ON THE ECOLOGICAL BASIS OF INTERSPECIFIC HOMOPLASY IN CAROTENOID-BEARING SIGNALS
Abstract
Evidence that similar color patterns occur in unrelated animals with different habits undermines the traditional view that homoplasy evolves through shared ecological selection pressures. Carotenoid pigments responsible for many yellow to red signals exhibit two related properties that could link ecology with appearance by nontraditional means. Ecologic homoplasy could arise through ecophenotypy because all animals must obtain carotenoids through their diet. Such homoplasy also could be hidden from view because increased carotenoid levels are more strongly encoded by decreased reflectance over ultraviolet (UV) wavelengths invisible to humans. To explore these possibilities, I examined apparent matches or mismatches between color and ecology among insectivorous (low carotenoid diet) and frugivorous (high carotenoid diet) bird species in relation to the typical yellow and black plumage pattern of insectivorous, UV-sensitive titmice (Paridae). Diagnostic features of reflectance spectra indicated that all yellow plumages resulted from carotenoids, black plumages from melanins, and olive green plumages from codeposition of both pigments. However, reflectance by carotenoid-bearing plumages correlated with diet independent of plumage pattern; compared to the insectivores, frugivores had reduced amounts of UV reflectance, and to a lesser extent, “red shifts” in longer-wavelength reflectance. Furthermore, an asymptotic decrease in amount of UV with increased redness implied that plumage reflectance of insectivorous species differed more over UV wavelengths, whereas that of frugivorous species differed more over longer wavelengths. I verified that dietary links to plumage reflectance resulted from greater amounts of plumage carotenoids in frugivores, presumably due to their carotenoid-rich diets. All of these ecological associations transcended post-mortem or post-breeding color change, and phylogeny. Thus, predictable associations between avian-visible plumage reflectance, pigmentation, and diet across evolutionary scales may arise directly (diet per se) or indirectly (honest signaling of diet) by ecophenotypy, although various genetic factors also may play a role.
The independent coupling of similar morphologies and ecologies in distantly related species provides classic comparative evidence for the role of environmental selection in the origin of adaptation (Mayr 1963, 1983; Futuyma 1998). Among the most famous examples of this phenomenon are the streamlined body forms of aquatic mammals and fishlike vertebrates, the specialized dentitions of marsupial and placental carnivorous mammals, and the behaviors and morphologies of eusocial rodents and insects (Ridley 1996; Futuyma 1998; West-Eberhardt 2003). Nevertheless, growing evidence suggests that the repeated evolution of similar attributes in different phyletic lines (homoplasy, through convergence or parallelism) does not necessarily imply a corresponding similarity in ecological habits (West-Eberhardt 2003). This caveat applies especially to homoplastic coloration (Omland and Hofmann 2006). Although some studies have demonstrated that similar color patterns evolve independently in similar environments (Cody 1970; Crochet et al. 2000; Dumbacher and Fleischer 2001; Bleiweiss et al. 2003; Weibel and Moore 2005; Omland and Hofmann 2006), many instances of similarity lack obvious ecological correlates (Omland 1997; Omland and Lanyon 2000; Omland and Hofmann 2006). Such apparent mismatches between appearance and ecological habits have focus attention instead on nonecological explanations based on sexual selection (Omland and Lanyon 2000; West-Eberhardt 2003), developmental constraints (West-Eberhardt 2003), or random genetic drift (Omland 1997).
Nevertheless, ecological causes of homoplasy may be underestimated due to past emphasis on direct environmental selection (Ridley 1996; Futuyma 1998). A consideration of alternative ecological processes seems warranted especially for signals based on carotenoid pigments, which are widespread constituents of yellow to red integumentary displays. A salient feature of carotenoids is that animals cannot synthesize these pigments, but must acquire them through their diets (Olson and Owens 1998; Stradi et al. 2001; Hill 2002). Thus, a strong connection between diet and carotenoid-based coloration has long been suspected (Price 2006). Explorations of this linkage have focused nearly exclusively on its implications for intraspecific variation because of interest in the relationship of signal form to mate choice (Endler 1983; Hill 2002). However, the ancient origin of ecophenotypy of animal carotenoids should make the phenomenon relevant to color evolution at any level of divergence. Thus, homoplasy in carotenoid-based color could arise through direct effects of a shared diet on plastic phenotypes.
Despite the obvious connection between carotenoids and diet, evidence for the ecophenotypic basis of carotenoid-based coloration remains surprisingly tenuous (Hill 1994; Hudon 1994; Olson and Owens 1998) because animals from virtually all trophic groups display carotenoids (Stradi 1998; Bleiweiss 2004a). However, links between ecology and carotenoid-based reflectance may simply escape the notice of human eyes. Although carotenoids reflect most strongly over longer wavelengths visible to humans and many other animals (400–700 nm), virtually all reflectance spectra of carotenoids deposited in biological materials are actually bimodal, with a variable secondary reflectance peak at near-ultraviolet wavelengths (UV, 320–400 nm) that is, visible to most animals except humans (Vorobyev et al. 1998; Cuthill et al. 2000; Hart et al. 2000; Osorio et al. 2001; Ödeen and Håstad 2003). Indeed, amounts of UV reflectance by carotenoid-bearing plumages may provide unique information about interspecific diets in certain avian clades; those species that consume a greater proportion of carotenoid-rich foods (fruit) have lower absolute and relative (to longer wavelengths) amounts of UV plumage reflectance, regardless of human-visible hue (Bleiweiss 2004a, 2007). Preliminary evidence suggests that avian frugivores deposit greater amounts of carotenoids in their feathers, which enhances UV absorption (Bleiweiss 2004a). Consistent with this interpretation, a taxonomic assortment of avian frugivores have higher carotenoid levels circulating in their blood plasma (Tella et al. 2004), a source of feather carotenoids (McGraw 2006).
Here I use physical measures of plumage reflectance to clarify apparent mismatches in dietary habits between species that humans perceive as having a strong physical resemblance, or the reverse situation in which human-visible colors differ but diets are similar. I focus on plumage color and pattern homoplasy in relation to certain titmice (“tits,” Paridae), an avian model for studies of coloration and UV vision. Tits are ideal for exploring the ecological basis of homoplasy because it is well established that carotenoids are deposited unmodified into their yellow plumages, and that the amount of the resulting pigmentation depends to varying degrees on the corresponding amount of ingested carotenoid (Slagsvold and Lifjeld 1985; Partali et al. 1987; Hörak et al. 2001; Fitze et al. 2003; Johnsen et al. 2003; Andersson et al. 2005). Less widely known is that the yellow and black plumage pattern characteristic of tits also has evolved among certain insectivorous and frugivorous species in other avian families. This transgression of tit-like plumage coloration across trophic habits again raises the specter of nonecological homoplasy. But for the same reason, these resemblances provide a critical test of dietary effects on plumage signals because fruits contain substantially greater amounts of carotenoids than do insects (Goodwin and Goad 1970). Thus, if diet exerts a strong influence on carotenoid-based signals, links between plumage carotenoids and diet should exist regardless of the birds' appearance (whether similar or different) to humans.
To explore this hypothesis, I evaluated the: (1) histories of change leading to tit-like plumage patterns (common ancestry vs. kind of homoplasy); (2) associations of diet with carotenoid-based plumage reflectance across the relevant avian visible spectrum (320–700 nm); (3) relative importance of different (UV, longer wavelength) reflectance components, and of plumage patterns, as biomarkers of diet; (4) utility of a practical in situ method to characterize carotenoids in broad comparative studies, which provides insight into proximate and evolutionary mechanisms of homoplasy.
Materials and Methods
TAXA, PLUMAGES, AND REFLECTANCE SPECTRA
My study focused on certain Asian tits with extensive carotenoid-bearing plumages, characterized by yellow cheeks and underparts, olive green backs, along with the black crowns and bibs that are typical of many tits (Table 1). Amazingly similar patterns have developed in a painted berrypecker (Oreocharis arfaki) and a wood-warbler (Wilsonia citrina) in other passerine clades. Although Wilsonia is insectivorous, Oreocharis is a specialist frugivore whose habits actually most closely resemble those of certain Neotropical finches (Euphonia), which have a dissimilar plumage pattern (Beehler et al. 1986; Coates 1990; Isler and Isler 1999). Comparisons among these taxa (Table 1) provide the requisite combinations of homoplastic and divergent plumage pattern elements (yellow, olive green, black) and diets (insects, fruits) to test the focal hypotheses. Females of most of these species lacked the tit-like pattern, so only males were analyzed. For data collection, plumage was subdivided into standard patches (cap, bib, cheek, breast, belly, back, rump) that contributed to the tit-like appearance. I defined a “patch” as a region of a standard feather tract (see Andersson and Prager 2006), which can be sampled on all individuals, is generally uniform in color (Omland and Lanyon 2000), and often evolves as a block (Price and Pavelka 1996).
| Family1 | Pattern2 | Carotenoid | Main diet3 | Distribution |
|---|---|---|---|---|
| Parinae, Paridae | ||||
| Periparus elegans | tit-like | yellow | insects | Philippines |
| Parus xanthogenys/spilonotus | tit-like | yellow | insects | Asia |
| Paramythiidae, Corvoidea | ||||
| Oreocharis arfaki | tit-like | mainly yellow | fruit | New Guinea |
| Emberizinae, Fringillidae | ||||
| Wilsonia citrina | tit-like | yellow | insects | Neotropical migrant |
| Euphoniinae, Fringillidae | ||||
| Euphonia spp. | not tit-like | mainly yellow | fruit | Neotropical |
- 1The four families are widely separated on the passerine phylogenetic tree (Sibley and Ahlquist 1980; Baker et al. 2004) and the parid genera are not sister taxa (Gill et al. 2005). Parus xanthogaster and P. spilonotus are allopatric (possibly parapatric) sister taxa.
- 2Tit-like = black cap and bib contrasting with brightly colored cheeks and underparts.
- 3Diet data for tits (Harrap and Quinn 1995), berrypecker (Coates 1990), wood warbler (Evans-Ogden and Stutchbury 1994), and Euphonia (Isler and Isler 1999) from standard references. Euphonia has been characterized as the ecological vicar of Oreocharis by Beehler et al. (1986).
The term “phenotype” is used here to refer to physical reflectance by plumage, as measured with a spectrophotometer. Quantitative reflectance spectra (n= 330) were recorded through a 60-mm integrating sphere attached to a Perkin-Elmer (Chicago, IL) lambda-9 UV-VIS spectrophotometer, with percent reflectance estimated relative to a BaSO4 white standard. Spectra were recorded over the wavelength range visible to UV-sensitive passerines (320–700 nm) in 1-nm intervals with a band pass of 2 nm and a slit width of 4 mm. Specimens were positioned in the (9 × 17 or 5 × 5 mm) sample acceptance port so that a visibly uniform patch filled the port (patches smaller than the port were excluded). Both the longer wavelength and UV lamps were activated prior to each recording session to allow for sufficient warm-up time. The background standard was measured prior to data acquisition for each specimen. Specimen and then patches within specimen were measured in random order to minimize any systematic measurement bias that might arise with respect to these factors. To improve accuracy of reflectance measurements, each patch was scanned two or three successive times, repositioning the specimen after each scan. Successive scans were averaged for use in subsequent analyses because of the high repeatability of spectra (< 2%, usually < 1%, different).
PHYSICAL PLUMAGE ATTRIBUTES
As with any pigment, carotenoids produce colors through selective absorption of particular wavelengths. When deposited in otherwise highly reflecting feather keratin, the absorptive properties of carotenoids produce a distinctly bimodal reflectance profile such that the perceived plumage signal comprises those wavelengths otherwise reflected by the keratin matrix and its microstructures (Bleiweiss 2005; Shawkey and Hill 2005; Andersson and Prager 2006; Montgomerie 2006). Thus, although most parameters considered herein describe plumage characteristics in terms of reflectance, no particular physical mechanisms is implied.
Bimodal reflectance by carotenoid-bearing plumage (Fig. 1) consists of a main reflectance plateau at long wavelengths (500–700 nm), a secondary peak at UV wavelengths (320–400 nm), and low reflectance over intervening wavelengths (400–500 nm). To assess distinctions between UV and “longer” (400–700 nm) wavelengths, I calculated corresponding pairs of variables for amounts and spectral locations of reflectance in each waveband (Bleiweiss 2004a). Total (R400–700) and maximum (Rvismax) reflectance components at longer wavelengths have corresponding total (R320–400) and maximum (RλUVpeak, where λRUVpeak is the spectral location of peak UV reflectance) reflectance components at UV wavelengths. However, the broad peak at longer wavelengths actually extends down from the infrared. This peak's spectral location is calculated by convention (Lipetz 1984) as the “cutoff wavelength” (λRvis50), measured as the wavelength located half way between minimum (Rmin) and maximum (Rvismax, at or near 700 nm) reflectance over longer wavelengths. I also estimated relative amounts of UV (Rcontrast) by the segment-based (sensu Endler 1990) formula [(RλRmin-700) – (R320-λRmin)/(R320–700)].
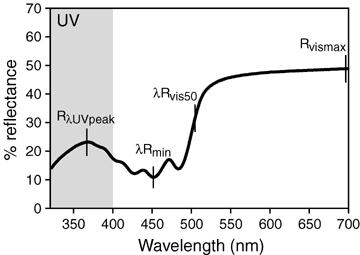
Typical reflectance spectrum of carotenoid-bearing plumage, indicating basic reflectance components used in analysis: λRvis50= cutoff wavelength, λRmin= wavelength of reflectance minimum, Rvismax= reflectance maximum at longer wavelengths, RλUVpeak= reflectance maximum at UV wavelengths. Shown is the mean (replicates × patch × individual × subspecies) spectrum for yellow cheek plumage of Periparus elegans (Paridae) across the avian-visible wavelength range. Shaded region indicates near-UV waveband. See text for further explanation.
Given that one of this study's aims is to compare subjective human, with objective physical, assessments of homoplasy, it is useful to apply human colors names to plumage patches. Pryke et al. (2001) observed that some reflectance components defined above also explain significant variation in human color perceptions of hue, or “redness” (λRvis50, 88%), and chroma (Rcontrast based on λRvis50, 75%) as estimated by the CIE (International Commission on Illumination 1971) system of human color space. Therefore, these components of reflectance provide an objective way to infer human-perceived color differences, even though the parameters differ from those employed in psychology. To help visualize plumage reflectance differences between UV and longer wavelengths, I recorded still-video images of museum study skins illuminated by each of the two wavebands (implemented by the methods described in Bleiweiss 2004b).
REFLECTANCE FINE STRUCTURE AND PIGMENT CHEMISTRY
Feather carotenoids and their amounts were determined by analyzing spectral shape and fine structure [(number and spectral location of the local absorption maxima = reflectance minima (Rmin)], which are diagnostic of different pigments (Rodriguez-Amaya 2001). This approach provides a practical basis for ascertaining general chemical features (in lieu of chemical extraction) across the large number of individuals and species in this comparative study. Intrinsic differences in fine structure generally correlate with the location of λRvis50 so that it is convenient to designate yellower (shorter λRvis50) versus redder (longer λRvis50) pigment classes (Inouye et al. 2001), although color also can depend on concentration and thickness of pigment (Rodriguez-Amaya 2001). For most carotenoids, the wavelength of the reflectance minimum (λRmin) resides above 440 nm whereas minima of noncarotenoid yellow to red pigments occur at shorter wavelengths [(psittacofulvins, λRmin located well below 440 nm, (Stradi et al. 2001; McGraw and Nogare 2005)] or lack minima over the relevant wavelengths (phaeomelanins)]. I also failed to detect any strong fluorescence indicative of pterins (Needham 1974). Melanins, including eumelanins responsible for many dark plumage patches by themselves (brown to black) or in combination with carotenoids (olive green) were identified by their flatter to concave reflectance profiles at longer wavelengths (Burkhardt 1989).
Increased amounts (pigment concentration or thickness) of pure carotenoids at levels below saturation are indexed mainly by increased absorption (reduced reflectance) at the short-wavelength (between 400 and 500 nm) minimum. However, to assess carotenoid amounts from the in situ reflectance spectra, it is necessary to take into account the potential confounding influences by feather keratins and melanins. The keratin proteins that comprise the feather matrix determine the upper bound to reflectance over all wavelengths. Melanin pigments absorb strongly over the entire relevant spectrum, acting to decrease both minimum and maximum reflectance. Thus, changes in the minimum (Rmin) relative to maximum (Rvismax) reflectance indicate primarily changes in amounts of carotenoids (Andersson and Prager 2006), calculated as the “carotenoid minimum” (Rcarmin) by the formula [(Rvismax−Rmin)/Rvismax].
FEATHER STRUCTURE
Many carotenoid-bearing (yellow to red) feathers have a simplified branching structure (Olson 1970; Johnson and Brush 1972; Brush 1990). These modifications appear to be pronounced in frugivores, and to correlate with their reduced amounts of UV reflectance (Bleiweiss 2004a, 2007). To further examine these associations, the feather mass within each patch was examined under a light microscope and ranked on an ordinal scale from one to four based upon reductions in the number of branching levels found in complete pennaceous contour feathers (Lucas and Stettenheim 1972), as before (see Bleiweiss 2004a): complete (rachis, ramus, barbule, barbicle) branching (1), lacking barbicels (2), lacking barbules (3), lacking rami (4).
CONFOUNDING VARIABLES
To further examine the robustness of links between plumage reflectance and diet, I assessed reflectance changes that might occur in relation to collection date (Bleiweiss 2005; McNett and Marchetti 2005) and breeding season (Figuerola and Senar 2005; Tubaro et al. 2005). Although collection date was available for all specimens, gonadal measures of breeding condition were available for few specimens (n= 17). Therefore, I also gauged breeding season based on each specimen's collecting locality, scoring breeding/nonbreeding season by the general rule that birds reproduce during the first half of the year north of the equator and the last half south of the equator (Isler and Isler 1999). This two-category, calendar-based measure was significantly associated with gonad size (t= 3.83, one-tailed P < 0.001), and allowed me to assess differences in reflectance in and out of the breeding season across the entire sample. Among other possible causes, post-mortem changes in UV reflectance may result from conversion of native trans-carotenoids to their cis isomers, which have distinctive “cis peaks” in the UV (McNett and Marchetti 2005). Cis isomers should be detectable by virtue of their λRmin values, which are 5 nm shorter than those for trans isomers.
ANALYSIS OF ANCESTRAL CHARACTER STATES
To determine the history of homoplasy, and to design appropriate statistical tests, I first ascertained the phylogenetic patterns of change (common ancestry vs. parallel or convergent homoplasy) for the relevant plumage pattern elements. I reconstructed plumage color pattern evolution for the relevant passerine clades (Table 1) with the focal tit-like pattern [two clades in titmice (Paridae), one each in wood warblers (Emberizinae) and painted berrypeckers (Paramythiidae)]. I used published phylogenies to reconstruct the evolution of plumage elements (Fig. 2). Preference was given to molecular-based phylogenetic hypotheses to avoid any circularity that follows from basing the phylogenies on the characters of interest (plumage). The phylogenies used defined a monophyletic Paridae (Gill et al. 2005), “Parulidae” (Lovette and Birmingham 2002), and the subclade that includes Paramythiidae within the larger Corvine radiation (Baker et al 2005). Euphonia belongs to yet another distinct clade with a variety of bold plumage patterns based on carotenoids (Euponiinae, Fringillidae), but as the tit-like pattern is absent from among those species, no reconstruction for that clade is presented. Minor differences in topology occur among published trees for each clade, depending on analytical methods and assumptions. I favored topologies based on likelihood or Bayesian methods (Fig. 2), although use of alternate topologies led to similar conclusions. Relationships among Wilsonia species had to be inferred from standard taxonomic treatments (Curson et al. 1994). Molecular hypotheses for paraphyly of traditional taxa also were accepted.

Phylogenies and ancestral character state reconstructions for living members of focal clades with tit-like plumage patterns. Reconstructions based on simple parsimony (unordered character states) for key male plumage elements that contribute to a tit-like appearance; patterns for belly and rump (not show) similar to those for breast and back, respectively. All trees are based on published molecular phylogenies (parids: Gill et al. 2005, figure 1B; parulids: Lovette and Birmingham 2002, figure 3, topology at left; paramythiids: Barker et al. 2005, figure 1 for relationships among genera, and Dumbacher and Fleisher 2001, figure 3 (Pitohui) and Moyle et al. 2006, figure 1 (Oriolus) for relationships within genera). “Equivocal” indicates two or more ancestral (or polymorphic) character states in the most parsimonious reconstruction. “Polymorphic” indicates two or more character states (one of which may be ancestral) in the extant taxon. To clarify presentation of the extensive parid phylogeny, some monophyletic clades that are monomorphic across character states were collapsed to single branches coded by ancestral character state for that clade: this reduction was applied to subspecies of Melanochlora sultanea, Parus niger, and Periparus ater, as well as to all 13 species of Poecile[monomorphic for all characters except the cap (black in all but two highly derived sister species)]. Bolded taxa have convergent tit-like appearance. Alternate coding schemes, and inclusion of more taxa based on traditional taxonomy, led to similar conclusions. The tit-like pattern apparently is lacking among all members of the Euphonia clade, whose species otherwise have extensive carotenoid-based coloration and distinctive plumage patterns.
Patches were scored for the tit-like (yellow, olive green, and black) and “other” colors. (Fig. 2) “Other” lumps certain distinctive phenotypes (white, brown, rufous, red), but captures the essential distinctions (more subdivided coding schemes gave similar results). The few patches of mixed color (crowns and backs of some parids and parulids) were scored for the predominant color. Intraspecific geographic variation in character states was scored as polymorphic. The evolution of plumage characters for each clade was then reconstructed using simple parsimony in MacClade (v. 4.03, Maddison and Maddison 2001), treating (multistate) characters as unordered, and gains and losses of character states as equally probable. Character transformations were polarized based on the root specified in the phylogeny for that clade.
STATISTICAL ANALYSES
I tested for associations between plumage reflectance and diet for the yellow, olive green, and black patches that comprise the male tit-like pattern, combining red (Oreocharis belly) and yellow patches for some analyses. Application of phylogeny-based statistical methods to control for effects of relatedness on standard statistical tests (e.g., Garland et al. 1998) is biologically unrealistic for the present comparisons because thousands of passerine species evolved between clades containing the focal taxa. Therefore, I employed a mixed effects general linear model to test the response of plumage reflectance components to diet for each of the color categories, and used the separate clades within each trophic class to detect clade-specific effects. This scheme treats diet (insectivorous vs. frugivorous) as a fixed effect, the separate clades as random effects nested within trophic class, and sources of variation below these levels as replicate sampling (Fig. 3). Notably, ancestral character state reconstructions indicated that plumage elements of the tit-like pattern evolved separately for each of the four occurrences except for the black markings in the two parid clades (Fig. 2). Thus, clade-specific and higher level effects are confounded with explicitly phylogenetic ones only for some black plumages.
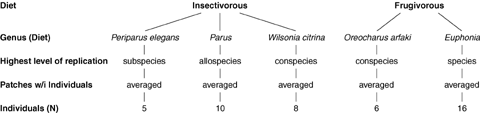
Experimental design used to test biomarkers of diet across passerine clades in which tit-like forms have (Periparus, Parus, Wilsonia, Oreocharis) or have not (Euphonia) evolved. Replicate individuals within clades are not taxonomically equivalent, but maximize variation within the higher-level effects (see MATERIALS AND METHODS for elaboration). Sampling of Euphonia comprised approximately 60% of species in that genus (Isler and Isler 1999), most of which have extensive yellow plumage. Note that the design is roughly balanced for sample sizes of individuals in each trophic class.
Replicate sampling within each clade/taxon was designed to maximize within-clade variation so that statistical tests would be conservative (Fig. 3; see the Appendix for enumeration of taxa and individuals). Thus, replicate individuals were drawn from different geographic localities for monotypic species (W. citrina, O. arfaki), from different subspecies for polytypic species (Periparus elegans), from different subspecies and allospecies (the Parus xanthogenys+P. spilonotus clade), and from different species for genera (Euphonia). To reduce pseudo-replication, mean reflectance values across patches of the same color grouping (yellow, olive green, black) were analyzed for each individual in the mixed model design (Fig. 3). Patches were analyzed as an ensemble only to estimate the interrelationships between reflectance variables because averaging patch values in this context would be misleading (results were qualitatively similar for analyses of maximum or minimum patch values).
Quantitative (year of collection, rank feather structure) and categorical (breeding status) confounding variables were added to the overall mixed model. Stepwise backward elimination revealed no significant higher-order interactions terms, so all models are based on first-order effects. Stepwise forward addition of confounding variables (Darlington and Smulders 2001) supported similar conclusions about their effects. Results were robust to a variety of data transformations, so results are based on raw data unless otherwise specified. All tests were two-tailed [(decrease or increase in reflectance in relation to confounding variables (McNett and Marchetti 2005), conservative assessment of plumage-diet hypothesis (Bleiweiss 2004a)]. Significance tests for general linear models were based on Type III sums of squares, and corrected for number of model parameters by standard Bonferroni procedures (Rice and Gaines 1994); separate probability statements were desirable for reflectance components, based on prior evidence for their significance. All statistical analyses were conducted in SAS 8.2 (SAS Institute Inc. 2003).
Results
PIGMENT IDENTITY
With few exceptions, yellow, red, and olive green plumage patches expressed λRmin values between 448 and 456 nm (Figs. 4, 5), and their spectra all had a “three-fingered” fine structure (when plotted at the appropriate scale) over intermediate wavelengths (Fig. 6). Both of these features distinguish “yellow”[hydroxy (λRmin > 445 nm) or canary xanthophyll (λRmin > 440 nm)] carotenoids (Rodriguez-Amaya 2001; Inouye et al. 2003) from “red”β-keto-carotenoids, which have a single broad Rmin located above 460 nm (Rodriguez-Amaya 2001).
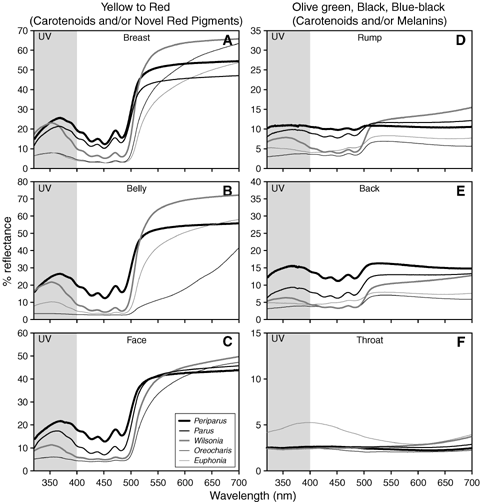
Reflectance spectra for selected plumage patches across the avian-visible wavelength range, including the UV (shaded, 320–400 nm). Shown are mean (replicates × patch × individual × subspecies × species) spectra for each color/pigment mechanism, by genus. See Bleiweiss (2004b) for additional details on recording equipment and methods. For carotenoid-bearing plumages (A–E), the secondary UV reflectance peak is separated from the high reflectance plateau at longer wavelengths by minimal reflectance over intermediate wavelengths (400–550 nm). At the longer wavelengths, concave reflectance in red (b, Oreocharis), olive green (D–E, all), and black (F, Periparus, Parus, Wilsonia, Oreocharis) to blue-black (F, Euphonia) plumage indicates the additional presences of melanins (see text). For carotenoid-bearing yellow, red, and olive green plumages, note variation in reflectance amplitude at shorter wavelengths, especially in the UV. See the Appendix for samples sizes and taxa averaged per genus.
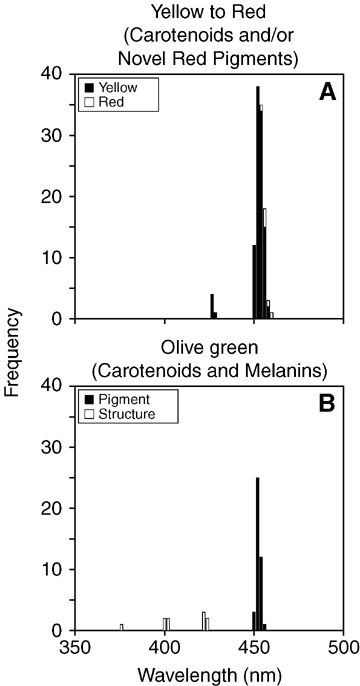
Spectral distribution of wavelength of reflectance minimum (λRmin= wavelength of minimum reflectance) for all patches × individuals × taxa for yellow to red (A) and olive green (B) plumages. Each observation represents the average value for replicate scans per patch. Note strong clustering of values around approximately 450 nm, a value diagnostic of carotenoids. Longer-wavelength shifts in minima for red compared to yellow plumages may result from the presence of additional pigments (including phaeomelanins) with absorption (strongly and inversely with wavelength) properties different from those of carotenoids (bimodal with wavelength). See RESULTS for additional details.
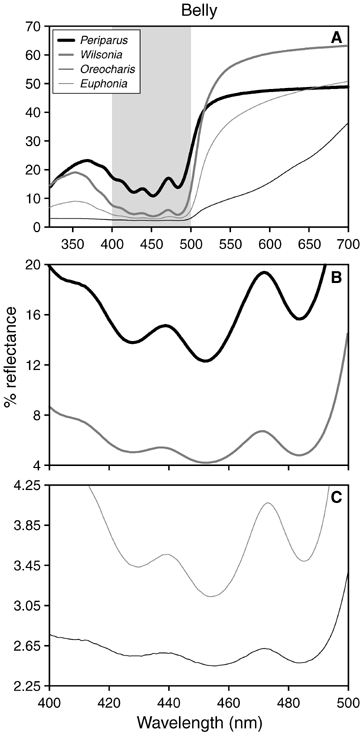
Comparison of reflectance spectra plotted on normative (A) and expanded (B–C) scales, highlighting spectral region of three-fingered fine structure (400–500 nm, shaded in A) typical of yellow carotenoids. Shown are mean (replicates × patch × individual × subspecies × species) spectra for belly patch, coded by genus. Species with low total reflectance across the relevant spectral window (Oreocharis, Euphonia) demonstrate characteristic carotenoid fine structure that parallels what is observed in species with higher total reflectance. Note changes in reflectance scale between panels.
The convex reflectance profiles for all yellow plumages are inconsistent with the presence of much if any melanin. However, melanins (presumably Phaeomelanins) and possibly β-keto-carotenoids, appeared to occur along with yellow carotenoids in the red belly plumage of Oreocharis; for this patch, the reflectance profile was more concave and had a muted fine structure (Figs. 4, 6). The lower and more concave reflectance profiles expressed by black plumages were typical of melanins (presumably eumelanins), whereas all olive green plumages appeared to combine features of carotenoids and eumelanins (Figs. 4, 5). The black and olive green plumages of Euphonia (e.g., Fig. 4F) had a strong bluish sheen suggestive of structural colors (Prum et al. 2003; Prum 2006), which could account for the shorter λRmin values for olive green plumages found in certain members (e.g., E. gouldi, E. mesochrysa, E. chrysopasta) of the genus (Fig. 5B).
OBJECTIVE ASSESSMENT OF PLUMAGE REFLECTANCE AND HOMOPLASY
For yellow plumage, most reflectance components were significantly associated with diet (Table 2; Figs. 4, 6). However, diet was more closely linked to reflectance components based on absolute (R320–400, RλUVpeak, λRUVpeak) and relative (Rcontrast) UV than on longer (R400–700, λRvis50, Rvismax) wavelengths (Table 2; Figs. 4, 6). The strong central tendency (little variation) in λRUVpeak (see Bleiweiss 2005) probably explains this component's weaker association with diet relative to other measures of UV. Thus as biomarkers, UV generally trumped longer wavelengths, and the magnitude of reflectance generally outperformed the spectral location of reflectance. These distinctions between UV versus longer wavelengths were evident in still-video images taken over the two wavebands (Fig. 7).
| Model1,2,3 | Longer wavelengths (400–700 nm) | UV wavelengths (320– 400 nm) | Combined | ||||
|---|---|---|---|---|---|---|---|
| R 400–700 | R vismax | λRvis50 | R 320–400 | R λUVpeak | λRUVpeak | R contrast | |
| Yellow | |||||||
| breed | 0.49 | 0.48 | 0.20 | 0.32 | 0.23 | 1.22 | 0.05 |
| year | 0.09 | 0.70 | 0.36 | 2.01 | 2.16 | 0.74 | 2.48 |
| diet | 18.29*** | 0.19 | 67.45*** | 90.75*** | 100.77*** | 6.39# | 85.89*** |
| genus (diet) | 2.27 | 6.79* | 2.36 | 0.78 | 0.71 | 20.29*** | 11.59*** |
| R 2 | 0.3881 | 0.3330 | 0.7271 | 0.7540 | 0.7657 | 0.6200 | 0.8032 |
| Yellow+Red | |||||||
| breed | 0.62 | 0.59 | 0.41 | 0.40 | 0.03 | 1.29 | 0.05 |
| year | 0.08 | 0.75 | 0.68 | 2.23 | 2.38 | 0.80 | 2.67 |
| diet | 35.80*** | 1.86 | 231.23*** | 101.73*** | 112.91*** | 31.88*** | 78.25*** |
| genus (diet) | 4.23# | 7.57** | 36.03*** | 1.44 | 1.62 | 29.09*** | 11.06*** |
| R 2 | 0.5091 | 0.3498 | 0.8864 | 0.7700 | 0.7819 | 0.7333 | 0.7965 |
- 1Significance of F-value: *=P < 0.05; **=P < 0.01; ***P < 0.001 (after Bonferroni correction for four simultaneous comparisons). Two degrees of freedom for genus (diet), one otherwise.
- 2Significance of F-value: #=P < 0.05 (only before Bonferroni correction for four simultaneous comparisons).
- 3PROC GLM in SAS.
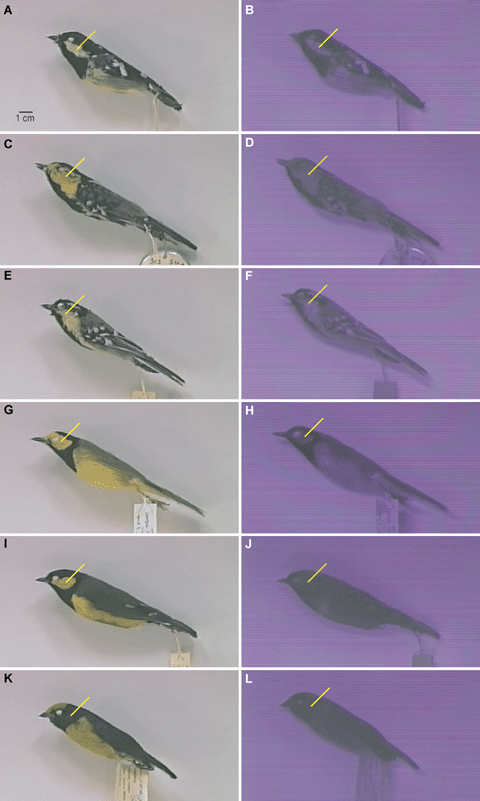
Human-visible (left) and UV (right) still-video images of adult male Periparus elegans (A–B); American Museum of Natural History 416839), Parus spilonotus (C–D; AMNH 306444), Parus xanthogenys (E–F; Los Angeles County Museum 33001, Wilsonia citrina (G–H; Delaware Natural History Museum 48216, Oreocharis arfaki (I–J; AMNH 421481), and Euphonia xanthogaster (K–L; University of Wisconsin Zoological Museum A20109). The UV images are transduced to violet blue by the video system. Note that UV reflectance by carotenoid-based plumages (yellow to red, olive green) is strong in insectivorous (Periparus, Parus, and Wilsonia) but weak in frugivorous (Oreocharis and Euphonia) taxa. Yellow pointers identify cheek region to facilitate comparisons of reflectance under the two illuminations. Images were made with an JVC GX S700U color video-camera (Wayne, NJ) fitted with a Takumar f1.8 lens (for details see Bleiweiss 1994, 2004b). UV images were obtained by capping the lens with a Kodak 18A Wratten filter (Kodak, Rochester, NY), which selectively passes UV between 310 and 400 nm and has a transmission maximum of 70% at 365 nm. This setup approximates passerine sensitivity to UV, which extends from 320 to 400 nm and is maximal around 370 nm (Cuthill et al. 2001). GretagMacbeth (New Windsor, NY) SpectraLight II and BLACK-RAY (San Gabriel, CA) UVL-56 lamps were used to provide UV versus human-visible illumination.
Red plumage was restricted to and within Oreocharis, and thus, was not a homoplastic feature among species. Nevertheless, the association of λRvis50 with diet increased dramatically for analyses that combined yellow with red patches because red plumage contributed disproportionately to the general red shift (quantitative shifts of λRvis50 to longer wavelengths) associated with frugivory (Table 2; Fig. 4). As part of this relationship, the red patch also extended the tail end of the general nonlinear (asymptotic) relationship between UV (R400–700 or RλUVpeak) and λRvis50 also expressed among yellow patches alone (Table 3; Fig. 8A). An important consequence of this functional relationship was that UV reflectance components varied more among insectivores whereas λRvis50 varied more among frugivores (Table 3; Fig. 8A).
| Parameter1,2,3 | Yellow4 | Yellow+Red4 | ||||
|---|---|---|---|---|---|---|
| F | t | Adj. R2 | F | t | Adj. R2 | |
| λRvis50 | 188.02 | −13.71 | 0.68302 | 78.19 | −8.84 | 0.45323 |
| λRvis50+(λRvis50)2 | 147.34 | −6.10+5.84 | 0.77166 | 160.13 | −11.90+11.46 | 0.77386 |
| 1/λRvis50 | 201.50 | 14.20 | 0.69788 | 95.95 | 9.80 | 0.50516 |
| (λRvis50)1/2 | 191.41 | −13.84 | 0.68689 | 82.28 | −9.07 | 0.46637 |
- 1All models significant at P<0.001 (after Bonferroni correction for four simultaneous comparisons).
- 2Results were similar for analyses based on R320–400 and Rcontrast as dependent variables.
- 3PROC REG in SAS.
- 4Note that R2 is greater in both relative and absolute terms for nonlinear quadratic polynomial compared to other models.
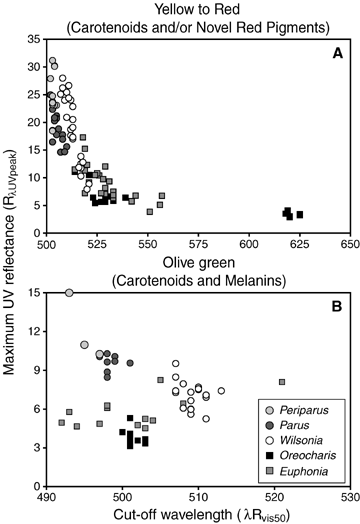
Maximum UV reflectance (RλUVpeak) versus cutoff wavelength (λRvis50) for yellow to red (A) and olive green (B) plumage patches; similar results obtained for total UV reflectance (R400–700) plotted against λRvis50. Each point represents the average value for replicate scans per patch, coded by genus (see MATERIALS AND METHODS for rational). Not all taxa have a particular patch color (olive green), so numbers of points were sometimes fewer than numbers of individuals for that genus (e.g., subspecies of Periparus elegans and Parus spilonotus). Statistical tests support a nonlinear response of UV to λRvis50 for either yellow or yellow plus red plumages (Table 3); this functional relationship is evident in the smooth transition between insectivores and frugivores over shorter λRvis50, but is accentuated by outlier points at the longest wavelengths (red belly plumage of Oreocharis), which may contain appreciable amounts of phaeomelanins as well as carotenoids. The nonlinear relationship observed between UV reflectance and λRvis50 changes associations between reflectance and diet as a function of spectral location (λRvis50); UV is a strong predictor of diet over shorter λRvis50, whereas λRvis50 improves as a predictor over longer λRvis50.
The similar functional relationships expressed by carotenoids alone or in combination with dissimilar red pigments suggest that dietary links to plumage reflectance are robust to certain differences in pigment composition. Consistent with this expectation, the general association between reflectance components and diet also held for olive green plumages (Table 4, Figs. 4, 6), which are produced by codeposition of carotenoids and eumelanins. However, the results for olive green plumage were distinctive in some respects. The biomarkers were weaker (especially for λRvis50), were less biased to UV components, and showed stronger taxonomic (nested) effects. Also, the strong covariation between UV reflectance components and λRvis50 observed among yellow to red plumage patches broke down for olive green ones (Fig. 8B). For pigment combinations, therefore, the biomarker patterns remained strong, but the covariance patterns among reflectance components became weak. Diet also was associated to some extent with reflectance properties of blackish plumages (Table 4). However, the iridescent blue–black plumages of most Euphonia (frugivores) contributed much short-wavelength reflectance (Fig. 4F) to this plumage × diet category (Tables 3 and 4). Thus, limitations to sampling design (resemblance among parids due to shared history, blueness of Euphonia) compromised interpretation of those results.
| Model1,2, 3 | Longer wavelengths (400–700 nm) | UV wavelengths (320–400 nm) | Combined | ||||
|---|---|---|---|---|---|---|---|
| R 400–700 | R vismax | λRvis50 | R 320–400 | R λUVpeak | λRUVpeak | R contrast | |
| Olive Green | |||||||
| breed | 1.35 | 0.06 | 1.99 | 5.51# | 4.95# | 0.04 | 9.96* |
| year | 1.28 | 1.70 | 0.55 | 0.44 | 0.55 | 0.05 | 0.03 |
| diet | 49.69*** | 45.77*** | 6.16# | 43.68*** | 48.15*** | 0.46 | 33.13*** |
| genus (diet) | 3.78# | 3.71# | 81.19*** | 7.00* | 7.18* | 100.59*** | 39.43*** |
| R2 | 0.8279 | 0.8014 | 0.9273 | 0.8291 | 0.8378 | 0.9285 | 0.8730 |
| Black | |||||||
| breed | 2.91 | 2.88 | 0.75 | 4.22# | 4.29# | 1.34 | 0.66 |
| year | 0.10 | 0.83 | 0.97 | 0.05 | 0.00 | 2.99 | 2.71 |
| diet | 9.62* | 18.28*** | 6.65 | 74.10*** | 108.14*** | 0.75 | 7.43* |
| genus (diet) | 41.34*** | 66.34*** | 12.94# | 68.86*** | 96.98*** | 0.53 | 14.65*** |
| R2 | 0.7731 | 0.8545 | 0.9432 | 0.8897 | 0.9205 | 0.5687 | 0.5382 |
- 1Significance of F-value: *=P < 0.05; **=P < 0.01; ***P < 0.001 (after Bonferroni correction for 4 simultaneous comparisons). Two degrees of freedom for genus (diet),one otherwise.
- 2Significance of F-value: #=P < 0.05 (only before Bonferroni correction for four simultaneous comparisons).
- 3PROC GLM in SAS.
FEATHER STRUCTURE, CHEMISTRY, AND HOMOPLASY
Values of Rcarmin for yellow and yellow + red plumages were strongly linked to diet (Table 5), indicating greater amounts of carotenoids in these feathers regardless of the confounding effects of other pigments and structures. Similar results were obtained with Rmin (Bleiweiss, unpubl. data), which supports evidence from the spectra that melanins are not major constituents in these same feathers (except possibly, red ones). Conversely, the relationship vanished when substantial melanins (olive green feathers) were present (Table 5). Chemistry (indexed by λRmin,) was not associated with diet, presumably because all groups used similar carotenoids (Table 5). Associations between frugivory and feather structure depended mostly on the highly modified red feathers of Oreocharis (Table 5).
| Model2,3 | Chemistry1 | Structure | |||||
|---|---|---|---|---|---|---|---|
| R carmin | λRmin | ||||||
| Yellow | Yellow + Red | Olive green | Yellow | Yellow+Red | Yellow | Yellow+Red | |
| breed | 0.00 | 0.01 | 2.27 | 2.23 | 1.74 | 0.13 | 0.08 |
| Year | 0.08 | 0.11 | 0.00 | 0.44 | 1.47 | 1.662.14 | 2.14 |
| diet | 22.74*** | 23.49*** | 0.86 | 0.01 | 2.77 | 3.12 | 7.14* |
| genus (diet) | 13.25*** | 13.23*** | 18.99*** | 10.99*** | 6.99* | 7.00* | 11.75*** |
| R 2 | 0.6518 | 0.6678 | 0.7704 | 0.4135 | 0.3987 | 0.3052 | 0.4331 |
- 1 R carmin is proxy measure for amount of carotenoids, λRmin is a proxy measure for cis-isomerization of native trans-carotenoid pigments.
- 2Significance of F-value: *=P < 0.05; **=P < 0.01; ***P < 0.001 (after Bonferroni correction for four simultaneous comparisons). Two degrees of freedom for genus (diet), one otherwise.
- 3PROC GLM in SAS.
EFFECTS OF CONFOUNDING VARIABLES
Date of collection was a poor predictor of all reflectance components (Tables 2, 4, and 5) despite a more than 50-year span in collection dates for all taxa (Table 6). Reflectance components for yellow patches of insectivores and frugivores overlapped mainly because of anomalously low values for three old (19th century) specimens of W. citrina (Fig. 8A). Nor did gonad-based (all P > 0.10) or calendar-based measures of breeding seasonality associate significantly with reflectance properties of plumage, except for one reflectance variable (Rcontrast) for olive green plumage (Tables 2–4). There were no significant shifts in the location of λRmin with collection date (Table 5), which would otherwise suggest cis-isomerization of native carotenoids.
| Genus1 | N | Mean | SD | Range |
|---|---|---|---|---|
| Periparus | 5 | 1936.4 | 29.712 | 1896–1965 |
| Parus | 10 | 1949.1 | 17.149 | 1924–1988 |
| Wilsonia | 8 | 1923.1 | 34.044 | 1880–1959 |
| Oreocharis | 6 | 1930.2 | 17.691 | 1899–1954 |
| Euphonia | 16 | 1940.4 | 32.786 | 1860–1977 |
- 1PROC MEANS in SAS.
HISTORY OF HOMOPLASY
Parsimony analyses supported convergent development of the tit-like color pattern in two ways. First, the yellow, olive green, and black pattern elements evolved independently in all cases (belly and rump not shown) except for black elements in the two parid subclades (Fig. 2). Second, the overall resemblance was achieved by a different sequence of changes across all of the different clades (Fig. 2). These different histories imply that color and pattern changed independently, rather than as a suite of correlated characters. With respect to diet evolution, insectivory is nearly universal in the parid and parulid clades, whereas frugivory developed independently in paramythiids, euphoniids, and their relatives (unpubl. data).
Dietary effects on resemblance are further implicated by failure to detect links between other ecological factors and color (Table 7), and by evidence for the carotenoid biomarkers in other, dissimilarly patterned, relatives: ample UV in insectivorous parids (Cuthill et al. 2000; Johnsen et al. 2003) and parulids (McNett and Marchetti 2005; unpubl. ms), meager UV in frugivorous euphoniids (Chlorophonia; unpubl. data) and corvids (Oriolus; unpubl. data). However, the widespread occurrence of the biomarkers allows that they antedate development of the tit-like pattern. Plumage pattern resemblance seems not to depend on diet or historical rates and durations of selection for convergence. Thus, Oreocharis and Euphonia share many features typical of specialized frugivores (chunky body, thick bill, short sturdy legs; Beehler et al. 1986; Coates 1990; Isler and Isler 1999) despite their radically dissimilar plumage patterns. One is led to conclude that intrapatch reflectance surpasses interpatch pattern as an indicator of diet.
| Taxon | Ecological habit5 | ||
|---|---|---|---|
| Elevation (m) | Habitat | Nest | |
| Parinae, Paridae1 | |||
| Periparus elegans | 0–2470 | broad-leaf lowland & montane tropical forest | tree-hole |
| Parus xanthogenys/spilonotus | 0–2620 | coniferous temperate forest | tree-hole |
| Paramythiidae, Corvoidea2 | |||
| Oreocharis arfaki | 1750–3000 | broad-leaf tropical montane forest | cup |
| Emberizinae, Fringillidae3 | |||
| Wilsonia citrina | 0–2500 | broad-leaf tropical & temperate forest | cup |
| Euphoniinae, Fringillidae4 | |||
| Euphonia spp. | 0–1500 | broad-leaf lowland & montane tropical forest | domed |
- 1Quinn and Harrap 1995.
- 2 Beehler et al. 1986.
- 3Howell and Webb1995.
- 4 Isler and Isler 1999.
- 5Putative selection pressures as follows: (1) elevation = increase in UV with elevation; (2) habitat = humidity, or conspicuousness relative to properties of the physical environment (as determined by ambient light qualities and broad secular trends with vegetation); (3) nest = exposure to predation (as determined by nest architecture); (4) dietary specializations, as summarized in Table 1= mate choice based on colors of important food items (insects vs. fruits, types of fruits). The small body size of all species should control for many metabolic and life-history constraints that could affect plumage color.
Discussion
My study reveals a suite of reflectance components that can resolve apparent mismatches between diet and plumage. These biomarkers highlight the importance of physical measures of plumage reflectance because key components are either invisible to (UV spectral region) or overlooked by (subtle shifts in λRvis50, feather structure) humans. By comparison, subjective human impressions of color or pattern bear little relationship to diet. The biomarkers likely have biological relevance because most species in the families examined here have superior color vision that extends to the UV spectral region (Cuthill 2000; Ödeen and Håstad 2003).
These findings support the general hypothesis that carotenoid-based plumage reflectance is linked to an animal's diet. But this ecological association could arise through at least three potentially overlapping mechanisms. First, direct selection on heritable genetic variation may act to match the appearance of sexually attractive traits to that of important food items (Rodd et al. 2002), or otherwise favor certain plumage colors in species with particular ecological habits. The available evidence seems to rule out the first possibility because Euphonia species specialize on mistletoe (Loranthaceae) berries (Isler and Isler 1999), which lack typical carotenoid-based coloration. The absence of any obvious associations between carotenoid-based reflectance and ecological variables other than what the birds actually consume (Tables 1 and 7) also suggests a more proximate cause for the dietary biomarkers. A second possibility is that genetic effects are an indirect consequence of selection for plastic phenotypes that track the amount of dietary pigment. Such ecophenotypes could be favored as honest indicators of genetic qualities that affect an individual's ability to acquire and use carotenoids, such as those that determine competitiveness, health, and parental care (Olsen and Owens 1996; Hill 2002). Under this “good genes” (including “good-parents”) hypothesis, use of carotenoids as indicators of individual quality could produce interspecific associations of color with diet because pigmentation will track the amount of dietary carotenoids. The hypothesis does not require that interspecific variation is adaptive, although such advantages are possible (social mimicry, signaling to predators, etc.). Third, the differences in plumage reflectance could simply be nonadaptive byproducts of the general inability of animals to synthesize or regulate epidermal carotenoids. This hypothesis does not imply any advantage to color differences among individual or species. Studies of carotenoid-based pigmentation in parids support both honest signaling (within populations; Senar et al. 2002), and nonadaptive (across habitats: Slagsvold and Lifjeld 1985; across pollution gradients: Eeva et al. 1998, 2005) interpretations. Here I consider the physical and biological origins for the dietary biomarkers as they relate to these hypotheses.
PROXIMATE BASIS FOR BIOMARKERS
A direct link between carotenoid levels in a bird's diet and in its yellow feathers is suggested by several findings. First, the proxy measure for amount of pigment (Rcarmin) indicates that frugivores have significantly greater amounts of plumage carotenoids. Second, plots of reflectance against carotenoid concentration typically produce the kinds of nonlinear (saturation) curves (Grether 2000) observed for plots of UV reflectance components versus λRvis50 (Table 3, Fig. 8A). Thus, the placement of frugivores at what would be the high concentration end of this distribution reinforces the other evidence that carotenoid-based plumage reflectance is a function of the amount of pigment. That these patterns relate to concentration per se more than to thickness of the pigmented layer in the feather (both of which influence total amount of pigment) is consistent with the weak association between reflectance and feather branching structure, as the latter is accompanied by changes to the thickness of the pigmented cortical layer (see below).
The direct connection between pigmentation and diet also is suggested by evidence that all species use the same principal carotenoid in their feathers (tight clustering of λRmin). This underlying commonality excludes the alternative possibility that species with different diets selectively deposit different carotenoids, distinguished by their intrinsic absorption properties. Indeed the λRmin values closely match those for the hydroxy-carotenoid lutein, one of the most widespread carotenoids, and one that is deposited unmodified in the plumage of many birds, including those of titmice and wood warblers (Partali et al. 1987; Mays et al. 2003; McGraw 2006). Thus, ecophenotypy seems to be responsible for some level of resemblance among species.
Although melanins generally are not thought to link to diet as closely as do carotenoids (Hill and Brawner 1998; Roulin et al. 1998; McGraw and Hill 2000), my data do not entirely rule out such a connection (see also Jawor and Breitwisch 2003). In particular, co-deposition of melanins with carotenoids (red, olive green) does not eliminate associations of reflectance with diet. Thus, either melanins do not entirely mask the carotenoid component of the signal, or melanins also are linked to diet, or both. With regard to the latter possibility, it is notable that reflectance by eumelanin-rich black plumage is lowest in the frugivore Oreocharis (Fig. 4F; the other frugivorous taxon Euphonia, lacks black plumage). Thus, if melanin deposition depends in part on diet, then combinations of melanins and carotenoids also should produce consistent dietary associations. These results suggest that frugivores may actually deposit larger quantities of all pigments.
Links between diet and feather branching structure were not as marked among tit-like, as compared to some other, birds (Olson 1970; Bleiweiss 2004b, 2007). When present, such modifications support the hypothesis that pigment amount (via diet) governs feather reflectance because reductions in branching appear to occur mostly in feather that are heavily laded with carotenoids (Brush and Seifried 1967; Johnson and Brush 1972; Brush 1990). How structural modifications reduce amounts of UV and red shift λRvis50 is uncertain. The corresponding thickening of the carotenoid-bearing cortical layer (Olson 1970; Finger 1995) that accompanies the gross changes in branching may increase absorption by augmenting optical density (concentration, thickness), by reducing the medullary layer responsible for structural colors (Mennill et al. 2003; Prum et al 2003; Prum 2006), or by some combination thereof. More work on proximate mechanisms is needed to untangle these possibilities.
IMPORTANCE OF UV VERSUS LONGER-WAVELENGTH BIOMARKERS OF DIET
In addition to clarifying the nature of plumage homoplasy, my results qualify earlier evidence that UV reflectance components (R320–700, RλUVpeak, Rcontrast) surpasses red shifts as dietary biomarkers (Bleiweiss 2004a). Although the earlier study (of tanager-finches) sampled extensively across rather than within species, controls on confounding factors were similar to those implemented here. Unlike tit-like birds, however, tanager-finches appear to achieve relatively redder (longer λRvis50) plumage by any one of several pigmentation strategies (Fig. 9; Bleiweiss 2007). As with frugivorous tit-like birds, frugivorous tanager-finches can deposit hydroxy-carotenoids at higher optical densities to achieve a redder plumage (see also Saks et al. 2003). But tanager-finches also appear capable of producing red plumage by depositing intrinsically redder β-keto-carotenoids at various optical densities (Fig. 9). This second pigmentation strategy occurs in either insectivores or frugivores, and apparently depends less on broad dietary distinctions than on use of unusual foods (Hudon 1989), or the ability to metabolize hydroxy-carotenoids into β-keto-carotenoids (Brush 1990; Bleiweiss 2007). Among species using such a wide variety of pigments, therefore, multiple routes to red coloration (high optical densities of yellow carotenoids, or a range of optical densities of red carotenoids) confound the diet-dependent [optical density-related (concentration or thickness of pigment)] red shift.
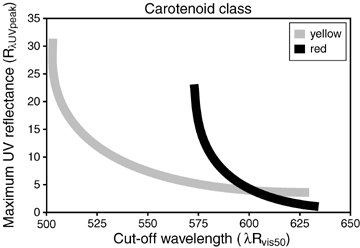
Schematic phenotype space defined by yellow (hydroxy, ɛ-keto) and red (β-keto) carotenoids [(based on tanager-finches (Bleiweiss 2007), and tit-like passerines (this study)]. Note that each pigment class describes an asymptotic curve in which the amount of UV saturates at higher cutoff wavelengths (λRvis50). Phenotypic variation among tit-like birds follows the curve for yellow pigments, whereas that for tanager-finches encompasses both curves. Because of these differences in pigmentation among taxa, interpretation of color variation in relation to diet depends on scale (portion of this variation expressed among species under consideration). See text for additional explanation.
For species that use carotenoid pigments with the same absorptive properties (within curves; Fig. 9), however, UV reflectance and λRvis50 are necessarily linked via their asymptotic interrelationship. At this scale, diet uniquely determines where a species falls along the bivariate reflectance curve that includes λRvis50. Indeed, for the more restricted pigmentation system, species with carotenoid-poor diets (high UV) are more divergent for UV components of reflectance, whereas species with carotenoid-rich diets (low UV) are more divergent for λRvis50 (Fig. 8A). Even among closest relatives, the sibling species of Parus (insectivores) are more divergent for UV whereas the siblings species of Euphonia (frugivores) are more divergent for λRvis50. More extensive (continuous) sampling indicates similar functional relationships in tanager-finches that employ one or the other broad class of carotenoid pigment (Bleiweiss 2007), and for certain fish (Grether 2000). These distinctions should be visible to most diurnal birds, given their superior vision across UV and longer wavelengths (Cuthill 2000). Thus, scale (e.g., within and across pigment classes, absolute value of λRvis50) is an important consideration for studies of signal evolution.
These various patterns reveal that use of yellow versus red carotenoid pigment classes as colorants translates the functional interrelationship between UV reflectance and λRvis50 along the redness axis (Fig. 9), but that any given pigment class preserves the covariance relationship between these reflectance variables. All other considerations being equal, the invariance of UV to changes in pigment class (redness) should make UV a superior indicator of carotenoid optical density, and hence, a more universal guide than redness to trophic status (Fig. 9).
ASSESSMENT OF PLUMAGE AS AN EVOLUTIONARY MOSAIC
Ultimately, the associations between plumage and diet at multiple evolutionary scales fulfill the basic expectation for ecological explanations of homoplasy that morphology and environment are linked. However, the results of this and earlier studies suggest that homoplasy may arise by far more subtle and varied processes than those considered in traditional models of adaptation (Grether et al. 2004). Thus, some genetic control on carotenoid pigmentation occurs even in parids whose plumage responds to dietary levels of these pigments (Fitze et al. 2003; Johnsen et al. 2003). For example genetically based metabolic pathways regulate feather pigmentation and structure (Brush 1990), and populations may evolve to use dietary carotenoids more efficiently (counter gradient selection; Craig and Foote 2001). More generally, a flexible developmental program may itself be an adaptation subject to selection (West-Eberhardt 2003). Related to this, peak shift models of genetic change under phenotypic plasticity indicate that trait comprised of both plastic and hard-wired elements facilitate such shifts (Price et al. 2003).
On the other hand, my study suggests that complex color-producing mechanism do not necessarily obscure unifying influences on plumage reflectance. For example, the different absorptive properties of carotenoids and melanins have led to their separate treatment in studies of color evolution (Hofmann et al. 2006). However, the similar (although not identical) associations between diet and combinations of carotenoids and melanins (yellow + red plumage, yellow + black) and carotenoids alone (yellow) suggest that ecologically relevant properties of reflectance can transcend combinations of qualitatively different pigments (Tables 3 and 4). Moreover, the discontinuity in λRvis50 values seen in plots of yellow + red plumages (Fig. 8A) probably relates to sampling design (choice of convergent forms) because more comprehensive samples of long-wavelength plumage signals fill this gap. (Bleiweiss 2007). Indeed, these other studies reveal that the nonlinear (saturation) response of the amount of UV to λRvis50 is typical of various carotenoids, deposited alone or in combination with similar (Grether 2000; Bleiweiss 2007) or different (pteridine) pigments (Grether 2004). Such consistency is less surprising if one considers that all pigments regulate reflectance through selective light absorption. Thus, comparative analyses of phenotypes based on pigment combinations can be biologically meaningful.
At the whole organism level, the uncoupling of color and pattern in relation to diet suggests that these two aspects of appearance are governed by some different selective or developmental factors. This is unsurprising because pigments and pigmentation patterns interact differently with physiology and environment. Plumage pattern (where pigments are deposited) may have a stronger genetic component than plumage reflectance (how much pigment is deposited in a pattern element). In that case, changes in pattern could be uncoupled from changes in either diet or within-patch reflectance. However, links between the tit-like pattern and nondietary ecological factors are no more evident than between reflectance and these other factors (Table 7); for example, the allopatry of tit-like species (Table 1) precludes their convergence through social mimicry (Moynihan 1968; Cody 1970). Moreover, interspecific variation in sexual dichromatism also suggests that both extrinsic (social, sexual behaviors) and intrinsic (metabolism, hormones) factors act on plumage patterns. Thus, interspecific plumage divergence is likely to be influenced by factors as varied as those acting within species (McGraw et al. 2002; Endler et al. 2005).
Associate Editor: M. Webster
ACKNOWLEDGMENTS
I thank M. Webster and two anonymous reviewers for helpful comments on the manuscript. The curators of the American Museum of Natural History (New York), Delaware Museum of Natural History (Greenville), Los Angeles County Museum (Los Angeles), Museum of Comparative Zoology (Cambridge), the United States National Museum (Washington, D.C.), and the University of Wisconsin Zoological Museum (Madison) made generous loans of specimens. P. Mathiaparanam (Appleton Ideas Company) and F. Padera (Perkin-Elmer Corporation) provided equipment. The Biology New Media Center (University of Wisconsin, Madison) facilitated video-to-digital image transfer, and W. Feeny drafted the figures.
Appendix
Specimens used to generate reflectance spectra. AMNH = American Museum of Natural History (New York, NY), DEL = Delaware Museum of Natural History (Greenville, DE), MCZ = Museum of Comparative Zoology (Cambridge, MA), USNM = United States National Museum (Washington, DC), UWZM = University of Wisconsin Zoological Museum (Madison, WI).
Periparus elegans elegans AMNH 784519; Periparus elegans albescens AMNH 782159, AMNH 680865; Periparus elegans elezani AMNH 680873; Periparus elegans gilliardi AMNH 459599, Parus spilonotus spilonotus AMNH 306444, AMNH 306451, AMNH 600821, USNM 586004, Parus spilonotus subviridis MCZ 265957. Parus xanthogenys xanthogenys AMNH 801090, AMNH 801092, AMNH 801093, MCZ 185464, MCZ 185475. Wilsonia citrina DEL 41705, DEL 45585, DEL 48213, DEL 48214, DEL 48216, MCZ 190027, MCZ 210388, MCZ 309957. Oreocharis arfaki AMNH 267778, AMNH 421481, AMNH 421495, AMNH 421497, AMNH 699229, AMNH 766084. Euphonia affinis UWZM 15389. Euphonia chlorotica serrirostris MCZ 266997. Euphonia trinitatus MCZ 105401. Euphonia concinna MCZ 34526. Euphonia saturata MCZ 299609. Euphonia violacea MCZ 176587. Euphonia laniirostris hypoxantha MCZ 299553. Euphonia hirundinacea MCZ 136946. Euphonia fulvicrissa MCZ 155979. Euphonia gouldi DEL 46667. Euphonia chrysopasta AMNH 232768. Euphonia mesochrysa DEL 65020. Euphonia anneae MCZ 109410. Euphonia xanthogaster brevirostris UWZM A20105. Euphonia rufiventris UWZM A20111. Euphonia cayennensis MZC 176608.




