The Interaction of Atovaquone with the P. carinii Cytochrome bc1 Complex
Atovaquone is an important second-line therapeutic and prophylactic agent for Pneumocystis carinii pneumonia although it was originally developed as an antimalarial [13]. For malaria, atovaquone is now only used in combination with proguanil, another antimalarial. This combination (Malarone®) is used because atovaquone monotherapy leads to resistance [10].
Atovaquone is structurally similar to ubiquinone (CoQ) (Fig. 1). UHDBT and stigmatellin (Fig. 1) are other CoQ analogs that have been well studied in experimental systems [2,3]. Both UHDBT and stigmatellin are known to inhibit electron transport by binding to the Q0 site of the cytochrome be1 complex. Atovaquone probably acts at the same site, since it has also been shown to inhibit electron transport in both maiaria and P. carinii [4,6]. This mechanism could also explain the rapid emergence of resistance to monotherapy; mutations could arise easily in cytochrome b, since it is encoded in the mitochondria! genome where the spontaneous mutation rate is lOx higher than in nucleus [1].
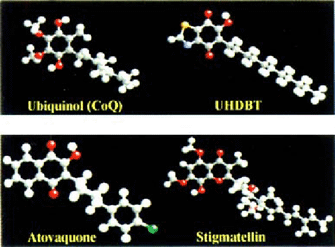
Structures of ubiquinone and its analogs.
In experimental systems, resistance to UHDBT and stigmatellin has been shown to arise by mutations leading to changes in amino acids in cytochrome b that line the Q0 site [2,3]. In 1998, we found mutations in the P. carinii cytochrome b Q0 site in isolates from patients exposed to atovaquone [16]. Subsequently, mutations in the cytochrome b Q0 site were found to be associated with atovaquone resistance in laboratory strains of plasmodium and toxoplasmosa [8,11,14,15]. We recently demonstrated a significant association between mutations in the P. carinii cytochrome b Q0 site and exposure to atovaquone in a retrospective cohort study suggesting that these mutations confer clinical resistance [7]. The mutation sites found in this and the previous study are shown in Figure 2.

Mutations found in the P. carinii cytochrome b Qo site [7,16].
Our goal now was to determine the location of these mutated amino acids in relation to the probable binding site of atovaquone. Since the P. carinii cytochrome bc1 complex has not been crystallized, we used the yeast and chicken bc1 complexes, whose 3-dimensional structures are known (PDB ID codes 2BCC and 1EZV) as models [5,17].
We first used a molecular mechanics energy minimization igram, MacSpartan Pro, to obtain the lowest energy conformation of vaquone (Fig. 3).
onformational energy profile for atovaquone.
Model-building based on the stigmatellin complexes suggests that atovaquone could bind to the be1 complex in either of two similar orientations. The correct binding mode will probably be unknown until co-crystals are obtained; however both orientations fit in the same cavity (data not shown). The interactions between atovaquone and the cytochrome be1 complex are shown for the yeast (Fig. 4) and chicken (Fig. 5) complexes in models made using Molscript [9] and Raster3D [12]. Amino acids which are homologous to the mutated amino acids are shown with bonds that are yellow (for P. carinii) and green (for Toxoplasma and Plasmodium). Amino acids in the yeast complex which are homologous to 5 of the P. carinii mutations (1147, T148, LI50, SI52, L275) in close proximity to atovaquone, while 2 (T127 and P266) are not. These latter mutations may affect atovaquone binding indirectly. For comparison, amino acids which are mutated in toxoplasma (M139 and 1269) and plasmodium (1269, F278, Y279, L282, and R283) are also shown as is the iron-sulfa protein (purple) and the active site histidine (HI81). This pattern is the same for the chicken structure (Fig. 5).
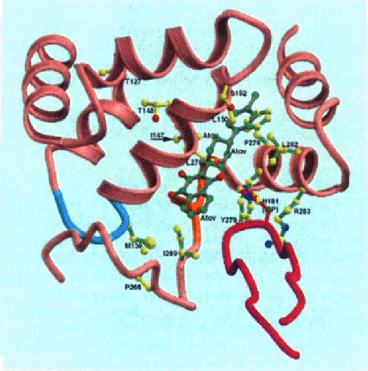
Interaction of atovaquone with yeast cytochrome bc1 complex.
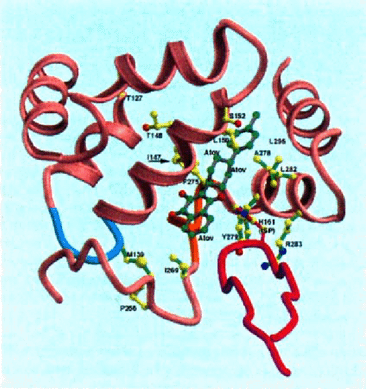
Interaction of atovaquone with the avian cytochrome bc1 complex.
We have also begun studying the effects of atovaquone on the yeast and bovine cytochrome bc1 complexes. As is shown in Figure 6, atovaquone is a competitive inhibitor. At a concentration of 0.1 μM, which inhibits the yeast enzyme approximately 50%, atovaquone increases the apparent Km for ubiquinol and has no effect on Vmax. In titration experiments, atovaquone causes 50% inhibition of the yeast enzyme at 90 nM and of the bovine enzyme at 170 nM. Thus, atovaquone appears to be selective for the yeast enzyme compared to the bovine enzyme. Of interest, yeast, P. carinii, Toxoplasma and Plasmodium (“sensitive”) cytochrome b's have aromatic residues at position 278, while the bovine, chicken and human cytochrome b's do not. In Plasmodium, resistance develops when this aromatic amino acid is changed to isoleucine. These data suggest that a pi-pi interaction between this residue and atovaquone might promote binding.
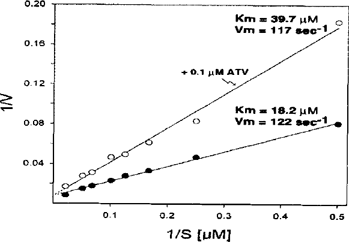
Lineweaver-Burke plot showing competitive inhibition of the yeast cytochrome bcl complex by atovaquone (ATV).
Further studies on the interaction between atovaquone and cytochrome bc1 may lead to a better understanding of the mechanism of resistance and aid in the development of new chemotherapeutic agents.
Acknowledgements
We would like to thank Wendy Armstrong, Paul A. Hossler, Ben Beard, Laurence Huang, Jane Carter, Jeffrey Duchin, Lawrence Crane, William Burman, James Richardson for technical help and access to patient samples. This work was supported by NIH grants R01 AI 46966, GM 20379, and R01 DK 44842.




