 1999; Engqvist 2006). However, much of the previous support from animals for the genetic compatibility hypothesis comes from studies that have failed to provide firm support for alternative (good genes) processes. Only a few animal studies have implicated genetic compatibility (i.e., nonadditive variance in fitness-related traits) within an experimental framework that employs statistical designs powerful enough to exclude the effects of good genes (Wedekind et al. 2001; Pitcher and Neff 2006; Ivy 2007). By contrast, the plant literature provides ample compelling evidence for incompatibility avoidance, particularly with respect to avoiding self-fertilization and promotion of outcrossing (reviews by Barrett 2003; Bernasconi et al. 2004). Our preliminary analysis of potential fitness traits in H. erythrogramma provides further support for the genetic compatibility hypothesis, at least with regard to embryo viability. The mechanisms underlying these nonadditive genetic effects are largely unknown, but may include incompatibilities due to selfish genetic elements, inbreeding and heterozygote deficiency (see reviews by Zeh and Zeh 1996; Tregenza and Wedell 2000).
Although variation in embryo viability was clearly influenced by nonadditive effects, our analysis of the number of juveniles that subsequently underwent metamorphosis revealed that the component of variance associated with sires was significant, accounting for 31% of the observed phenotypic variance for this trait (Table 1). Although it is not clear whether early (or indeed delayed) metamorphosis, as measured in our study, has fitness implications for juvenile urchins, there is anecdotal evidence from work on the settlement ecology of this species which suggests that spontaneous metamorphosis in the absence of settlement cues (originating from the coralline alga Amphiroa anceps) results in lower postmetamorphic survival (D. J. Marshall, unpubl. data). Indeed, several studies on other marine invertebrates show a strong effect of settlement choice on the subsequent performance of settlers (Elkin and Marshall 2007). Consequently, variation in settlement rates may provide an important target for natural selection in H. erythrogramma. More generally, the settlement “choices” that marine invertebrate larvae make are thought to have important implications for fitness, and delaying settlement in the absence of cues indicating suitable habitats may be an adaptation to avoid high rates of mortality (Pechenik 1990; Elkin and Marshall 2007).
Interestingly, embryo viability and metamorphosis (at six days) were negatively correlated (Fig. 1C), raising the possibility that larvae from crosses with high hatching rates were more likely to reject poor settlement cues than those from less viable crosses. Alternatively, and equally plausible, rates of larval metamorphosis may have been influenced by larval density, which would have varied according to differences in hatching rates among crosses. Unfortunately, our study cannot distinguish between these possibilities. Nevertheless, our work does underscore the need for studies specifically aimed at understanding the fitness implications of variation in spontaneous juvenile metamorphosis, as such information is crucial before concluding that additive genetic variance in this trait is due to paternal good genes.
We also uncovered a positive association between fertilization and metamorphosis rates (the proportion of surviving larvae that successfully metamorphosed into the juvenile form). This finding is potentially informative in the context of hypotheses that link male fertilizing success to the genetic benefits they bestow on their offspring (Yasui 1997) (reviewed by Evans and Simmons 2007). However, it is important to emphasize two important limitations of our study with respect to its relevance for such “good sperm” processes. First, the importance of metamorphosis (as measured in our study) in influencing offspring fitness is unknown (see above), and therefore it is unclear whether the selection of males imposing high (or low) rates of spontaneous metamorphosis on their offspring actually represents a benefit. Second, and more crucially with respect to good sperm processes, we estimated fertilization rates during noncompetitive IVF assays; presently we do not know whether the performance of sperm undergoing noncompetitive IVF trials predicts their success during sperm competition. Our ongoing research addresses both of these questions in H. erythrogramma in an attempt to specifically address the good sperm hypothesis.
Finally, we found no association between fertilization rates, which were influenced by intrinsic male and female effects, and embryo viability, which was strongly influenced by interacting parental effects. This finding has potentially important ramifications for studies that use paternity data to infer fertilization rates, particularly in internal fertilizing species in which paternity is typically estimated from newly hatched offspring rather than recently fertilized eggs. Although we stress again that our fertilization data come from noncompetitive mating trials, and that the link between noncompetitive and competitive fertilization success needs to be made, our data may inform recent debates on the importance of estimating fertilization at conception rather than from the proportion of viable offspring sired by focal males, because the latter may be influenced by a range of (differential) effects that affect embryo survival (e.g., see Gilchrist and Partridge 1997; Olsson et al. 1999; García-González and Simmons 2007).
Associate Editor: D. Ayre
ACKNOWLEDGMENTS
We thank L. Simmons, D. Ayre, and two anonymous referees for comments on the manuscript, C. Duggin for assistance in the laboratory and W. Gibb and T. Stewart for collecting the sea urchins. This work was supported by grants to JPE, FG-G and DJM from the Australian Research Council.




