Evaluating Degradation with Fragment Formation of Prehospital Succinylcholine by Mass Spectrometry
Presented at the Society for Academic Emergency Medicine regional meeting, Newark, DE, 2009; to be presented at the North American Association of Clinical Toxicologists meeting, San Antonio, TX, 2009.
This study received no grants or financial support. No conflict of interest exists or copyright constraints with any author.
Abstract
Objectives: Pharmaceutical manufacturers recommend refrigerating succinylcholine at a temperature range of 2–8°C. With widespread use of prehospital succinylcholine on ambulances without refrigeration, it is important to understand the stability of this drug. Using mass spectrometry, this study investigated the degradation of the succinylcholine compound before and after its exposure to ambulance cabin temperatures, while removing light exposure. A 10% degradation threshold was set as not appropriate for human use, in accordance with U.S. Food and Drug Administration guidelines.
Methods: The study used 17 vials of succinylcholine sealed with duct tape in light-resistant bags. The bags were placed in climate controlled compartments in two ambulances: one stationed in a garage and the other stationed outdoors. Mass spectrometry analysis was used to examine drug degradation at Time 0, the 14th day of the first month, and monthly from Time 0 to 7 months.
Results: The degradation products of succinyl monocholine (SMC) and choline are already present at Day 0. Ten percent degradation was achieved at approximately 90 days into the experiment. Temperature in the ambulance climate controlled compartment was 70°F, with a range from 56 to 89°F during the 6-month time period.
Conclusions: Identifiable breakdown fragments of succinylcholine have been identified using mass spectrometry with fresh drug upon receipt from the manufacturer. Ten percent degradation was not observed until approximately 90 days after being placed on ambulances. Temperature variations did not significantly contribute to degradation of succinylcholine, and it is safe for injection until approximately 90 days in similar climates.
ACADEMIC EMERGENCY MEDICINE 2010; 17:631–637 © 2010 by the Society for Academic Emergency Medicine
Succinylcholine is a depolarizing neuromuscular blocking agent used to assist endotracheal intubation. Previous degradation studies apply to the hospital setting, where succinylcholine is stored at the recommended temperature. However, in the prehospital setting, no current literature exists involving succinylcholine degradation and fragment formation as measured by mass spectrometry.
It is essential to understand the factors that can lead to degradation of the drug. It is estimated that 85% of succinylcholine is destroyed within the 30 seconds of injection into the blood, and only 5% remains present 2 minutes after injection.1 Studies have shown that the drug is susceptible to a loss of potency with increases in temperature and light and decreases in pH.2–5 While light affects the stability of succinylcholine injections only slightly, decreased pH has a larger effect because the hydrolysis of the drug is catalyzed by hydrogen ions. One of the degradation products is succinic acid, which further lowers the pH and accelerates the degradation reaction.6 Extreme temperatures in countries with hot climates have demonstrated decreases in effectiveness of the drug well before the expiration date.7–14
While studies have shown increased rates of degradation of succinylcholine at high temperatures, little data are present on the effect of temperature fluctuations present in the prehospital environment. As opposed to the existing literature, this study evaluates the use of succinylcholine on ambulances that were in service and responding to emergency calls. Real temperature variations in standardized climate-controlled cabinets were evaluated, and in contrast to the prior literature, high-technology mass spectrometry was used to determine the specific fragment formation.2,6,9,14
Because succinylcholine is stored on paramedic vehicles throughout the country, the procedure for removing unused vials needs to be standardized and based on scientific literature. Currently, pharmaceutical manufacturers recommend refrigerating succinylcholine at a temperature range of 2–8°C and state that the multidose vials are stable for up to 14 days at room temperature without significant decomposition.4 Significant protocol inconsistency exists within regions; some replace succinylcholine daily or monthly, while some adhere to expiration dates. Emergency medical services (EMS) vehicles are climate-controlled, but can easily be exposed to temperatures greater than 100°F when the air conditioning is not turned on during the summer months. For this reason, determining when the drug is no longer safe for human injection after being exposed to typical ambulance temperature fluctuations is critical. According to U.S. Food and Drug Administration standards, greater than 90% potency (i.e., loss of no more than 10% of potency) is used to determine medication shelf life.15–18 While the clinical efficacy of this potency is not established for succinylcholine, it remains important as a guideline for appropriate use in patients.
Methods
Study Design
This was a prospective observational study using mass spectrometry on succinylcholine degradation in ambulances. The study was approved by the institutional review board at our institution.
Study Setting and Environment
Our two-tiered system is composed of six advanced life support units staffed by two paramedics per ambulance. Our Type II ambulances contain built-in climate-controlled compartments that are installed prior to delivery (First Priority Emergency Vehicles, Manchester, NJ). EMS providers are unable to alter the temperature in this compartment. The system is located in suburban New Jersey, an area with an average daily high temperature of 86°F in late July and an average daily low temperature of 20°F in late January. Summers are hot and humid, and the region receives an average of 30 to 40 inches of snowfall each winter.
Study Protocol
In addition to analyzing the drug stability within the ambulance, we assessed the temperature variations in our prehospital environment. Seventeen vials were obtained from a fresh batch of succinylcholine delivered to our pharmacy department; all vials contained identical lot numbers. The vials were covered with duct tape to minimize light exposure. One vial was made our control and was analyzed immediately by the mass spectrometer. The remaining 16 vials were divided and stored among two ambulances, one stationed in an outdoor parking lot and another within a garage. The ambulances continued to respond to daily 9-1-1 requests during the course of the study. The vials were labeled 1 through 7 with a subscript O (outdoor–parking lot) and 1 through 7 with a subscript I (indoor–garage). In each vehicle the eight vials were placed in climate-controlled compartments that are installed by the manufacturer prior to delivery of new ambulances and are designed to maintain an average temperature of 70°F. These vials were not used on any patients. The samples were removed from the ambulances and analyzed at 2 weeks and at 1-, 2-, 3-, 4-, 5-, 6-, and 7-month intervals.
Succinylcholine and two of its principal breakdown products, succinyl monocholine (SMC) and choline (Figure 1), were analyzed by high-performance liquid chromatography (HPLC) coupled to an ion trap mass spectrometer (Thermo Finnigan Deca XP, ThermoFischer Scientific, San Jose, CA).
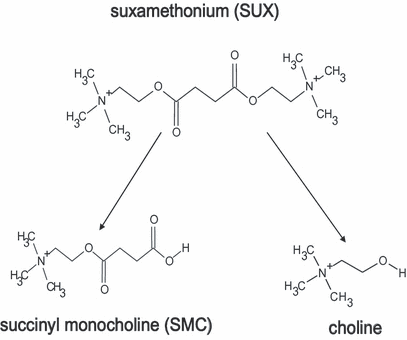
Succinylcholine and its two degradation products, SMC and choline, all measured with mass spectrometry. SMC = succinyl monocholine.
Samples were transported to the analytical laboratory on ice after removal from the ambulance at the designated intervals, were stored at −20°C until analyzed, and were protected from light infiltration to insure no further degradation of the sample during analysis. The succinylcholine and its breakdown products were measured with liquid chromatography/mass spectrometry. The liquid chromatography is used to separate each compound from the others, and the mass spectrometer identifies and quantifies it based on its unique mass to charge ratio m/z. By counting the number of observations for each compound and comparing it to counts from a known quantity of pure material, precise quantitation can be achieved. If the pure material cannot be obtained (e.g., for breakdown products), an approximate concentration can be estimated by assuming that the number of observations from a similar pure compound will have approximately the same response as the unobtainable compound.
The succinylcholine was separated from the SMC and choline using a Surveyor mass spectrometry pump (Thermo Finnigan) with gradient elution. The system was equipped with a vacuum degasser and a Surveyor photodiode array detector (Thermo Finnigan). The separation was carried out on a Waters HSPgel AQ 2.5 6.0 × 150-mm column (Waters, Milford, MA). An isocratic elution of 80% acetonitrile in water was at a constant flow of 150 μL/min. Two microliters of sample was injected. With this elution program, succinylcholine had a retention time of 14.8 minutes, choline 15.6 minutes, and SMC 16.9 minutes.
The eluate of the HPLC system was directed into a LCQ DECAXP ITMS (Thermo Finnigan) using an electrospray ionization source operated using Xcalibur 1.3 software. Mass spectrometry settings were optimized for detection using a direct infusion experiment with the T0 sample. The mass spectrometer was configured for electrospray ionization in the positive ion mode. Capillary voltage, sheath gas flow rate, tube lens offset, and octapole voltages were optimized before each analysis. Data acquisition was performed in mass spectrometry/mass spectrometry mode with quantitation ions of 115 m/z (mass to charge ratio) for succinylcholine, m/z 145 for SMC, and m/z 104 for choline with an isolation width of 1 m/z. Chromatogram and mass spectrum of all three analytes are shown in Figure 2. Integrated peak areas were used for all three analytes. Integration was performed using Xcalibur (Reston, VA) software with Genesis peak integration method. Quantitation of succinylcholine degradation was accomplished as ratios of the total succinylcholine T0 signal.
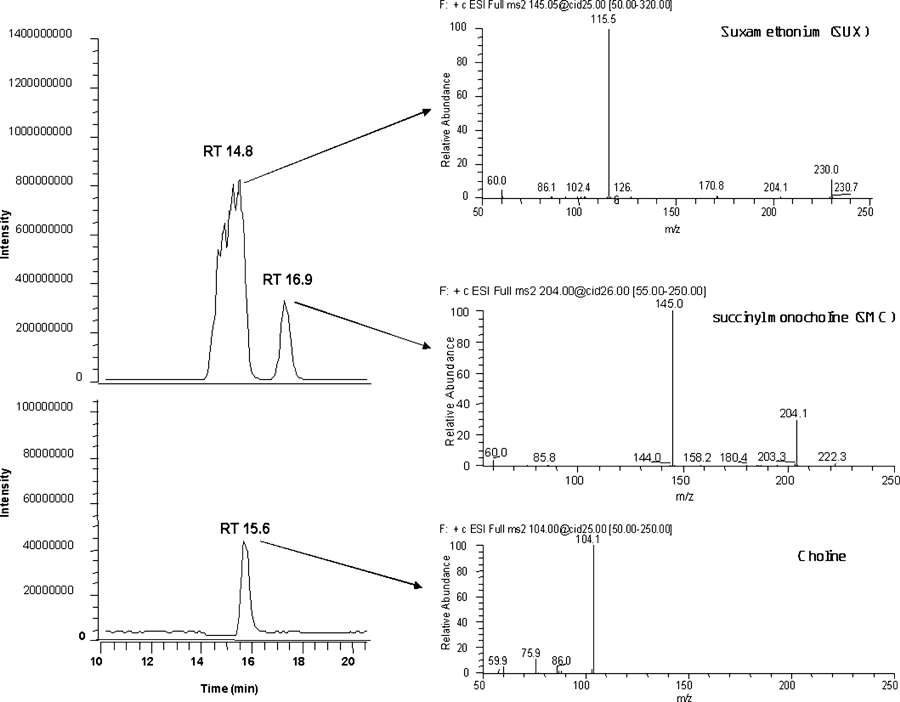
Mass chromatogram and individual mass spectrum for succinylcholine and its two degradation products, both present in the commercial standard. SMC = succinyl monocholine; SUX = succinylcholine.
Degradation products were present in all samples analyzed including T0 and a powder form of the drug (Sigma-Aldrich Co., St. Louis, MO). Quantification of the amount of degradation of succinylcholine was accomplished by assuming that 100% of the labeled concentration was present at the initial measurement (T0). While it may be possible to estimate the concentration of the two breakdown products by assuming that a ratio of 1:1 exists for the peak area created by breakdown production to that of the succinylcholine ion, this was not done for the data plots because of the imprecision associated with such an estimate. The data are plotted as a percentage degradation of the succinylcholine based on the loss of peak area and as the raw peak area increase for each of the two degradation products (Figure 3). In addition to these measurements, a log of the temperature within the ambulance climate-controlled compartments was recorded by a JR-1 Avatel temperature and humidity recorder throughout the duration of the study (Avatel Inc., Fort Bragg, CA).
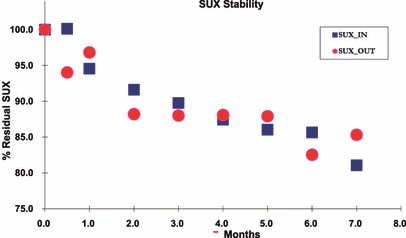
Percentage of succinylcholine present at sampled time points over the course of the study. The percentage was calculated based on the percent of integrated mass spectral signal present at T0. SUX = succinylcholine.
Data Analysis
An investigator trained in Microsoft Access and the Emergency Department Information Management (EDIM) database collected all data. Statistical analysis was carried out utilizing SAS 9.1 TS level 1M0, XP_PRO platform (SAS Institute Inc., Cary, NC) and MINITAB 15 (MINITAB Inc., State College, PA). Scatter plots of area for succinylcholine, SMC, and choline versus time by location (indoors or outdoors) were created. The first time at which 10% degradation occurred was noted for both locations.
Regression models were used to establish mean trends in degradation, to compare degradation when succinylcholine was stored in indoors versus outdoors ambulances, and to confirm that there was no cumulative effect of ambient temperature that was not attributable to a quadratic effect of time. Specifically, percentage of residual succinylcholine, calculated as the area of succinylcholine at each time point divided by the area at baseline, was used as the response. In the latter case, the cumulative average ambient temperature was calculated for each time point and included in the regression model after including linear and quadratic predictors of time. A t-test of the regression parameter associated with cumulative ambient temperature was used to study the effect of ambient temperature.
Summary statistics were calculated for temperatures from within the indoors and outdoors units, as well as for ambient temperatures. Regression statistics examined whether and to what degree the unit temperatures were dependent on the ambient temperatures by modeling the daily mean temperature within a unit as a function of the ambient temperature. The degree of the polynomial appropriate for describing that relationship was selected using forward stepwise regression.
Results
All three components were present in every sample analyzed, including at T0. Plots of the changes in amount of succinylcholine and its degradation products across time are given in 3-5.
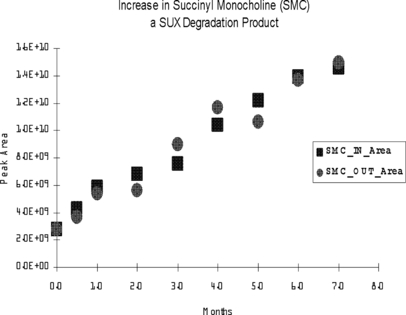
Appearance of SMC as a function of storage time in vehicles generally stored inside (in) a building when not in use and in vehicles that remained outside (out) during their time in service. Data are presented as the integrated peak area for the primary quantitation ion. SMC = succinyl monocholine; SUX = succinylcholine.
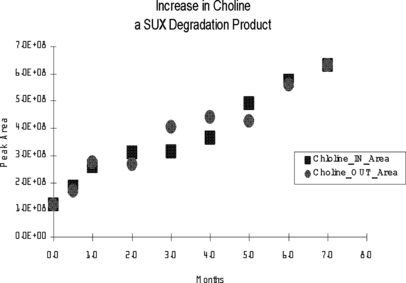
Appearance of choline as a function of storage time in vehicles generally stored inside (in) a building when not in use and in vehicles that remained outside (out) during their time in service. Data are presented as the integrated peak area for the primary quantitation ion. SUX = succinylcholine.
The samples from the indoors and outdoors units realized at least 10% degradation by 3 and 2 months, respectively. Via regression, the exact times to 10% degradation were estimated to be 3.0 and 2.4 months, respectively (Figure 3). To test whether the trends were the same between indoors and outdoors, a Wald chi-square statistic was calculated comparing the regression parameters when two separate quadratic regression functions were created for each location. This resulted in marginal significance (chi-square (df = 3) = 7.60, p = 0.05), indicating a possible difference in trends of degradation due to location.
Finally, there was no evidence that ambient temperature (during the 7-month study period, mean ± SD= 49.9 ± 16.5 °F, range = 16.0–84.5°F) contributed to the degradation of succinylcholine in either location (p = 0.22 and 0.45 for indoors and outdoors, respectively). There is also no evidence that the indoors unit internal temperature (for complete 6 months of data, mean ± SD= 70.1 ± 5.8°F, range = 56.4–89.3°F) contributed to the degradation of succinylcholine (p = 0.47). The seventh month is excluded from the immediately previous analysis because indoors unit temperatures were only collected 10 days into the seventh month.
Temperatures (from December 15, 2007, through June 25, 2008) within the indoors unit ranged from 56.4 to 89.3 F with mean ± SD of 70.3 ± 5.7°F). These temperatures did correlate highly with outside temperatures. Specifically, a quadratic function of the ambient temperature explained roughly 45% of the variance in the unit temperature, such that there was more variability among unit temperatures at lower ambient temperature but less variability with a higher overall mean, with higher ambient temperatures. Figure 6 is a scatter plot of the unit’s internal temperature and the ambient temperature. Figure 7 shows the correlation between the ambient temperature and the unit’s internal temperature.
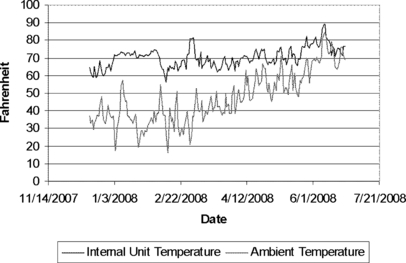
Time plot for both the unit temperature and ambient temperatures during the 6-month study period (°F).
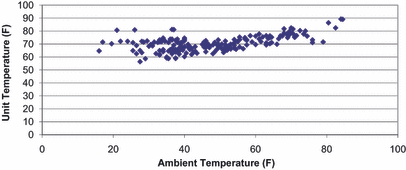
Unit temperature versus ambient temperature during the 6-month study period (°F).
Discussion
This is the first study known to us evaluating prehospital succinylcholine prospectively, using real temperature variations, by mass spectrometry with degradation and measured fragment formation. Because the decision to remove succinylcholine from ambulances at various time intervals is based on limited literature, this investigation provides evidence to support removing it at precise time intervals.
Succinylcholine appeared to degrade independent from internal and external temperature fluctuations, leading us to conclude that temperature may have less effect than initially believed. Because internal and external temperature did not correlate, it is probable that the internal temperature of the vehicle was altered based on the comfort level of individual EMS providers. The climate-controlled compartments worked well during temperature extremes in our region.
EMS systems may station vehicles indoors or outdoors, so it is notable that only a marginal significance existed between these vehicles (3 months vs. 2.4 months). Indoor vehicles may have less temperature variation than outdoor vehicles, explaining this small difference. We did not have temperature data from the outdoors climate-controlled compartment to compare to the data from the indoors compartment.
During the study there were two possible approaches to quantifying the degradation of succinylcholine. The active component (parent compound) was measured directly, as well as the two principal degradation products, SMC and choline. Mass spectrometry was chosen to measure these compounds because we believed the appearance of the degradation products was just as important as the disappearance of the parent compound. Most of the previous studies had only utilized light absorbance-based spectroscopy to quantify the disappearance of the parent compound as a means to measure the breakdown. This may have been because the product compounds do not absorb light well. Measuring the appearance of the product compounds allowed us to quantify smaller changes in the concentration of the parent. While a 1% decrease in the parent is difficult to quantify, an increase from background to 1% of the product compounds was very evident. It also allowed us to determine that the product compounds were present in all samples (even the freshest powder form), suggesting that they may be a part of a residue of the manufacturing process.
The degradation of the succinylcholine was essentially linear. This suggested a degradation mechanism largely independent of external influences such as light or temperature. While there was slight correlation with respect to temperature, the rate of degradation did not differ significantly whether the material was stored in the indoors vehicle or the outdoors vehicle. If the stability of the succinylcholine was significantly dependant on temperature, there should have been a greater difference in the degree of degradation. Based on the structure of the compound, it can be assumed that light may have played a role in the degradation of succinylcholine even though we made extreme efforts to control this variable. Like most compounds that photodegrade, a compound that contains multiple double bonds in conjugation is more likely to efficiently absorb light and dissipate the light energy through breakdown of the compound. While the conjugated double bonds are limited in succinylcholine, its measurement by other investigators using absorption spectroscopy suggests enough of a system to efficiently absorb photons of light. The vials were covered from light by coating the vials with duct tape preventing much of the light from infiltrating the sample. While it is possible that some light penetrated the samples, it is unlikely to be a significant contributor in the degradation process.
Agitation is an unlikely cause of degradation because it does not produce enough energy to break the bond that links the two degradation products in the succinylcholine molecule. In addition, there was no evidence of oxidation, as might be expected if the oxygen concentration in solution was significantly increased by mixing of the drug solution with its vial headspace. The degradation products indicate only cleavage of the succinylcholine molecule into two clean degradation products, and agitation is not an energetic enough process to cleave this bond.
Limitations
These data can only be applied to institutions with climates similar to our geographic region. Our climate-controlled compartment did have some temperature variations, some of which was attributable to external temperatures. Although there was no evidence of an effect of internal temperature on degradation, our small sample precludes a conclusive statement about temperature effects. Thus, it is possible that in other climates, higher external temperature variations could yield more variation in internal temperatures that, in turn, could theoretically decrease the time to achieve 10% degradation. Even in our climate, the time to achieve 10% degradation may have been shorter than 90 days if we had started in the summer, when temperatures are higher.
Vials were placed in ambulances stationed indoors and outdoors to replicate EMS systems that primarily store vehicles in this way; however, based on a system’s call volume and ambient temperature, it is difficult to compare our results to other systems. In addition, our relatively small sample sizes could have produced different results if additional data points were obtained. If our samples were tested over the course of several years of changing climates, it is possible that various data points would yield different results.
Finally, although every attempt to keep light exposure at a minimum was made, it is possible that some light came through, accelerating product breakdown. This is not likely to be a significant factor because the vials were wrapped in duct tape and placed in light-resistant bags within dark compartments.
Conclusions
In ambulances that are based indoors, succinylcholine degrades by 10% at 3 months if placed in light-resistant environments and climate-controlled compartments. In ambulances that are based outdoors, succinylcholine degrades by 10% at 2.4 months when placed in light-resistant environments and climate-controlled compartments. Ambient temperature and internal controlled temperatures do not correlate with succinylcholine degradation and fragment formation. Although further research is needed to confirm our results, we recommend succinylcholine be removed from paramedic vehicles no later than these time frames to prevent a lack of paralysis or potential untoward effects from drug degradation.




