Brain MRI Lesion Load at 1.5T and 3T versus Clinical Status in Multiple Sclerosis
[Correction added after online publication 10-November-2009: Second sentence in the Abstract has been reworded]
Funding sources: This work was supported by research grants to Dr. Bakshi from the National Institutes of Health (1R01NS055083-01) and National Multiple Sclerosis Society (RG3705A1; RG3798A2). Authors have nothing to disclose.
J Neuroimaging 2011;21:e50-e56.
Abstract
ABSTRACT
BACKGROUND/PURPOSE
To assess correlation between brain lesions and clinical status with 1.5T and 3T magnetic resonance imaging (MRI).
METHODS
Brain MRI fluid-attenuated inversion-recovery (FLAIR) sequences were performed in 32 multiple sclerosis (MS) patients. Expanded Disability Status Scale (EDSS) score (mean ± standard deviation) was 2 ± 2.0 (range 0-8), disease duration 9.3 ± 8.0 (range .8-29) years.
RESULTS
FLAIR lesion volume (FLLV) at 3T was higher than at 1.5T (P= .01). Correlation between 1.5T FLLV and EDSS score was poor, while 3T FLLV correlated moderately and significantly (rs= .39, P= .03). When controlling for age and depression, correlations between FLLV and cognitive measures were significant at 1.5T for the Judgment of Line Orientation test (JLO) (rs=−.44, P= .05), the Symbol Digit Modalities Test (SDMT) (rs=−.49, P= .02), and the California Verbal Learning Test Delayed Free Recall (CVLT DR) (rs=−.44, P= .04). Correlations at 3T were also significant for these tests, but of greater magnitude: JLO (rs=−.70, P= .0005), SDMT (rs=−.73, P= .0001), CVLT DR (rs=−.061, P= .003). Additional significant correlations obtained only at 3T included the 2 second-paced auditory serial addition test (rs=−.55, P= .01), the Brief Visuospatial Memory Test-Delayed Free Recall (rs=−.56, P= .007), and the California Verbal Learning Test Total Recall (rs=−.42, P= .05).
CONCLUSION
MRI at 3T may boost sensitivity and improve validity in MS brain lesion assessment.
Introduction
Cognitive impairment occurs in 40-70% of patients with multiple sclerosis (MS)1,2 and frequently includes limitations in mental processing speed, learning, and both working and episodic memory. Impairment in these and other cognitive domains can impact activities of daily living, quality of life,3 and employability.4,5 Cognitive deficits can present early in the disease course6 and can afflict patients despite a relative lack of physical disability.7
Studies attempting to link magnetic resonance imaging (MRI) defined MS brain pathology as assessed by total brain T2 lesion volume and cognitive dysfunction have had varying degrees of success.8,9 Regional T2 analysis has not significantly enhanced correlations.10,11 Though a potential explanation for this may be that cerebral T2 hyperintensities lack pathologic specificity, another possibility is that conventional MRI platforms at 1.5T do not adequately capture the full extent of MS-related damage.
There is a growing interest in the use of 3T and higher MRI field strengths to increase diagnostic yield in the evaluation of a host of neurologic disorders.12,13 With Food and Drug Administration approval of 3T for clinical use,14 3T MRI platforms are becoming increasingly available. Studies in MS indicate that 3T or higher field strengths increase sensitivity to MS brain lesions when compared to 1.5T.12,15–18 On the other hand, 3T also shows increased sensitivity to age-related and incidental hyperintensities in normal subjects.19 Thus, the purpose of this study was to assess the validity of both 1.5T and 3T in the detection of lesions in patients with MS by comparing hyperintense lesions detected with fluid-attenuated inversion recovery (FLAIR) to clinical measures of physical disability and cognitive function.
Methods
Subjects
Demographic and clinical characteristics of the subjects are summarized in Table 1. We performed brain MRI in 32 patients who met the International Panel criteria for either MS or a clinically isolated syndrome (CIS),20 and 17 normal controls. Among patients with MRI and neurologic examination, we were also able to obtain cognitive testing in 24 within 2 weeks of MRI. All 17 normal controls underwent cognitive testing and MRI. MS patients were enrolled consecutively from a community-based, university-affiliated MS clinic. Controls were recruited by using an Institutional Review Board (IRB)-approved advertisement that was posted in a local newspaper and our hospital website. Telephone interview was conducted by using a questionnaire. Control subjects did not differ demographically from the MS group [control mean ± standard deviation age = 45.5 ± 7 years, education = 16.5 ± 1.9 years (P= .74), 71% female (P= .79)]. Our IRB approved this study and informed consent was obtained on all subjects. Within 1 month of MRI, MS disease course21 and clinical measures including Expanded Disability Status Scale (EDSS) score22 and timed 25-foot walk (T25FW)23 (Table 1) were assigned by a treating neurologist. This study was part of a larger ongoing study in which patients are being recruited to assess the relationship between MRI findings and the development of sustained disability 3 years later. For this reason, patients were included only if they had “active disease.” This was defined as a clinical relapse, new or enlarging MRI-defined central nervous system lesion, or an increase in EDSS score of at least .5 in the year prior to recruitment. Only patients aged 18-55 years were included to minimize confounding effects from age-related phenomena. To avoid confounding neuropsychological testing, we excluded any potential participants (both MS patients and controls) with a history of major medical, neurologic, or neuropsychiatric disorders or a history of substance abuse or motor/sensory deficits that may impact cognitive testing.
| Total Number of Patients (n) | 32 |
|---|---|
| Number with cognitive testing | 24 |
| Sex ratio (men/women) | 0.45 (10/22) |
| Age (mean ± SD) | 41.7 ± 8.7 |
| Multiple Sclerosis (MS) Disease Course | |
| Clinically isolated syndrome | (n= 1) 3.1% |
| Primary progressive MS | (n= 2) 6.3% |
| Relapsing remitting MS | (n= 25) 78.1% |
| Secondary progressive MS | (n= 4) 12.5% |
| Disease duration, years, mean ± SD (range) | 9.3 ± 8.0 (0.8-29.0) |
| EDSS score, mean ± SD (range) | 2.1 ± 2.0 (0-8.0) |
| Timed 25-foot walk, mean ± SD (range) | 4.9 ± 1.5 (0-8.0) |
| Education (years), mean ± SD (range) | 15.9 ± 2.7 (8-20) |
- EDSS = expanded disability status scale; SD = standard deviation.
MRI acquisition
Whole brain axial 2-dimensional fast FLAIR was performed in all subjects at 1.5T and 3T using the scan protocols shown in Table 2. The primary goal of this study was to determine correlation between 3T lesion burden and clinical measures rather than to directly compare 1.5T and 3T platforms. Attention was paid to achieving feasible scan time with optimized image quality on both platforms. Because of the potential at 3T to exceed specific absorption rate (SAR) patient safety limitations24 and scanning time considerations, repetition time (TR), echo time (TE), and echo train length varied between the 2 platforms, although voxel size was nearly equivalent.
| 1.5T | 3T | |
|---|---|---|
| Scanner manufacturer | Philips | General Electric |
| Head coil | Quadrature | Receive-only phased array |
| Number of channels | 4 | 8 |
| Type of sequence | 2D fast FLAIR | 2D fast FLAIR |
| Field of view (cm) | 24 × 24 | 25 × 25 |
| Matrix size | 256 × 256 | 256 × 256 |
| Slice thickness (mm) (no gap) | 2 | 2 |
| TR (ms) | 10,000 | 9,000 |
| TE (ms) | 125 | 157 |
| Inversion time (ms) | 2,700 | 2,250 |
| NSA | 3 | 1 |
| Flip angle | 90 | 90 |
| Pixel size (mm) | 0.938 × 0.938 × 2 | 0.976 × 0.976 × 2 |
| Scan time (minutes) | 8.2 | 9.1 |
- FLAIR = fluid-attenuated inversion recovery; TR = repetition time; TE = echo time.
Brain Lesion Analysis
Image analysis was performed using the software package Jim (Version 3.0, Xinapse Systems Ltd., Thorpe Waterville, UK, http://www.xinapse.com). Scans were pooled and all images were anonymized, intermixed, and randomized for analysis. FLAIR hyperintense lesions were identified by the consensus of 2 trained observers (J.S., M.N.) and differences were resolved by an experienced observer (R.B.). Whole brain FLAIR lesion volume (FLLV) was then obtained by a semiautomated method using an edge-finding tool based on local thresholding on each axial slice with manual adjustments as necessary.
Cognitive Analysis
Neuropsychological testing was according to consensus panel recommendations25 using well-established, reliable, and valid tests.2,26,27 This battery, known as the Minimal Assessment of Cognitive Function in MS, includes the Controlled Oral Word Association Test (COWAT),28 Judgment of Line Orientation Test (JLO),28 California Verbal Learning Test, second edition (CVLT),29 Brief Visuospatial Memory Test—Revised (BVMT),30 Paced Auditory Serial Addition Test (PASAT),31 Symbol Digit Modalities Test (SDMT),32 and Delis-Kaplan Sorting Test (DKEFS).33 In addition, patients were evaluated for depressive symptoms using the Center for Epidemiologic Studies Depression scale34 a test that has been successfully employed in an MS population.6 A measure of premorbid IQ, the North American Adult Reading Test (NAART) was also obtained.35 It was decided a priori to control for depressive symptoms in the analysis of MRI-cognition relationships due to the fact that depression may affect cognition in MS.36 The patients undergoing cognitive testing were not significantly different from the overall MS cohort in terms of age, gender, EDSS, or T25FW (P > .5 for all comparisons). Subjects had not previously been exposed to any of the tests from the MACFIMS battery.
Statistical Analysis
MRI platform (1.5T vs. 3T) lesion volume and MS subgroup differences were compared using the Wilcoxon signed rank test and Wilcoxon rank sum test as appropriate. The Spearman rank correlation tested associations between FLLV and clinical measures including EDSS score, T25FW, and disease duration. In addition, the association between the cognitive tests and lesion volume measured at 1.5T and 3T were determined using Spearman partial correlation coefficients controlling for age and depression score. Given the sample size, formal interplatform statistical comparison testing of correlation coefficients was not employed. A P-value less than .05 was considered statistically significant. Since this was an exploratory study, no corrections for multiple comparisons were performed. The analysis for this article was generated using SAS software version 9.1 of the SAS System for Windows (Copyright 2002, SAS Institute Inc., Cary, NC).
Results
Brain Lesions
Brain FLLV at 3T (10,800 ± 14,799 mm3) was higher when compared to 1.5T (8,834 ± 13,210 mm3, P= .01). This improved sensitivity at 3T was likely driven by the fact that small FLAIR hyperintensities not seen at 1.5T could more frequently be visualized at 3T (Fig 1), although this was not formally quantified on a lesion-by-lesion basis. Typically, lesions uniquely detected at 3T were in the periventricular white matter, cortical, or juxtacortical region.
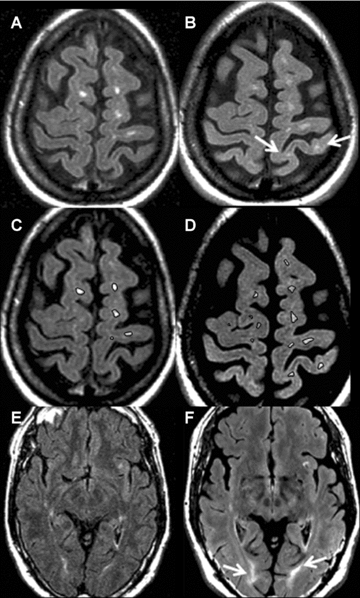
Lesions not well visualized at 1.5T can be at times seen at 3T in patients with MS. Paired FLAIR images were taken from 2 relapsing remitting MS patients, with intrasubject between platform scans taken an average of 15 days apart. Images on the left (A, C, E) were obtained at 1.5T, and, on the right (B, D, F), at 3T. Images A-D are from a 34-year-old woman with disease duration of 1.3 years and Expanded Disability Status Scale (EDSS) score of 1.5. A few cortical lesions (arrows) are visualized at 3T but not at 1.5T. Images C and D show the same slices in A and B after lesion segmentation (note borders around lesions). Image C (1.5T) had a calculated slice lesion area of 56.29 mm2 for the slice shown, image D (3T) a slice lesion area of 82.01 mm2. Images E and F are from a 52-year-old man with disease duration of 15 years and EDSS score of 1.0. This case illustrated the increased sensitivity of 3T in detecting FLAIR hyperintense lesions (arrows) adjacent to the occipital horns of the lateral ventricle.
Disability-MRI Associations
Correlations between disease duration, EDSS score, or T25FW with 1.5T and 3T FLLV were weak and nonsignificant except for a moderate association between EDSS score and 3T FLLV (Spearman's r= .39, P= .03) (Table 3). There was no significant difference in FLLV between relapsing (n= 26) and progressive patients (n= 6) at 1.5T (Relapsing: 8,967 ± 13,814; progressive: 8,257 ± 11,282 mm3; P= .69) or 3T (Relapsing: 10,859 ± 15,876; progressive: 10,544 ± 9,883 mm3; P= .33), but this comparison was likely underpowered due to a small sample size.
| 1.5T FLLV | 3T FLLV | |||
|---|---|---|---|---|
| R | P-value | R | P-value | |
| EDSS score | .19 | .29 | .39 | .03* |
| Timed 25-foot walk | .25 | .19 | .15 | .42 |
| Disease duration | .24 | .18 | .18 | .31 |
- EDSS = expanded disability status scale; FLLV = fluid-attenuated inversion-recovery hyperintense lesion volume.
- Spearman rank correlation r values are reported.
- *P < 0.05.
Cognitive-MRI Associations
Mean NAART full scale interquartile score was similar for both patient (111 ± 8.5) and control populations (111 ± 10.5) (P > .05). Both control (16.5 ± 1.9 years) and patient populations (15.9 ± 2.7 years) were highly educated (P > .05). Three patients (13%) scored less than 1.5 standard deviations below the mean on 2 or more cognitive tasks when compared to normal controls, meeting established criteria2 for cognitive impairment. Six patients (25%) were impaired on at least 1 cognitive measure. Significant lesion-cognition associations for PASAT2, BVMT DR, JLO, SDMT, CVLT DR, and CVLT TL (Table 4, Fig 2) were observed at 3T after controlling for age and depression score, and the partial correlation coefficients were strong for SDMT and JLO (rs > .65). Conversely, only JLO, SDMT, and CVLT DR were significantly associated with FLLV at 1.5T after controlling for age and depression score, but the strength of association was less in each case. Other cognitive measures did not significantly correlate with FLLV at either 1.5T or 3T (Table 4). Results were not substantially altered when age alone or age + depression + gender were controlled for in the modeling (data not shown).
| Cognitive Test | 1.5T FLLV | 3T FLLV | ||
|---|---|---|---|---|
| R | P-value | R | P-value | |
| PASAT2 | −.37 | .09 | −.55 | .01* |
| PASAT3 | −.28 | .21 | −.30 | .16 |
| COWAT | −.19 | .40 | −.27 | .22 |
| BVMT DR | −.40 | .06 | −.56 | .007* |
| BVMT TL | −.41 | .06 | −.37 | .09 |
| JLO | −.44 | .04* | −.66 | .0008* |
| SDMT | −.49 | .02* | −.73 | .0001* |
| CVLT TL | −.23 | .30 | −.42 | .05* |
| CVLT DR | −.44 | .04* | −.61 | .003* |
| DKEFS CS | −.26 | .24 | −.36 | .10 |
| DKEFS DS | −.22 | .33 | −.33 | .13 |
- r is Spearman partial correlation coefficient controlling for age and depression.
- FLLV = fluid-attenuated inversion-recovery hyperintense lesion volume; PASAT = Paced Auditory Serial Addition Test, 2- and 3-second delay; COWAT = Controlled Oral Word Association Test; BVMT = Brief VisuoSpatial Memory Test (DR = delayed free recall, version TL = total recall); JLO = Judgment of Line Orientation; SDMT = Symbol Digit Modalities Test; CVLT = California Verbal Learning Test (TL = 5-trial recall, DR = delayed recall); DKEFS = Delis-Kaplan Sorting test (CS = total confirmed correct sorts, DS = total description score).
- *Indicates P < 0.05 statistical significance.
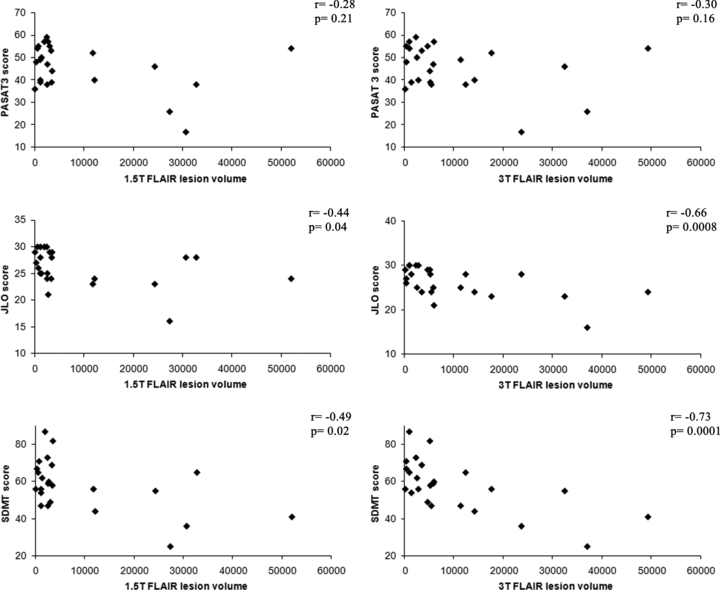
Correlation between brain MRI lesion load and cognitive function: 1.5T versus 3T. Correlations are shown between global cerebral FLAIR hyperintense lesion volume (FLLV, mm3) and Paced Auditory Serial Addition Test, 3 second delay (PASAT3), Judgment of Line Orientation test (JLO), and Symbol Digit Modalities Test (SDMT) at both 1.5T and 3T. Correlations were nonsignificant for PASAT3 at both 1.5T and 3T. Higher correlations with brain FLLV were obtained for JLO and SDMT on the 3T platform.
Discussion
In this preliminary MRI study, the first to examine 3T brain lesion load-clinical correlations, we report 3 major observations. The first is that in an MS population a 3T platform yielded a higher cerebral lesion load than a 1.5T platform using FLAIR. Second, when assessing the validity of the MRI-based lesion load measurement in relation to physical disability, the 3T platform showed the only significant correlation. Third, when assessing the relationship between MRI-determined lesions and cognitive function, the 3T platform showed stronger and more frequent association between cognitive domains than the 1.5T platform. Our findings of increased sensitivity at 3T for detecting MS-related cerebral structural changes extend previous observations that found enhanced sensitivity at 3T when compared to 1.5T. Wattjes et al.12 reported an increased number of CIS patients who met MS diagnostic criteria at 3T. Enhanced sensitivity for cortical lesions17 and white matter lesions (both unenhanced and gadolinium enhanced)15,16 has also been reported.
The relationship between conventional MRI-defined lesion measures and cognitive status has been generally weak to moderate at best and unreliable based on studies employing 1.5T or lower field platforms.37 In our study, the 3T platform showed promise in improving such correlations, particularly when compared to 1.5T in the same subjects. Correlations between FLLV at 3T and multiple cognitive domains including visual perception and spatial processing (JLO), informational processing speed/working memory (SDMT, PASAT2), verbal learning and memory (CVLT LD), and executive function (DKEFS CS) suggest that global high field assessments of MS brain lesional pathology are valuable. Though moderate Spearman rank correlations with PASAT3 (Lazeron r=−.41, P < .001; Sperling r=−.66, P=.001) and SDMT (Lazeron r=−.50, P < .001; Sperling r=−.45, P= .02) have been reported using T2 lesion assessments at 1.5T,10,11 the cohorts were more disabled and contained more progressive than found in the present study. Lazeron et al.'s population was also less educated (mean = 11 years) while the level of education in Sperling et al.'s study mirrored our own. Though many other studies have explored MRI cognition-correlations, differing cognitive tests or reported results did not permit a more direct comparison. Two studies in this regard should be specifically noted because they employed FLAIR rather than T2 sequences. Rovaris et al.38 demonstrated that cognitively impaired patients had a significantly higher FLAIR lesion load when compared with patients classified as unimpaired by cognitive testing, while Lazeron et al.39 were unable to obtain a correlation between overall FLAIR lesion volume and cognitive impairment using the Brief Repeatable Battery. At 3T our findings of a relationship between lesion volume and cognitively impairment subgroup were similar to those reported by Rovaris et al., though this was not the case at 1.5T. Like Lazeron et al., at 1.5T correlations between cognitive tests and FLAIR lesion volume were generally nonsignificant in our study.
The most likely cause of the increased sensitivity of 3T versus 1.5T in the demonstration of FLAIR hyperintense lesions in our preliminary study was the improved detection of small lesions missed by 1.5T, particularly those in the periventricular white matter, cortical, or juxtacortical areas. In view of the fact that correlations with clinical status were stronger at 3T, we hypothesize that these small lesions, detected mostly at 3T only, are clinically relevant. Several studies have emphasized the generally poor correlations between conventional MRI-defined cerebral lesion load and measures of physical disability such as EDSS score.8,9 Most of these studies showing this clinical-MRI paradox are based on 1.5T or lower field strength systems and spin-echo T2-weighted images. With the advent of FLAIR and its ability to better detect lesions than T2-weighted images,19,40,41 combined with the use of higher MRI field strength, we report improved correlations with EDSS score at 3T. This might be based in part on increased detection of cortical and juxtacortical lesions, particularly given the increasingly recognized important role of gray matter involvement in determining MS clinical status.42 However, it should be emphasized that we did not formally evaluate size or location between platforms on a lesion-by-lesion basis. Furthermore, we did not formally quantify differences in contrast-to-noise between platforms. Many of the lesions missed or seen as smaller on the 1.5T platform may be because of higher noise on the 3T platform.
There are several limitations of our study worth noting. Overall sample size was small, particularly when considering the subgroup that underwent cognitive testing. This urges caution in interpreting our results. Of further concern, when compared to typical MS populations, our patients were less cognitively impaired (only 13% compared to a more commonly reported 40-70% prevalence of cognitive impairment), better educated (mean level of education 15.9 ± 2.7 years, when compared to a recent cohort (mean 14.2 ± 2.1 years),43 and were preselected to have active disease. Thus, our sample had a restricted range of cognitive function and other aspects perhaps not generalizable to a typical MS cohort. Given these issues and sample size, further studies are necessary to confirm and extend our findings, particularly with regard to the cognition-MRI associations. We did not assess hypointense lesions on FLAIR images, which perhaps should be assessed in future studies. Our population was less disabled and earlier in the disease course as compared to a general MS sample typically encountered in neurology practice.44 Disease duration and physical impairment as measured by EDSS score were relatively low, potentially restricting the range available to show appropriate clinical-MRI correlations. We also did not include spinal scanning, which can also contribute to disability.45 Regarding the comparison between 1.5T and 3T, although there were many similarities between the protocols used, the comparison between the 2 approaches was not completely optimal. Though scan reviewers were blinded to magnet strength, image quality did differ between 1.5T and 3T platforms, so full blinding was impossible, possibly introducing bias. Additionally, hardware and acquisition software differed between the 2 platforms. For example, our 3T MRI was equipped with an 8-channel coil while the 1.5T was equipped with a 4-channel one, which could have contributed to increased resolution on the 3T scanner independent of magnet strength. While voxel size and other major parameters were similar, differences in gradient strength, TR, and TE were made in order to optimize image quality and stay within scan time and SAR limits. Raters did not independently review each scan which could potentially introduce bias. Contrast-to-noise, and intrarater, interrater, and scan/rescan reliability measurements also were not obtained and should be compared between platforms in future studies. As increased optimization of 3T occurs one would expect further improvements in sensitivity and validity.
Our preliminary results indicate that brain 3T FLAIR lesion detection likely was not sufficient to uncover the full extent of clinically relevant tissue damage, as correlations with both clinical and cognitive measures remained moderate. Consistent with this hypothesis, we have reported separately that 3T FLAIR lesion assessments do not capture the full extent of white matter pathology, which can be detected with advanced MRI measures such as T2 relaxometry.46 Additional techniques useful for detecting diffuse occult damage, such as diffusion tensor imaging,47,48 magnetization transfer,49 and MR spectroscopy,50 have shown relationships with cognitive measures. Brain activation and adaptive cortical changes related to cognitive function between MS patients and normal controls are also being elucidated with functional MRI.51 Volumetric MRI analysis also has shown promise in helping to link cognitive impairment and MS-related damage, such as regional atrophy in the hippocampus,52 thalamus,53 and general gray matter42 showing stronger correlations than conventional measures.




