The Effects of Ponazuril on Development of Apicomplexans In Vitro
Abstract
Abstract. We examined the effects of 5 μg/ml ponazuril treatment on developing tachyzoites of Neospora caninum and merozoites of Sarcocystis neurona to better determine the mode of action of this anticoccidial drug. Both parasites develop asexually by endogenesis. Neospora caninum was selected for study because it develops by endodyogeny, which results in two tachyzoites being produced internally, and S. neurona was selected because it develops by endopolygeny which results in many merozoites being produced internally. Ponazuril inhibited development of N. caninum after approximately 48 h post-exposure. Treated tachyzoites of N. caninum developed vacuoles and underwent degeneration. Ponazuril also inhibited development of merozoites of S. neurona. Treated merozoites and maturing schizonts of S. neurona developed vacuoles and underwent degeneration. The ability of S. neurona schizonts to undergo cytokinesis was inhibited. Our results are discussed in relation to previous ultrastructural research on endogenesis of tachyzoites of Toxoplasma gondii undergoing endodyogeny which indicated that ponazuril induced multinucleate stage formation and inhibited cytokinesis. Ponazuril is believed to act on the apicoplast and our study demonstrates that this agent may express its inhibitory effects in different phenotypic manners on different apicomplexan parasites. The enzyme/enzyme systems that are the inhibitory target of ponazuril may be different in these apicomplexans, or the results of inhibition may affect different pathways downstream of its initial site of action in these parasites.
The tissue cyst-forming coccidia Toxoplasma gondii, Neospora caninum, and Sarcocystis neurona are economically important apicomplexan parasites. Congenital toxoplasmosis in humans has long been recognized by its devastating results, including the triad of hydrocephalus, blindness, and mental retardation in severely infected infants (Jones et al. 2001b). Additionally, congenitally infected children who are less severely infected may suffer from a variety of neurological-related ailments throughout their lives (Roberts and Frenkel 1990). In the United States it is estimated that 85% of women of child-bearing age are at risk for toxoplasmosis (Jones et al. 2001a) and that up to 4,000 cases of congenital toxoplasmosis occur each year (Jones et al. 2001b). Toxoplasmosis is also a frequent and fatal complication in patients that receive organ transplantation (Soave 2001) and toxoplasmic encephalitis is still an important neurological component of AIDS (Luft and Chua 2000). The annual economic impact of toxoplasmosis on the human population in the U.S. is about $7.7 billion (Buzby and Roberts 1996).
Neospora caninum was first recognized as a cause of neonatal paralysis in dogs but was soon found to be a major cause of bovine abortions worldwide (Dubey and Lindsay 1996). It is structurally and biologically similar to T. gondii and was confused with that parasite for almost a century (Dubey and Lindsay 1996). Dogs are the definitive host of N. caninum (Lindsay, Dubey, and Duncan 1999a; McAllister et al. 1998).
Equine protozoal myeloencephalitis (EPM) is caused by S. neurona. This disease, known since the early 1960's, is a major neurological syndrome of horses in the Americas (Dubey et al. 2001). It was not named until 1991, when it was isolated and grown in cell culture (Dubey et al. 1991). Horses are accidental hosts whose central nervous system is invaded by the schizonts and merozoites of S. neurona. The sarcocyst stages of S. neurona have not been found in any tissues of the horse. The Virginia opossum, Didelphis virginiana, is the only known definitive host in North America (Dubey and Lindsay 1998).
Apicomplexans divide asexually by two basic mechanisms: endogenesis and exogenesis (Chobotar and Scholtyseck 1982). We are interested in endogenesis because it represents the most common mode of asexual replication of the pathogenic stages in the tissue cyst-forming coccidia of mammals. In endogenesis, merozoites are produced internally in association with nuclei, centrioles, and centrocones (Chobotar and Scholtyseck 1982; Dubey, Lindsay, and Speer 1998; Speer and Dubey 2001, 2005). The membranes of the future merozoites (tachyzoites) and apical complex develop internally. In contrast, in exogenesis merozoite formation is initiated by a thickening of the inner membrane complex with the merozoites developing in association with this complex. They are eventually extruded from the surface of the schizont (Chobotar and Scholtyseck 1982). Development by endogenesis can further be subdivided into endodyogeny and endopolygeny. Endodyogeny is production of two organisms internally by endogenesis. Endopolygeny is production of many organisms internally by endogenesis.
Ponazuril is a triazine anticoccidial that is used to treat EPM, and it is a major metabolite of toltrazuril. Toltrazuril is an anticoccidial drug that is used to prevent coccidiosis in poultry in many parts of the world. In vitro (Darius, Mehlhorn, and Heydorn 2004a; Lindsay, Dubey, and Kennedy 2000) and in vivo (Darius, Mehlhorn, and Heydorn 2004b; Franklin et al. 2003; Gottstein et al. 2001) studies indicate that ponazuril is active against N. caninum and S. neurona. We have previously demonstrated that ponazuril is highly active against T. gondii in vitro and in vivo (Mitchell et al. 2004) and that 5 μg/ml ponazuril affects the ability of T. gondii tachyzoites to undergo endodyogeny (Mitchell et al. 2003). Multinucleate schizont-like stages are induced in T. gondii treated with ponazuril, indicating an effect on parasite cytokinesis (Mitchell et al. 2003).
The present study was done to better understand the mode of action of ponazuril against tissue cyst-forming coccidia. Our first hypothesis was that since N. caninum and T. gondii both divide by endodyogeny (Dubey, Lindsay, and Speer 1998; Lindsay et al. 1993), the effects of ponazuril treatment would be similar if not identical for these apicomplexans. Our second hypothesis was that since S. neurona divides by endogenesis, although it is characterized as endopolygeny (Speer and Dubey 2001), the effects of ponazuril treatment would be similar to those observed for other apicomplexans that divide by endogenesis, even if by endodyogeny.
MATERIALS AND METHODS
Light microscopy studies of ponazuril inhibition. Light microscopy studies were undertaken to determine when ponazuril exerted its inhibitory effects on developing Neospora caninum and Sarcocystis neurona. Monolayers of African green monkey (Cercopithecus aethiops, American Type Culture Collection, CCL-70) kidney (CV-1) cells were grown in RPMI 1640 medium containing 10% fetal calf serum and antibiotics (Mitchell et al. 2003) on 22-mm2 cover slips, placed on the bottom of two 6-well plates. The CV-1 cells were infected with 2 × 105N. caninum (NC-1 isolate) tachyzoites. After a 3-h incubation period at 37°C to allow the tachyzoites to enter host cells, the infected media were removed and replaced with a maintenance medium (RPMI 1640 medium containing 2% fetal calf serum and antibiotics) without ponazuril or with a maintenance medium containing 5 μg/ml ponazuril. Ponazuril (lot PFA101; Bayer HealthCare Animal Health, Shawnee Mission, KS) was dissolved in DMSO and then made up to a stock solution of 1 mg/ml. Plates were incubated at 37°C in a humidified incubator containing 5% CO2 and 95% air. One cover slip was removed from both the control plate and the ponazuril-treated plate at 13, 24, 48, 72, and 94 h post-treatment and placed in 10% (v/v) buffered formalin for 1 h at room temperature. Cover slips were then placed in 100% methanol until staining.
Cover slips were stained using Diff-Quick stain (Dade Behring Inc., Newark, DE) and allowed to dry before mounting onto slides with Permount. The number of parasites in 100 host cells was determined at each examination. The number of divisions that had occurred by endodyogeny was calculated as follows: if 1 tachyzoite was present, it was recorded as 0 divisions; if 2 tachyzoites were present, it was recorded as 1 division; if 3 or 4 tachyzoites were present, it was recorded as 2 divisions; if 5–8 tachyzoites were present, it was recorded as 3 divisions; if 9–16 tachyzoites were present, it was recorded as 4 divisions; and if 17 or more tachyzoites were present, it was recorded as >5 divisions. Similar studies were conducted using Hs68 cells (human foreskin fibroblast, CRL-1635, American Type Culture Collection, Manassas, VA).
Similar methods were used to examine the effects of ponazuril treatment on development of S. neurona. The CV-1 cells were grown on 22-mm2 cover slips placed on the bottom of 6-well plates. Cover slips were stained and the numbers of immature and mature schizonts (=schizonts with fully formed merozoites) present were recorded for the first 100 infected cells. Sarcocystis neurona does not grow in Hs68 cells so comparative studies were not done in this cell type.
Transmission electron microscopy. Host CV-1 cells were grown to confluence in eleven 25-cm2 plastic cell culture flasks. Host cells were infected with 2 × 106 tachyzoites of N. caninum or merozoites of S. neurona for 3 h at 37°C, after which the infected media were removed and replaced with control media or maintenance media containing 5 μg/ml ponazuril (as above). Flasks were incubated at above conditions.
Flasks were scraped on days 5, 6, 7, 8, and 9 post-treatment using a cell scraper to remove CV-1 cell monolayers infected with N. caninum or S. neurona. Control cells infected with N. caninum and S. neurona were collected 5- and 6- d post-treatment, respectively. Experiments were repeated using NC-1 N. caninum tachyzoites and Hs68 cells. The scraped media were removed and pelleted by centrifugation. The pellets were fixed in 3% (v/v) glutaraldehyde in PBS (pH 7.4) for transmission electron microscopy (TEM). Pellets were then fixed in 1% (w/v) osmium tetroxide in 0.1 M sodium phosphate buffer and rinsed twice with this buffer. The cell pellets were dehydrated in an ethanol series and were cleared by being passed through two changes of propylene oxide. Pellets were embedded in Poly/Bed 812 resin (Polysciences Inc., Warrington, PA) and thin sections were stained with uranyl acetate and lead citrate. Samples were examined with a Zeiss 10CA TEM operating at 60 kV and digital images were taken using an ATM camera system (Advanced Microscopy Techniques Corp., Danvers, MA).
RESULTS
Effects of ponazuril on apicomplexans. Multiplication rates of tachyzoites of N. caninuim were similar for the first 24 h (Table 1). Between 24 and 48 h, ponazuril began affecting tachyzoite development. At 72 h, the distinction between individual tachyzoites was obscured in most ponazuril-treated cells, making quantitative counts difficult. The numbers of host cells containing more than 5 divisional cycles remained >25% in ponazuril-treated cells but was never >10% in infected controls (Table 1), suggesting that host cell lysis was not occurring as readily in ponazuril-treated cells due to the presence of non-viable tachyzoites. Similar results were obtained in Hs68 cells (data not shown).
| Examination time (h) | Treatment | Number of divisions observed per infected cell | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | >5 | ||
| 13 | Control | 20 | 31 | 46 | 3 | 0 | 0 |
| 13 | Ponazuril | 21 | 43 | 34 | 2 | 0 | 0 |
| 24 | Control | 6 | 14 | 34 | 32 | 8 | 6 |
| 24 | Ponazuril | 18 | 19 | 31 | 20 | 8 | 4 |
| 48 | Control | 30 | 4 | 6 | 7 | 5 | 8 |
| 48 | Ponazuril | 24 | 22 | 6 | 9 | 14 | 25 |
| 72 | Control | 18 | 41 | 15 | 10 | 10 | 6 |
| 72 | Ponazuril | 9 | 14 | 11 | 10 | 5 | 51 |
| 94 | Control | 15 | 21 | 25 | 21 | 10 | 8 |
| 94 | Ponazuril | 13 | 11 | 9 | 1 | 0 | 66 |
There was little visible difference between the development of ponazuril-treated and control schizonts of S. neurona using light microscopy of cell cultures. Four separate experiments were conducted, and no conclusive results were obtained (data not presented).
Transmission electron microscopy. For N. caninum, results are based on examination of 14 micrographs of control parasites in CV-1 cells and 40 micrographs of ponazuril-treated stages in CV-1 cells. Control NC-1 tachyzoites grown in maintenance medium were crescent-shaped or ovoid, and contained a conoid, rhoptries, dense granules, micronemes, and other organelles typical of apicomplexan tachyzoites. Some contained lipid or glycogen-like vacuoles. Ponazuril treatment caused the degeneration of tachyzoites. Multiple large vacuoles were present in the cytoplasm of degenerating tachyzoites (Fig. 1). These vacuoles may have originated from the apicoplast, the mitochondrion or from the fusion of lipid or glycogen-like vacuoles or combinations of these occurrences. The nuclear membrane often appeared to be swollen. Some tachyzoites viewed 7- and 8-d post-treatment maintained their natural shape and appeared normal. Ponazuril did interfere with normal tachyzoite division in a few parasites causing the presence of multiple nuclei, but this was not a frequent occurrence. Similar results were obtained for N. caninum that had infected ponazuril-treated Hs68 cells (data not shown).
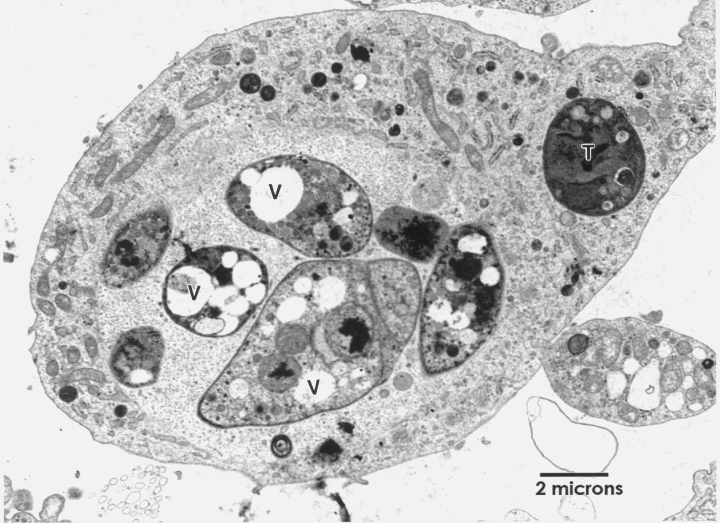
Transmission electron micrograph of a group of degenerating tachyzoites of Neospora caninum in a CV-1 cell treated with ponazuril. Note the vacuoles (V) in individual tachyzoites. A tachyzoite (T) that contains few vacuoles is also present.
For S. neurona, results are based on examination of 12 micrographs of control parasites in infected CV-1 cells and 52 micrographs of ponazuril-treated stages. Control infected host cells examined 5–9 d post-treatment appeared normal, and various stages of endopolygeny were observed. Ponazuril-treated and infected CV-1 cells either appeared normal or contained degenerating schizonts or groups of degenerating merozoites. Vacuoles were present in the schizonts and in developing merozoites (Fig. 2). Merozoites were often in the process of budding from the surface of these degenerating schizonts. Occasionally, apparently viable merozoites could be seen in the same host cell as degenerating schizonts with budding merozoites. This suggests that some merozoites may have completed development before the complete effects of ponazuril were expressed on the schizont.

Transmission electron micrograph of a group of two degenerating schizonts of Sarcocystis neurona in a CV-1 cell treated with ponazuril. Large vacuoles (VA) (VB) are present in central portions of the schizonts. The anterior portions of some merozoites appear normal (open arrows), while others (arrows) appear to be degenerating due to increased vacuolization.
DISCUSSION
Light microscopic studies. Ponazuril has minimal effect on N. caninum endogenesis up to 48 h, approximately the time needed for four divisional cycles. This contrasts with the findings of Mitchell et al. (2003) who found that ponazuril inhibited endogenesis of T. gondii after the second division. These delayed effects of inhibitory action have been found for many different classes of chemical agents that inhibit endogenesis of T. gondii (Beckers et al. 1995; Lindsay and Blagburn 1994). These findings might be due to the differences in divisional cell cycles between the 2 parasites. The cell cycle of T. gondii is 8–10 h, while that of N. caninum is 14–15 h (Sundermann and Estridge 1999). The rounded appearance of ponazuril-treated tachyzoites of N. caninum was similar to the description of Darius et al. (2004a) who observed ponazuril-treated tachyzoites of N. caninum using light microscopy.
Our light microscopic studies with ponazuril and S. neurona were inconclusive. The cell cycle of S. neurona takes 3 d (Lindsay et al. 1999b) and division is by endopolygeny. Since we examined ponazuril-treated S. neurona infected CV-1 cells up to 11-d post-treatment, this should have allowed for a minimum of three divisional cycles of S. neurona, sufficient time for ponazuril to exert its antiparasitic effect. Lindsay et al. (2000) used a merozoite production assay conducted at 10-d post-treatment to determine that ponazuril inhibited merozoite production of S. neurona. Lindsay et al. (2000) also determined that ponazuril did not cause mortality of S. neurona in cell cultures, but it did inhibit the growth rate as measured by merozoite production. Higher doses may be completely lethal, but we were unable to examine doses higher than 5 μg/ml ponazuril because ponazuril induced changes in the CV-1 host cells at doses >5 μg/ml (data not presented).
Transmissin electron microscopy. The present study determined that ponazuril affects endogenesis of N. caninum differently than endogenesis of T. gondii, despite the fact that both develop by endodyogeny. The development of both species was inhibited at 5 μg/ml ponazuril. However, the drug had a distinct effect on the cytoplasmic divisional process of T. gondii (Mitchell et al. 2003), preventing cytoplasmic division and inducing the formation of multinucleate schizont-like stages. Only one instance of a multinucleate tachyzoite of N. caninum was observed in the present study. Ponazuril had a more direct effect on tachyzoites of N. caninum, causing their degeneration. Darius et al. (2004a) examined the effects of 30 μg/ml ponazuril on developing N. caninum tachyzoites, and their findings are similar to those we observed with 5 μg/ml ponazuril. They attributed the action of ponazuril to adverse effects on the apicoplast and the tubular mitochondrion, and noted that ponazuril caused a swelling of these important organelles, which eventually caused tachyzoite death. The vacuoles that we observed in ponazuril-treated tachyzoites might possibly have been in the apicoplast and mitochondrion, but we could not conclusively demonstrate that the ponazuril-induced lesions were always confined to these two organelles. Darius et al. (2004a) did not report the presence of multinucleate stages of N. caninum, and we observed only one such stage, suggesting that this is not the usual effect of ponazuril treatment on N. caninum.
Using TEM, we were able to determine that ponazuril affected developing schizonts of S. neurona. The schizonts developed vacuoles that eventually led to their degeneration. Inhibition of schizont cytokinesis was also observed, similar to that observed in ponazuril-treated T. gondii (Mitchell et al. 2003). The presence of some normal-appearing schizonts in ponazuril-treated infected CV-1 cells using TEM further supported the findings of Lindsay et al. (2000), who showed that ponazuril was not completely cidal (kills) for all stages, but that it was static (inhibits) at this dose. The distinction between cidal and static is often dose dependent (Lindsay and Blagburn 2001). Additionally, agents that are static in vitro may be cidal in vivo because of assistance from the host's immune system (Mitchell et al. 2004).
Ponazuril may act on the apicoplast of coccidial parasites (Darius et al. 2004a; Hackstein et al. 1995). The apicoplast is an exciting new drug target for apicomplexan parasites, and its metabolic functions are many (Gornicki 2003; Seeber 2003). Our study has demonstrated that ponazuril may exert its inhibitory effect in phenotypically distinct manners on closely related apicomplexan parasites. The molecular reasons for these phenotypic differences await further study.
In conclusion, dramatic differences were observed for ponazuril treatment of the two apicomplexans that develop by endodyogeny—tachyzoites of N. caninum versus tachyzoites of T. gondii. Thus, we must reject our first hypothesis that endodyogenous apicomplexans are similarly affected by ponazuril. When we compared two apicomplexans that develop by endogenesis, but by endodyogeny as in T. gondii and by endopolygeny as in S. neurona, the effects of ponazuril treatment were very different for these two parasites. Thus, we must reject our second hypothesis that all endogenetic apicomplexans are similarly affected by ponazuril.
Acknowledgments
SMM was supported by a graduate student fellowship from Bayer HealthCare Animal Health.




