Plateins: A Novel Family of Signal Peptide-Containing Articulins in Euplotid Ciliates1
This paper was originally presented as part of the symposium on “Novel Cytoskeletal Proteins in Protists”, June 24, 2000, during the 53rd Annual Meeting of the Society of Protozoologists, San Juan, Puerto Rico ( Kloetzel et al. 2001).
GenBank Accession Numbers: αl-Platein, AY124989; α2-Platein, AY 124990; β/γ-Platein, AY 124991
Abstract
ABSTRACT. In euplotid ciliates, the cortex is reinforced by alveolar plates—proteinaceous scales located within the membranous alveolar sacs, forming a monolayer just below the plasma membrane. This system appears to play a cytoskeletal role analogous to that provided by the fibrous epiplasm found beneath the cortical alveoli in other ciliates. In Euplotes aediculatus, the major alveolar plate proteins (termed α-,β-, and γ-plateins) have been identified. Using anti-platein antibodies, an expression library of Euplotes genes was screened, and a platein gene identified, cloned, and completely sequenced. Comparison of its derived amino acid sequence with micro-sequences obtained directly from purified plateins identified this gene as encoding one of the closely related β-or μ-plateins. The derived protein, of 644 amino acids (74.9 kDa), is very acidic (pI = 4.88). Microsequences from authentic ot-platein were then used to design oligonucleotide primers, which yielded, via a PCR-based approach, the sequences of two α-platein genes from E. aediculatus. Even more acidic proteins, the derived al- and a2-plateins contain 536 and 501 residues, respectively. Analyses of their amino acid sequences revealed the plateins to be members of the articulin superfamily of cytoskeletal proteins, first described in Euglena and now identified in the ciliate Pseudomicrothorax and in Plasmodium. The hallmark articulin repetitive motifs (based on degenerate valine-and proline-rich 12-mers) are present in all three plateins. In β/γ-platein this primary motif domain (27 repeats) is central in the molecule, whereas the primary repeats in the α-plateins lie near their C-termini. A cluster of proline-rich pentameric secondary repeats is found in the C-terminus of pAy-platein, but near the N-terminus of a-plateins. All three plateins contain canonical N-terminal signal sequences, unique among known cytoskeletal proteins. The presence of start-transfer sequences correlates well with the final intra-alveolar location of these proteins. This feature, and significant differences from known articulins in amino acid usage and arrangement within the repeat domains, lead us to propose that the plateins comprise a new family of articulin-related proteins. Efforts to follow microscopically the assembly of plateins into new alveolar plates during pre-fission morphogenesis are underway.
Eukaryotic cells typically possess specialized protein complexes associated with their plasma membranes that appear to provide supportive and pattern-forming functions. Due to their large sizes and free-living lifestyles, unicellular eukaryotes (protists) display particularly well developed surface complexes (variously termed ‘pellicle’, ‘epiplasm”, or‘membrane skeleton’ in different organisms), with a varied array of cortical cytoskeletal elements. The arrangement and protein composition of these protistan surface assemblages have been subject to considerable analysis, and in recent years several novel cytoskeletal proteins have been identified that play important structural roles (and likely roles in pattern generation) within the cortex of these unicells (Brimmer and Weber 2000; Coffe et al. 1996; Honts and Williams 1990; Huttenlauch, Peck, and Stick 1998; for reviews, see Adoutte and Fleury 1996; Beis-son 1994; Bouck and Ngo 1996; Fleury, this symposium).
Numerous additional studies have described in some detail, cytologically, the processes used by protists (particularly the ciliates) in remodeling their cortical cytoskeletons in preparation for cell division (Eigner 1997; Foissner 1996; Frankel 1989; Grimes and Aufderheide 1991; Ruffolo 1976). This process, termed‘cortical morphogenesis”, offers significant experimental advantages for better understanding the more general process of cellular morphogenesis: the cells are frequently large, their cortical elements are readily accessible to a wide range of staining protocols and cytological monitoring, and the entire process is often drawn out over a period of many hours, enabling intermediate steps in the process to be dissected and scrutinized in detail. Moreover, these steps typically follow a rigid, quite stereotypical sequence over a predictable time course, providing the considerable advantage of reproducibility and the potential for drawing samples from synchronized groups of cells.
Euplotes cortical structure: alveolar plates and platein proteins.
Our recent studies have focused on the cortex of the hypotrich ciliate Euplotes aediculatus. Ciliates typically possess a monolayer of flattened membranous cisternae (termed ‘cortical alveoli’), tightly abutted to one another, just beneath the plasma membrane. Euplotids differ from most ciliates in that they construct one of the main cytoskeletal elements of their cortexes within the cortical alveoli in the form of rigid (intra) alveolar plates (AP; see Geyer and Kloetzel 1987; Hausmann and Hiilsmann 1996; Hausmann and Kaiser 1979). This assemblage of alveolus-AP units is integrated into a contiguous mosaic surface (Fig. 1) by a ‘grouting’ of fibrous material revealed by classical inorganic silver staining and termed the ‘silverline system’ or ‘argyrome’. From earlier studies (Ruffolo 1976; Wise 1965), it appears that virtually the entire cortex (at least on the ventral surface) is replaced with each cell fission, as new duplicate sets of AP (and compound ciliary organelles) mature and spread across the cell surfaces of the future daughter cells. In order to better understand the assembly of new pellicle in Euplotes, we set out to identify the proteins of which the major cytoskeletal elements are composed. The initial studies focused on the AP.
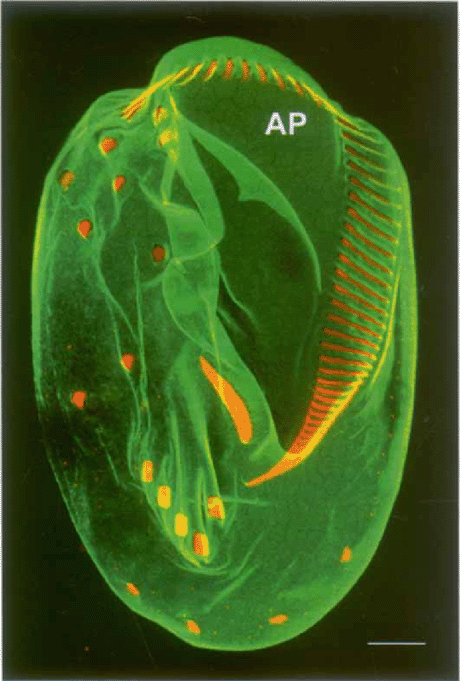
Ventral surface of Euplotes aediculatus, decorated with antibodies against plateins (alveolar plate proteins; in green) and against centrins, concentrated at the ciliary bases of cirri and oral membranelles (in red; anticentrin 2 courtesy of Michel Bornens). Polygonal alveolar plates (AP) are seen most clearly at the anterior end of the cell. Confocal micrograph. Scale bar = 10 μn.
Williams et al. (1989) were the first to identify proteins of the AP in Euplotes eurystomus immunologically, and showed that they were represented by a pair of prominent electropho-retic forms of approximately 116 and 110 kDa. Subsequently, Williams (1991) showed that these two bands could be resolved as three individual polypeptides on two-dimensional gels, matching the three E. aediculatus bands distinguishable on both 1-D and 2-D gels. Kloetzel (1991) confirmed a similar pattern of AP protein bands in E. aediculatus, using monoclonal antibodies (mAbs). Peptide mapping and genetic studies (Kloetzel 1991; Kloetzel, Hill, and Kosaka 1992; Kloetzel 1993) led to the conclusion that three separately encoded forms of AP proteins exist in E. aediculatus. These proteins were named ‘plateins’, and comprised an α-platein form, migrating with an apparent 125-kDa mass in SDS-PAGE, and two more closely related forms (termed β and -γ) migrating with apparent Mrs of 99- and 95-97 kDa, respectively (Fig. 2). Direct peptide microsequencing was performed on α-, β and γ-platein bands separated electrophoretically and transferred to PVDF membranes; following trypsin digestion, several internal peptide sequences were obtained for each platein form (Kloetzel et al. 2003). Significant sequence overlap was noted within some peptides derived from the β-and γ-platein bands (reflecting their similar 1-D peptide maps; Fig. 2C). The a-platein peptide map showed no similarity to those of the β or γ forms, nor did any of the a-peptide sequences overlap the others.
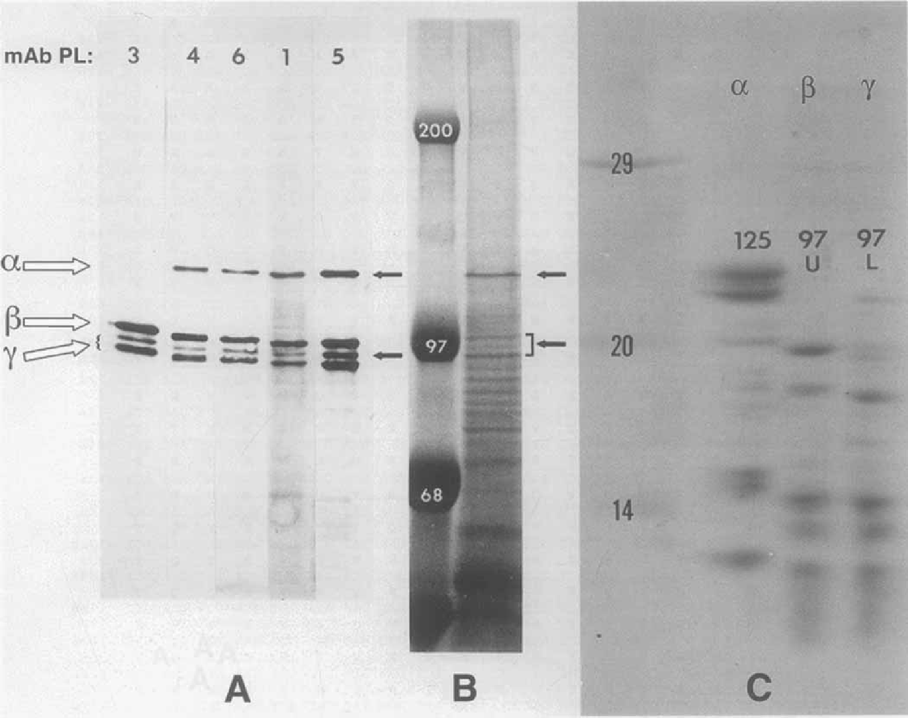
Characterization of E. aediculatus platein proteins by SDS-PAGE, immunoblotting, and peptide mapping (from Kloetzel 1991). A. Immunoblot detection by 5 separate alveolar plate-reactive mAbs (PL-series) of platein protein bands in electrophoretically separated whole-cell extracts. Most anti-AP mAbs react with platein forms designated α, β and γ7 (here with two allelic variants); PL-3 is distinct in that it recognizes only the β and γ-plateins. B. Coomassie blue-stained whole-cell extracts following separation by SDS-PAGE. Platein bands (arrows) represent prominent, major proteins. Molecular mass standards are given in kDa. C. One-dimensional peptide map of individual platein forms digested with S. aureux V-8 protease, re-electrophoresed and stained with Coomassie blue. The ct-platein peptides are quite distinct from those of the β and γ-forms, which resemble each other closely. Molecular mass standards in kDa
Sequencing of platein genes.
A cDNA expression library of E. aediculatus was constructed in àgt11 This library was screened with antibodies generated against plate proteins of both E. eurystomus (‘anti-E’ sera; generously provided by N. Williams) and E. aediculatus. Several positive X clones were thus identified, which led (following subcloning and the screening of a genomic Euplotes library) to the identification of the first full-length macronuclear gene encoding a platein (Kloetzel et al. 2000; Kloetzel et al. 2003). Conceptual translation of this gene revealed an encoded protein (ORF) of 644 amino acids (a. a.); several of the β-platein peptides mapped precisely onto the derived protein sequence, as did several γ-peptides. Since it remains unclear which of these two plateins is specifically encoded by the sequenced gene, we refer to it as a ‘β/γ-platein’ gene (Fig. 3). There was no similarity between the encoded product of this gene and any of the microsequenced a-platein peptides.
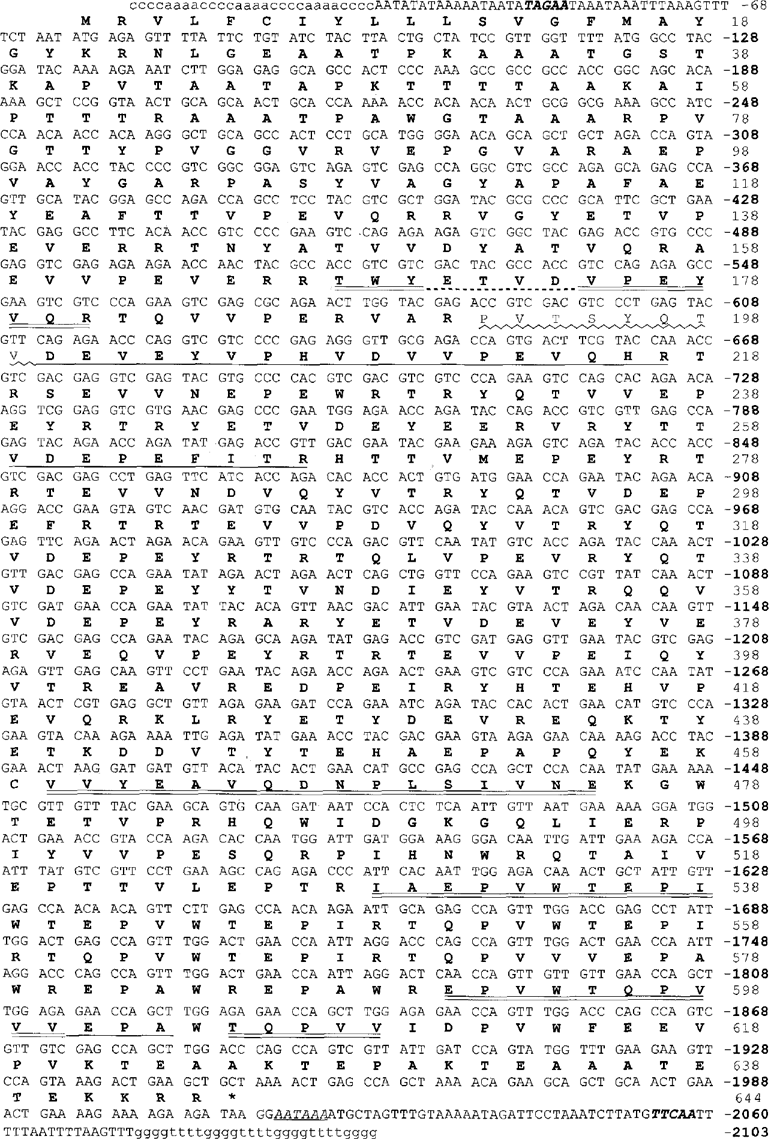
The macronuclear gene sequence of the β/γ-platein gene, with the derived amino acid sequence indicated above. The normal euplotid telomeric repeats (5′-C4A4 and G4T4-3′) are added in lowercase. A putative polyadenylation signal (AATAAA) is italicized and underlined; the typical consensus cleavage-signal sequences 17 nucleotides internal to the telomeres are in bold italics. Sequences corresponding to directly sequenced β- or γ-platein peptides are underlined (doubly for β-, singly for -β-peptides. Dashed underline indicates uncertain sequence within one p-peptide.) Nucleotide numbering is based on the assumed euplotid telomere at the 5′ end (C4A4)35.
With the sequence of one of the related β- or /γ-platein genes in hand, a different approach was utilized to obtain the sequence of an α-platein gene. Oligonucleotide primers were designed from the known sequence of authentic a-platein peptides, and a PCR strategy devised that yielded the sequence of an α-platein gene (onto which all of the a-platein peptides were found to map; Fig. 4; see Barchetta, Kloetzel, and Miceli 2000; Kloetzel et al. 2003). Surprisingly, in the course of the sequencing studies, the presence of a second α-platein gene was discovered. The first gene, encoding a larger polypeptide (536 a. a.), was denoted the al gene; the second, a2, shows a great deal of similarity with the first, but encodes a product 35 a. a. shorter (Fig. 4). Colonies containing both forms were equally represented when RT-PCR products were cloned, indicating that both genes are transcribed. Presumably their similar migration in SDS-PAGE masked the presence of the two α-platein forms in previous studies. Since the predicted pis for αl and α2 are 4.75 and 4.77, respectively (Kloetzel et al. 2003), even 2-D gels may not resolve these two forms successfully. In Williams (1991) the α-plateins of E. aediculatus show significant charge heterogeneity on 2-D gels, although covering a more basic pi range (ca. 5.7–6.2) than predicted from the conceptual translation of the genes presented here. All the platein proteins migrate much more slowly in SDS-PAGE Trisglycine gels (Fig. 2) than would be expected for the molecular masses predicted from the sequenced genes (56–61 kDa for the α-plateins; 72.8 kDa for the β/γ-platein).
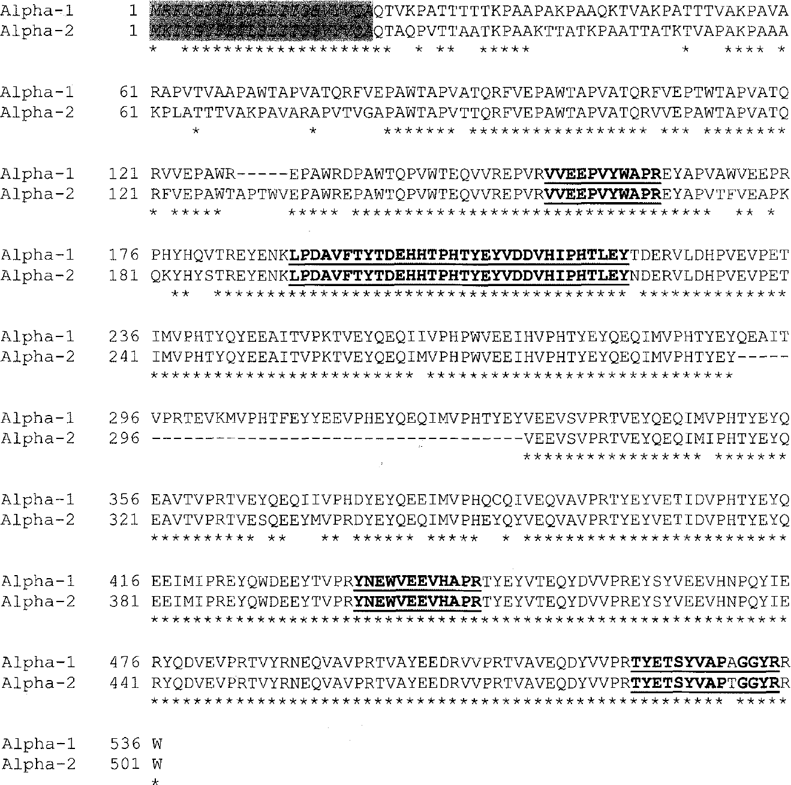
Amino acid sequence alignment of the cd- and ct2-plateins predicted from the sequenced genes. Sequences corresponding to the tryptic peptides from a-platein are in bold underline. Predicted N-terminal signal sequences are italicized (gray highlight). Gaps in the alignment (amino acids deleted in one of the two sequences) are indicated by “-”.
Properties of the platein genes and their projected proteins: a new family of articulins.
In hypotrichous ciliates, macronuclear DNA is composed of molecules ranging from approximately 0.5 to 20kb. Each molecule consists of a “mini-chromosome”, generally carrying a single transcription unit without introns, flanked by 5′ and 3′ non-coding regulatory sequences, with telomeres at both ends (Hoffman et al. 1995). Probing a Southern blot of total cellular DNA reveals the length of the relevant “minichromosome”, and allows an estimation of the upper limit of the gene product size. Southern blot hybridization of total E. aediculatus DNA with one of the antiplatein-selected X clones as probe gave a single band at around 2kb (Fig. 5A), agreeing well with the determined gene size of 2103 nt (Fig. 3). Similarly, the existence of two α-platein genes was confirmed by Southern blot analysis; probing with the insert of a clone selected from the à library with an α-platein-specific antibody, a tight doublet of bands appeared (Fig. 5B). The nucleotide sequences of these platein genes have been submitted to GenBank under accession numbers AY124989 (αl), AY124990 (α2), and AY124991 (β/γ).
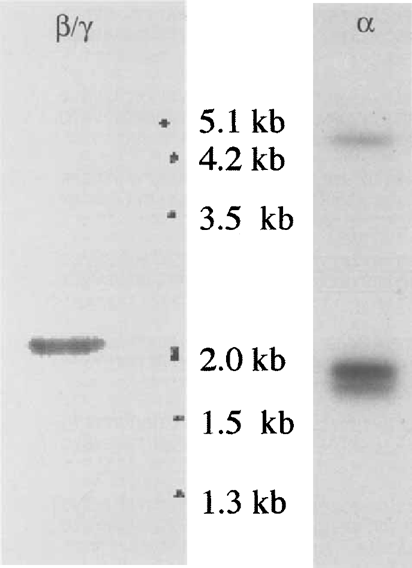
Southern blots using DNA isolated from E. aediculatus. LEFT: Probe is a β/γ-platem-specific cDNA clone obtained from the expression library. DNA marker sizes are indicated on the right. RIGHT: Probe is a X-clone insert selected from the expression library using an α-platein-specific antibody (AP-2). Two bands of similar size are clearly distinguishable. The nature of the pale high molecular weight band is unknown. Size standards are not shown, but direct comparisons of relative migrations indicate that the a-specific bands are of smaller size than the β/γ-specific band shown at the left.
Nucleotide sequence analysis.
As typically observed in hypotrichs, the mean G C content of the three platein coding sequences (ca. 50%) is much higher than in their flanking noncoding sequences (10–20%). Putative polyadenylation signals (AATAAA) are found downstream from the TAA stop codons. The 5′ leader sequences are very short, as in most hypotrich genes, and putative TATAA boxes are found upstream of the ATG initiation codons. In addition, the typical consensus sequence of Euplotes internal to telomeres (TTGAA; Klobutcher et al. 1998) is found at 15–17 bp from both terminal repeats (e.g. Fig. 3). Examination of the platein genes’ codon usage shows the strong bias against a number of G-ending codons (GCG, AGG, ACG, GTGr…) classically observed in ciliates, with a general preference for using A-ending codons (AGA, CCA, CAA). Overall, the three platein sequences show a similar pattern of codon usage. Only αl-platein shows an in-frame TGA codon (cysteine in the euplotid code), at residue number 388; α2 is devoid of cysteine, and β/γ-platein's two cysteines are encoded by standard codons.
Platein polypeptide sequence analysis.
Closer examination of the projected platein protein sequences reveals a considerable degree of sequence redundancy. Another protistan cytoskeletal protein featuring many repetitive sequences has been identified and named ‘articulin’. First discovered in Euglena gracilis (Marrs and Bouck 1992) and since described in the ciliate Pseu-domicrothorax dubius as well (Huttenlauch et al. 1995, 1998), articulins are characterized by a core domain of 12-mer repeats with a consensus alternating valine/proline (VPVPV…) sequence. The extent of this repetitive zone was demonstrated graphically by Marrs and Bouck (1992) using dot-plots. Plotting the β/γ-platein sequence similarly reveals an extensive repetitive core domain (Fig. 6), as well as a secondary repetitive domain—another feature common to the described articulins—C-terminally. The α-plateins likewise possess prominent repeat domains, although these are located at the extreme C-terminus of the molecule, rather than in a more central ‘core’ typical of all other articulins (including β/γ-platem).
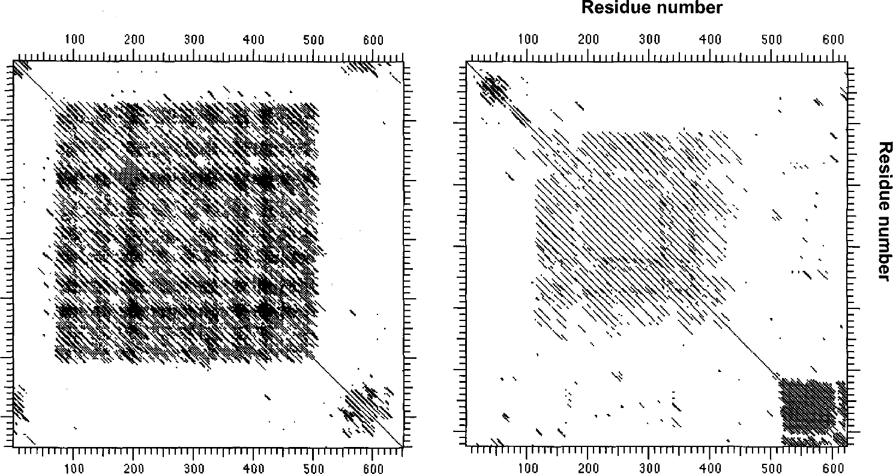
Intrasequence comparisons (matrix or ‘dot’ plots, using the DNA Stricter program; 15-residue windows). Left plot is for articulin 1 from the ciliate Pseudomicrothorax dubius (Huttenlauch et al. 1998); right plot is β/γ-platem from Euplotes aediculatus. Similarities of the proteins are apparent in terms of their tripartite organization, repeats in their central cores, and additional, unrelated repetitive motifs near their C-termini.
BLAST searches (Altschul et al. 1997) using a-platein sequences yielded the strongest similarity scores among the Euglena and P. dubius articulins (as well as close relatives in Plasmodium; Bowman et al. 1999). Like the articulins, plateins are V/P-rich (Fig. 7), and a major domain of each can be arranged readily as repeating dodecamer units (with some degeneracy, in the form of occasional interspersed 8-, 10-, or 14-mers). However, the consensus of these repeats differs significantly from the alternating VP repeat of the articulins. Figure 8 compares the consensus of the 27 tandem 12-mer repeats of the pAy-platein sequence with the comparable domain of P. dubius articulin 1, and highlights another difference: unlike the articulins’ strict consensus alternation of positive and negatively charged residues (substituting for V or P within the 12-mer repeats), the plateins often favor negative residues at adjoining positions within the 12-mer (e.g. residues 8, 9 and 10 in the α-plateins; residues 2 and 3 in β/γ, Fig. 8). This reflects the overall much greater proportion of acidic a. a. in the plateins, with predicted pi values of 4.75–4.88 for the three plateins (or 4.43-4.66 for their even more acidic primary core domains). Notable by their virtual absence in these platein primary repeat domains are glycine residues. For example, no Gs are found in the primary repeat domains of αl- or α2-plateins (28 and 24 tandem 12-mers, respectively), and of 15 Gs in the total length of β/γ platein, only one is found in the 27 repeats forming the core domain (a run of 316 residues). This feature seems to characterize articulins generally: neither E. gracilis articulin (80 or 86) contains a single glycine within the 33 repeats of their central cores. Since glycine can act as a hinge or swivel in the peptide backbone, it can be inferred that, in terms of the molecule's functional properties, a premium is demanded in restricting flexibility within the platein/articulin primary domain. Additionally, proline residues, abundant in the core domains of both articulins and plateins, are known for the rigid, inflexible bond conferred by their pyrrolidine backbone ring structure (Williamson 1994).
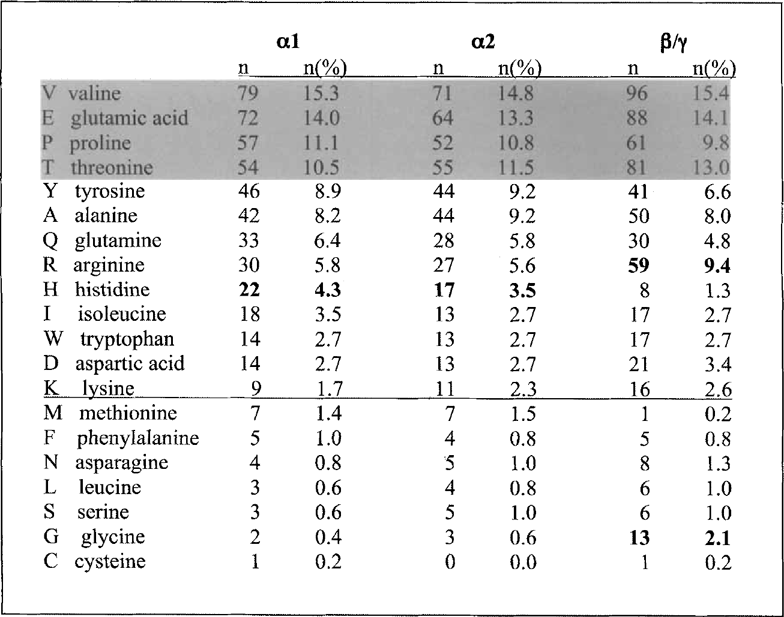
Amino acid composition of the plateins described here (minus their predicted signal sequences), arranged in rank order of residue abundance in oil. Although the absolute rank differs slightly among the platein forms, the same 4 major residues (highlighted) make up 50% or more of the total proteins. Similarly, the 7 least used amino acids (below the line) total 6.6% or less of the total residues in each platein, again indicating their overall similarity in composition. The β/γ-platein differs from the a forms in its relatively greater abundance of arginines and glycines, and lesser utilization of histidines (bold).
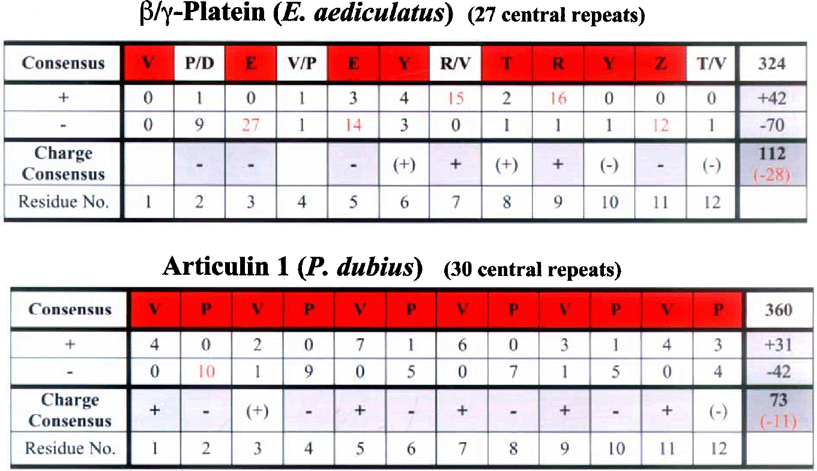
Analysis of a. a. use within the primary 12-mer repetitive domains of β/γ-platein, with articulin 1 of P. dubius for comparison. Consensus residues at each position within the 12-mer repeat (highlighted in red) are those whose frequency at that position is more than twice that of the next most common residue (minimum of 10 uses for the consensus residue). Charged residues (+= R or K; – D or E) at each position are also noted, and summed for all repeats in the right-hand column. Notable is this platein's deviation from articulin's VPVPV… consensus, and from articulin's strict alternation of positively and negatively charged residues (substituting for V and P, respectively, at particular repeat positions). Note also the very large excess of negatively charged residues (red numerals) in platein, compared with articulin (Z = E or Q.).
As noted above, the dot-plot of the β/γ-platein sequence (Fig. 6) reveals a prominent secondary repetitive domain near the protein's C-terminus. The described articulins also feature a second repeat domain. In E. gracilis (cf. Fig. 6) these comprise four heptads with a general APVT… consensus (N- or C-termi-nally, depending on the articulin form); in P. dubius articulins there are 9–13 glycine-rich C-terminal hexamers (Huttenlauch et al. 1998). The Euplotes secondary domains, upon closer analysis, appear to be made up of even more numerous pentamers, each proline-initiated. While present in α-plateins as well, the pentameric repeats are located toward the W-terminus in the αl and α2 forms. In this respect the two categories of plateins mirror the alternative ‘head-tail’ arrangements of primary vs. secondary repetitive domains found in the two E. gracilis articulins (Marrs and Bouck 1992). Contrary to the absence of glycines from the primary core repeat domain, the secondary repeat domains of most articulins have an abundance of Gs, implying considerable flexibility within these portions of the molecules. In this regard the plateins differ fundamentally from the other articulins in their exclusion of glycines from the secondary repeat domains. Instead, these motifs feature prolines uniformly spaced five residues apart [(PX4)n] over a span of 14-17 tandem repeats. Presumably this would yield a kinked, more rigidly extended structure (Williamson 1994), whose role in intermolecular binding and/or assembly would be predicted to differ entirely from that of the secondary domains of other articulins. Rather than the glycines common in the articulin secondary repeats, the Euplotes platein pentamers are rich in tryp-tophan, in addition to proline. (This contrasts with 0-1 trypto-phan residues total in each of the other articulins.) It seems worthy of note that each of the 17 pentameric repeats in pAy-platein ends with an acidic residue (D/E), or glutamine, whereas the secondary domains of the previously described articulins are completely devoid of acidic residues. The two a-plateins are intermediate in this regard, and (unlike pAy-platein) also have short tetrameric stretches interspersed within their secondary domains (cf. Fig. 9).

Amino acid sequences of β/γ-platein (left) and αl-platein (right), derived from their respective nucleotide sequences. The proteins are arrayed to display their respective domain structures and regions of repetitive sequences. The N-terminal signal sequences are highlighted in yellow. The primary repetitive sequences (VP-rich 12-mers, with some degeneracy) are indicated in red; the secondary proline-rich pentameric repeats are shown in blue. Note the inversion of primary and secondary repeat domains between the two platein forms. (The structure of α2-platein, not shown, is very similar to that of oil, with 4 fewer VP core repeats and one additional pentameric repeat, reflecting its 35 fewer a. a). Sequences corresponding to tryptic peptides from the respective plateins are underlined; for β/γ-pIatein platein, double underline indicates p peptides, single underline corresponds to -y peptides; wavy underline indicates sequences common to both. Residues that show very high prediction values as phosphorylation substrates (≥ 0.9 output values using NetPhos 2.0) are highlighted in gray.
Perhaps the most distinctive difference between the plateins and articulins lies at their N-termini. Hydropathy plots of all three platein polypeptides reveal marked hydrophobic regions of 20-25 residues at their N-terminal ends, contrasting with the otherwise quite hydrophilic nature of these proteins. When analyzed by the SignalP vl. l program (Nielsen et al. 1997) these terminal regions are identified as canonical signal (start-transfer) peptides. It can be predicted, therefore, that the plateins should be translocated across a rough ER membrane system during their synthesis, and that the mature proteins should lack these signal sequences (assuming the functioning of a signal peptidase). The projected presence of signal sequences accords well with the intra-alveolar location of the final assembled platein products—as alveolar plates. This further distinguishes the plateins from the other articulins, which lack signal sequences and assemble into epiplasmic skeletal elements free in the cytoplasm, subtending the plasma membrane in Euglena (cf. Bouck and Ngo 1996), or beneath the cortical alveoli in the other ciliate studied, Pseudomicrothorax (Peck et al. 1991). In Fig. 9 the derived sequences of the al- and pAy-plateins are displayed in a form emphasizing their design according to the ‘modules’ described above: signal sequences, primary (V/P 12-mer) and secondary (P-pentamer) repetitive domains.
The NetPhos 2.0 program (Blom, Gammeltoft, and Brunak 1999) can be used to search for potential phosphorylation sites in eukaryotic protein sequences. Although the program identifies serines, threonines and tyrosines (S/T/Y) with threshold scores above 0.5 (within a range of 0–1) as potentially phos-phorylable, we applied a much more stringent threshold in a search for predicted phosphorylation sites within the plateins. In Fig. 9 those S/T/Y sites with output values of 0.9 or greater are highlighted. Note particularly the large number of such residues (primarily Ts and Ys) in pAy-platein and their distribution within the core 12-mer sequences. The potential significance of these sites in the control of platein polymerization is considered below. Post-translational modifications, if they indeed occur, could help explain as well the anomalous retardation of the plateins on electrophoretic gels noted above. In addition to phosphorylation, covalently bound oligosaccharides could be an explanation for such retarded gel migration. While the plateins contain no consensus sequences for N-glycosylation, the NetOGlyc 2.0 program (Hansen et al. 1998) indicates that the plateins have a very high potential for O-glycosylation N-terminally. Retarded electrophoretic migration is a general property of all articulins reported to date. Given the unusual amino acid composition of the articulins and plateins (particularly their high proline content), a persistent, extended secondary structure (see Williamson 1994) offers another explanation for their anomalous behavior under standard electrophoretic conditions.
In summary, then, the Euplotes plateins possess many sequence features indicating their membership in the articulin class of cytoskeletal proteins. However, the many differences between the plateins and the articulin archetypes, detailed above, indicate clearly that the plateins belong in a separate family. Figure 10 compares in diagrammatic form the similarities and differences in molecular architecture between the plateins and those articulins that have been well characterized to date.
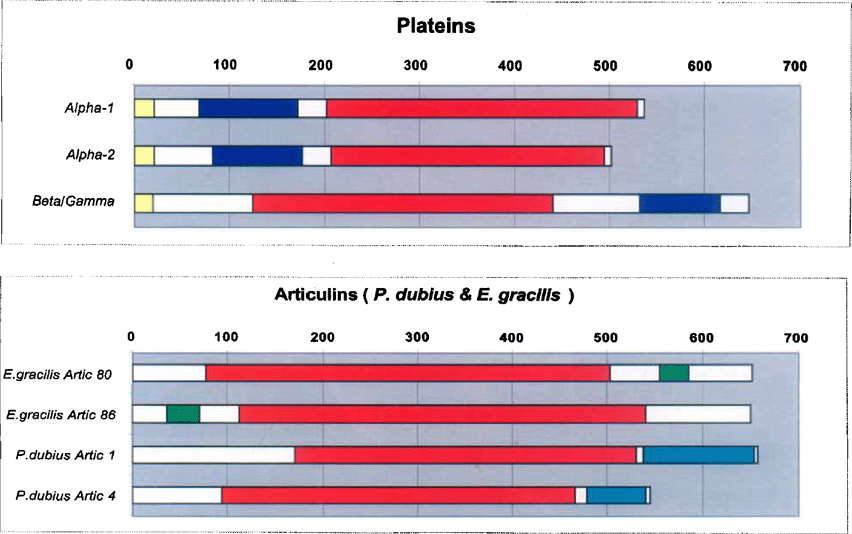
Models of the molecular architecture (‘modules’) of the plateins (upper) and the previously sequenced articulins (Euglena gracilis’. Marrs and Bouck 1992; Pseudomicrothorax dubius: Huttenlauch et al. 1998). Primary repetitive domains (V/P-rich 12-mers) are indicated in red; blue and green represent the secondary repeat domains (the nature of which differs between species). The N-terminal signals, unique to the plateins, are shown in yellow.
Visualization of alveolar plates by immunofluorescence: plateins vary in solubility.
Antibodies specific to a-plateins (polyclonal—pAb AP-2) and β/γ-plateins (mAb PL-3) are available (cf. Kloetzel 1991), as are both mAbs (PL-5; Kloetzel) and pAbs (anti-E; Williams et al. 1989) that react with all the platein forms. These have been used to study by immunofluorescence the cellular localization of the plateins, both in mor-phostatic (non-dividing) cells and during morphogenesis of new cortex in growing cultures.
As reported earlier by Williams (1991), all platein forms co-localize (at the light microscopic level) within the population of AP in non-dividing cells. Our recent studies have provided additional information. It appears that the α-plateins are both more readily accessible within the alveoli and more readily solubilized from the AP than are the β/γ forms. This is based on the observation that low (0.25%) concentrations of Triton X-100 (the membrane permeant used in immunofluorescence studies) permit full staining of the AP using β-platein-specific AP-2 pAb, but only partial staining with β/γ-specific mAb PL-3 (Fig. 11 A, C). In cells pretreated with higher concentrations of Triton (1%), the α-platein staining is greatly reduced, revealing only a fainter surface stain (no individual plates) and subsurface vesicles (Fig. 11D). By contrast, this more stringent permea-bilization reveals intact, fully stained plates when the β/γ-specific antibody is used (Fig. 11B).
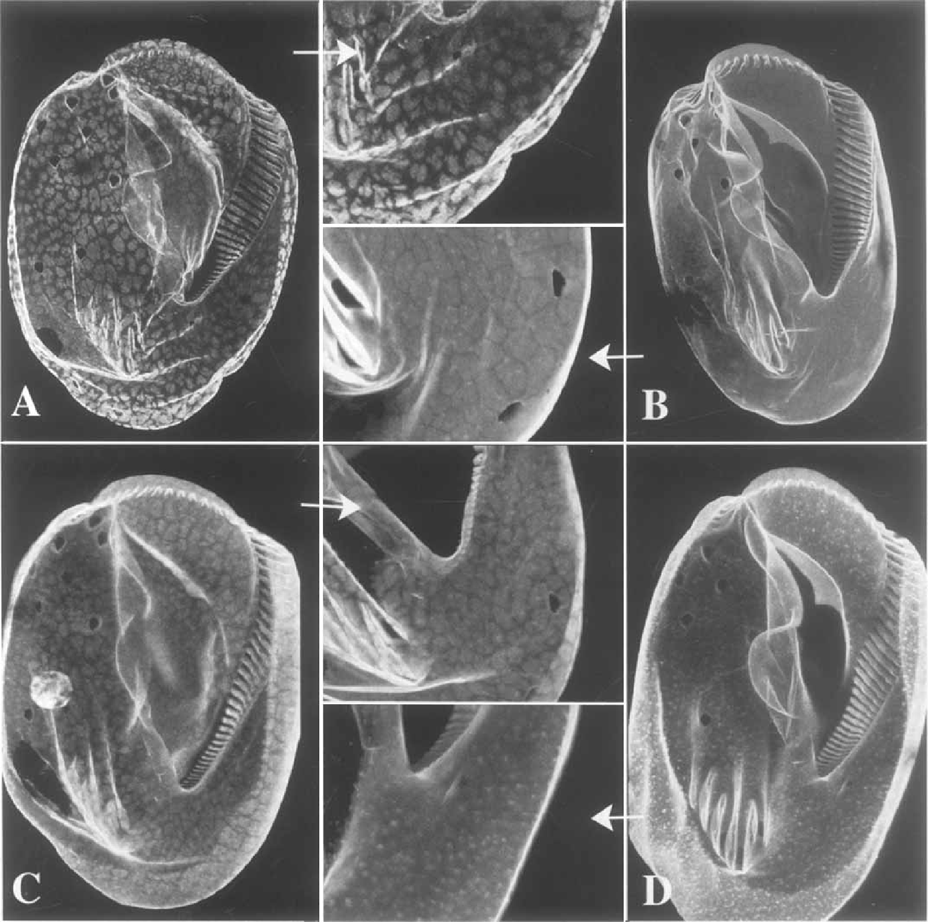
Confocal immunofluorescence localization of α-and β/γ-plateins on the ventral surfaces of morphostatic cells. Before staining, cells were permeabilized/extracted with either mild (0.25% A, C) or stronger (1 % B, D) concentrations of Triton X-100. After mild permeabilization, plates appear only irregularly decorated with the anti-Plyplatein (A; inset), but are clearly visible after decoration with the α-platein antibody (C; inset). After strong extraction the plates are fully and strongly decorated with the anti-β/γ-platein (B; inset), although plates are no longer detected with the anti-α-platein (D; inset); many small vesicles appear just beneath the cell cortex.
Platein solubility (and/or accessibility to immunoglobulin probes) appears to be altered during cortical morphogenesis (Fig. 12). With α-platein antibodies, newly forming plates appear partially extracted (reticulated) after even mild (0.25% Triton) treatment. No AP-2 (α-platein) staining remains in these developing plates following 1 % Triton pre-treatment. These detergent treatments are performing a selective extraction of the α-plateins from the AP, rather than plate dissolution: reaction with PL-3 (β/γ-specific) reveals fully stained new plates after either Triton extraction condition. Thus the β/γ-plateins are less soluble than the α forms. Since the β/γ forms in assembling plates are fully stained even after mild permeabilization, their epitopes appear to be more accessible to antibodies during platein polymerization than they are in fully assembled, mature plates. At the end of morphogenesis, just after cell separation, α-plateins remain relatively more soluble within the new plates, as observed by the contrast in AP-2 staining between old and new plates. Interestingly, at the time of cell separation the limit between the presumptive ‘old’ and ‘new’ plates is closer to the poles than has been described with traditional silver staining; plateins remained insoluble within only the anterior-most ranks of parental plates (Fig. 13) instead of the ten or so expected for the anterior cell. In the posterior daughter, a peculiar new-plate staining pattern was observed around the posterior-most parental basal bodies, which do not undergo multiplication during division and which are surrounded by plates thought to be transmitted from one generation to the next without change. Thus, solubility of plateins in the plates does not seem to follow the pattern of renewal of the plates as previously characterized by the classical inorganic silver impregnation techniques.
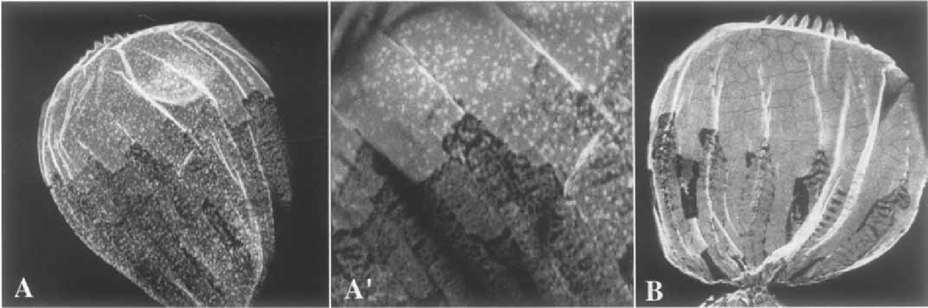
Confocal immunofluorescence localization of α- and β/γ-plateins in dividing cells. Shown are the dorsal surfaces of cells decorated with anti-α- (A, enlarged in A') and anti-β/γ-plateins (B). The difference in labelling between mature plates (anterior in these proters) and newly forming plates closer to the fission furrow is apparent with the anti-β-platein stain. Both new (along the dorsal ciliary rows) and old AP are decorated with the anti-β/γ-platein antibody.

Confocal immunofluorescence localization of plateins in a proter following division, using mAb PL-5 (which reacts with all platein forms). The border between new and old plates is evident: the majority of plateins detectable with this antibody have been solubilized, illustrating the extent of the field of new dorsal plates following the second wave of AP remodeling described in the text. Only a few anterior ranks of plates retain the mature plate staining appearance.
In order to clarify this paradox, we undertook an analysis of the late stages of pre-division morphogenesis, using silver nitrate impregnation. We observed that, indeed, almost all parental dorsal plates undergo a replacement following the first wave of proliferation. This unexpected second wave of remodeling occurs within the plates surrounding those basal bodies that do not duplicate; argentophilic circular lines appear around adjacent basal bodies and enlarge until they merge. At the end of the process, all plates adjoining basal bodies on the dorsal surface have been renewed, and only the anterior-most ranks of plates (located between the anterior end of the kineties and the oral ciliature) remain unmodified. Full AP stability (interphase staining/solubility properties of all plates) is not achieved until cell remodeling is completed, about 30 min after cell separation.
In sum, these immunofluorescence studies suggest that plateins are in an intermediate, more plastic state of polymerization as growing AP spread across the cortex of cells undergoing morphogenesis. Full insolubility (and presumably rigidity) of the AP seems not to be achieved until the plates have obtained their final sizes and tightly abutted polygonal contours within mature cortical fields.
Models of alveolar plate assembly and its regulation.
Mature AP exist in a form sufficiently stable that they can be isolated intact and examined by electron microscopy (Haus-mann and Kaiser 1979). This apparent sturdiness is presumed to be the basis by which the linked monolayer of AP lend rigidity and form to the Euplotes cortex. To date, little is known about the process by which plateins (and presumably other accessory plate proteins) polymerize to form AP. As stressed by Williamson (1994), many proline-rich proteins are ‘sticky’, with the ability to bind rapidly and tightly to other proteins, forming “interlocking networks of high overall strength”. With both primary and secondary domains of platein being proline-rich, such multiple protein associations (often reversible through phosphorylation) may well be important in the AP polymerization process. Additionally, several other factors must be taken into account when considering models of plate assembly:
- 1
evidence for plate precursor vesicles, based on previous cytological work;
- 2
evidence for disulfide cross-linking of platein monomers;
- 3
the unusual environment (alveolar cisternae) within which the plates are constructed;
- 4
potential for platein phosphorylation in regulating poly merization state.
Postulated plate precursor pathway.
New AP are inserted into, and old AP removed from, the Eupldtes cell surface with each cell reproduction, vegetative or sexual (Ruffolo 1976; Wise 1965). Current models propose the assembly of new al-veolus-AP complexes via the fusion of aggregates of dense-cored membranous vesicles (“alveolar precursor vesicles”: Geyer and Kloetzel 1987; Ruffolo 1976). These vesicles, apparently derived from specialized ER without Golgi involvement, accumulate specifically at times of cortical morphogenesis, and their contents have been shown to react with anti-platein antibodies by immunoelectron microscopy (Kloetzel 1990). It appears then that the initial steps in platein polymerization may occur within small membranous precursors even prior to their insertion into growing AP; however, from the immunochemical results described here, the plates do not develop their full resistance to solubilization until they are of mature size. A gradual, stepwise sequence of polymerization/condensation events thus can be envisioned—a sequence that evidently can be reversed as old plates are removed/replaced with each cell reproduction.
Potential disulfide cross-linkages.
Although cysteines are rare in the plateins, both α- and β/γ-plateins contain one such residue (a second cysteine in pAy-platem presumably is removed with the signal sequence). When cortical extracts are subjected to SDS-PAGE in the absence of reducing agents, it has been observed that protein bands with higher molecular weights than platein monomers are observed; these bands react with antiplatein antibodies (both mAbs and pAbs). Isolation and re-elec-trophoresis of these high Mr bands in the presence of β-mer-captoethanol resolves these proteins into platein monomers; evidence for both homo- and heterodimeric plateins in the non-reduced state was obtained (JAK, unpubl. data; see Kloetzel 1997). Although the oxidation-reduction state within the cortical alveoli (and within the AP precursor vesicles described above) is unknown, formation of disulfide-bridged multimers may be involved in the polymerization of mature AP.
Alveolar environment and potential Ca2+ involvement.
Numerous studies have shown that the cortical alveoli in at least some ciliates serve as reservoirs for calcium ions (Plattner et al. 1997; Stelly et al. 1991). This means that, unlike other cytoskeletal proteins (including articulins) that assemble in a cytoplasmic environment with very low free Ca2+ concentrations, plateins may exist and polymerize into AP in a Ca2+-rich environment. It would thus seem that Ca2+ ions potentially could be used in the polymerization process, and/or are a component of the assembled AP. Gumpel and Smith (1992) describe a Ca2+-binding protein from Euglena gracilis whose features include acidic repeat domains and a very low pi. Considering that the plateins have very large net negative charges (accounting for the low pi values reported above), it seems highly likely that the responsible aspartate and glutamate residues may play roles in binding Ca2+ ions—perhaps as part of the polymerization process. These considerations bring to mind the calcareous scales that assemble within the cortical alveoli in the ciliate Coleps (Huttenlauch 1985).
Phosphorylation and plate assembly/disassembly?
Finally, another factor in the control of platein polymerization could be a reversible phosphorylation of one or more of the plate proteins. Experimental evidence has shown that phosphorylation of the lamin A intermediate filaments (IF) of the nuclear lamina leads to depolymerization of the IF meshwork and breakdown of the nuclear envelope at mitosis (Heald and McKeon 1990). The polymerization state of other IF classes has been shown to be similarly regulated by phosphorylation (Inagaki et al. 1996). In the plateins a significant pool of potentially phosphorylable residues has been detected. In Fig. 9, likely sites of serine, threonine, and tyrosine phosphorylation (S/T/Y with high-stringency prediction values) are highlighted. Most striking is the high number of such residues (32) in β/γ-platein, and the distribution of these sites within the core dodecamer repeat domain. In the arrangement of this region depicted in Fig. 9A, ten of the 27 repeats terminate with Ts predicted to be phosphorylation substrates; these are scattered through the core domain. Fourteen other highlighted residues (mostly Ys) are scattered through the core at ‘internal’ sites in the depicted repeat arrangement. The α-plateins have many fewer potential phosphorylable sites, primarily in the core domain, grouped noticeably toward the C terminus. If the stringency threshold for predicted phosphorylation is lowered to values of 0.8 and above, there are 10 potential S/T/Y sites in the final 9 core repeats of α2 platein.
Articulins in Euglena are phosphorylated in vivo (Fazio et al. 1995), albeit while residing in a different cellular environment (cytosol) than the plateins. However, protein kinases that function within membrane-enclosed cisternae (on acidic, pro-line-rich substrates) are known in the secretory pathway (Drzymala et al. 2000); if accompanied by similarly localized protein phosphatases, such a level of AP assembly regulation within the environment of the alveolar sacs is feasible. To date there is little information on the phosphorylation state of the plateins. Williams (1991) demonstrated that all the platein iso-forms of E. aediculatus show significant charge heterogeneity on 2-D gels. Surprisingly, in his figure the plateins of the closely related E. eurystomus did not show this. However, if platein phosphorylation is transient and reversible (e.g. as a function of new alveolar plate assembly and old plate disassembly), the levels and pi range of charged platein isoforms could reflect the growth state of the cultures from which the cytoskeletal proteins were isolated. It is relevant to note that a “transcellular wave” of phosphorylation, involving cytoskeletal proteins as substrates, has been reported to accompany cortical morphogenesis in Paramecium (Sperling et al. 1991).
Plateins in other cell types?
Although articulin-like proteins are found in many taxa, the question arises as to whether plateins, as a clearly separable family within the articulin class, also have homologs beyond the genus Euplotes. A much larger family of hypotrich ciliates, collectively termed the euplotids, also display plates within their cortical alveoli similar to those of Euplotes (at least ultrastructurally). If, as discussed above, the alveoli function in Ca2+ storage, the intra-alveolar space would provide an unusual environment in which to assemble elements of the cytoskeleton. It seems reasonable, then, to expect similarities in the basic properties of the cytoskeletal proteins themselves—if not strictly at the level of primary a. a. sequence, then at the level of the composite molecular plan: overall a. a. composition and/or secondary and tertiary structure, yielding similar functional domains. Thus, while their biochemical composition is not yet known, we suggest that the AP in such hypotrich ciliate genera as Aspidisca (Rosati et al. 1987), Diophrys (Walker and Maugel 1980), and Certesia (Wicklow 1983) will be found to be composed of platein-like molecules. Additionally, some non-euplotid ciliates display intra-alveolar cytoskeletal elements. One well-documented case is that of the ‘armored’ gymnostome Coleps, which contains rigid calcified scales within its alveoli (Huttenlauch 1985). These plates most likely include a proteinaceous matrix, perhaps of specialized platein/articulins. Whether these considerations might apply even to the thecal plates within the ‘alveolus equivalents’ of dinoflagellates (cortical amphiesmal vesicles; Bouck and Ngô 1996; Bricheux, Mahoney, and Gibbs 1992) will require further studies.
A corollary to these predictions is that cells that do not have intra-alveolar cytoskeletal elements, and assemble a membrane skeleton or epiplasm free in the cytoplasm, would be expected to utilize articulins more similar to those of Euglena or Pseudomicrothorax, rather than plateins (or, possibly, epiplasmin-type proteins instead of articulins: see Coffe et al. 1996; Hut-tenlauch, Peck, and Stick 1998).
Utility of expression libraries in identifying genes in hypotrich ciliates.
In ciliates, attempts to isolate genes using antibody probes have not been profitable because the altered ciliate genetic code (one of the few deviating from the standard or ‘universal’ code) prevents the expression of their genes in heterologous systems. In some ciliate species, the standard stop codons UAA and UAG are translated into glutamine; in other species (like the euplotids) the glutamine codon usage is normal, but UGA is translated into cysteine (Baroin-Tourancheau et al. 1995). Hence, up to the time of this study, no ciliate gene had been isolated from an expression library. In E. aediculatus, the extent to which the UGA codon is used does not appear very high, since very few or no abnormal cysteine codons were found in the sequences of several coding genes. Accordingly, we assumed that this species would be likely to provide an exploitable expression library in E. coli for genes having rare UGA codons, with many of the truncated polypeptides being of sufficient length to carry epitopes reactive with antibodies.
The present work demonstrates that in Euplotes, at least for genes having few cysteine codons, the approach is viable. A set of monoclonal and polyclonal antibodies raised against other cytoskeletal proteins has also been tested; for example, a cDNA encoding an α-tubulin gene has been fully characterized using a specific mAb (this gene also lacks any in-frame UGA). We currently are trying to identify several other cortex-associated protein-encoding genes, using antibody screening of the Euplotes expression library.
ACKNOWLEDGMENTS
Thanks are due to Norman Williams for his gift of Euplotes anti-E antiserum, to Yang Tie for making available the genomic library of E. aediculatus, and to Ghislaine Fryd-Versavel for carrying out the silver stain protocols. JAK acknowledges with gratitude the financial assistance of the CNRS for his residence in Orsay, and from the Universitæ di Camerino for his visit there. The corresponding author would like to dedicate this work to the memory of the late André Adoutte, whose inspired leadership and steady encouragement was an essential ingredient of our collaboration at the Universite Paris-Sud.




