SL-11158, a Synthetic Oligoamine, Inhibits Polyamine Metabolism of Encephalitozoon cuniculi
Polyamine metabolism as a target for drug intervention in rapidly proliferating cells has evolved within the past two decades. The initial impetus was to block de novo synthesis of putrescine and spermidine, targeting ornithine decarboxylase and S-adenosylmethionine decar-boxylase (Fig. 1). This, however, did not take into account the ability of many cells, especially tumor cells, to transport spermidine or spermine, interconverting them as needed using the highly inducible enzyme spermidine/spermine N1-acetyltransferase (SSAT) and poly-amine oxidase. Thus present attempts to interfere with polyamine metabolism are now directed to the use of poly-amine analogs which are transported, induce SSAT, and cause reductions in cellular polyamine levels but do not replace polyamines in function [7,9]. Agents such as bis-ethylspermine, bis-ethylhomospermine and BE-4X4 [1,20-(ethylamine)-5,10, 15-triazanonadecane] have become the forerunners of a rapidly evolving synthetic strategy. These and other polyamine analogs are now in clinical trials for various types of cancers [6,7] and as anti-spasmodic agents in AIDS-related diarrhea [4].
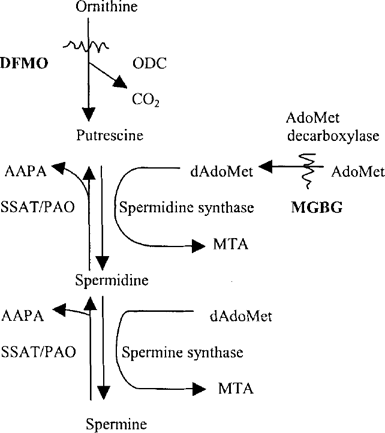
Polyamine synthesis and interconversion in Encephalitozoon cuniculi. Abbreviations: ODC, ornithine decarboxylase; AdoMet, S-adenosylmethionine; dAdoMet, decarboxylated AdoMet, AdoMetdc, AdoMet decarboxylase; MTA, methylthioadenosine; SSAT, spermidine/spermine N1acetyltransferase; PAO, polyamine oxidase; AAPA, 3-acetamidoproprion-aldehyde; DFMO, DL-α-difluoromethylornithine; MGBG, methylglyoxal-(bis)guanylhydrazone.
Polyamine analogs as chemotherapeutic agents have recently been applied experimentally to opportunistic pathogens, including Pneumocystis carinii [10], Cryptosporidium parvum [11], and the microsporidian Encephalitozoon cuniculi [2]. One class of polyamine analogs are the oligoamines—those having the general structure: C2H5[HN-(CH2)4]NHN-(CH2)4HN[(CH2)4NH]NC2H5 (7). A series of these compounds has been synthesized and several found to be growth inhibitory in vitro and in vivo in a laboratory model infection of Enc. cuniculi. One of these, SL-11158, is an octamine having the structure: C2H5[HN-(CH2)4]3HN-(CH2)4HN-]3C2H5. This compound gave an IC50 value of 8.2 μM in a standard Enc. cuniculi growth screen, and was curative in two in vivo screen using nude (Balb/cNu/Nu) or CD-8 knocknout (C57B/6JΔCD8) mice infected with this organism [2].
In the present study we examined pre-emergent spore stages of Enc. cuniculi for polyamine uptake and interconversion, and for possible effects of SL-11158 on polyamine metabolism.
MATERIALS AND METHODS
Organism
Encephalilozoon cuniculi (ATCC 50612) is maintained on RK-13 cells which are grown to 80% confluency in Coming T-25 or Falcon T-75 flasks and infected with 5 X 105 spores. RK-13 cells are grown and maintained on Minimal Eagles Medium (+Earle's salts + L-glutamine + 7% fetal bovine serum: [3].
Gradient techniques
Isolation of two fractions of Enc. cuniculi from homogenates of infected RK-13 cells was done using a Percoll gradient technique modified from that of Green et al. [8], Infected RK 13 cells were homogenized in pH 7.4, 0.15 M KPO4 buffer + 0.9% NaCl containing a protease inhibitor cocktail of TLCK, aprotinin, leupeptin, and dithiothreitol. The two fractions sedimented at 1.018–1.035 g/ml (light fraction) and 1.102–1.110 g/ml (heavy fraction). Details of this procedure have been described [3].
Incubation studies
Purified gradient preparations were incubated in pH 7.4, 0.15 M KPO4 buffer + 0.9% NaCl containing: 3 mM ATP, 1 mM GTP, 0.5 mM NAD+, 2 mM glucose-6-PO4, 0.5 mM acetyl CoA, 1 mM Na pyruvate and 2 mM glucose. Either 0.5 μCi (0.2 nmol) [terminal methylenes 3H(N)]spermidine or 0.25 μCi (2.1 nmol) [1–14C] spermine with or without 10 uM SL-11158 was added to start the incubations. Suspensions were pelleted and extracted overnight with 10% TCA. The supernatants from these incubations were also saved for analysis.
HPLC techniques
We used a solvent system which separates putrescine (RT 23:30) spermidine (34:30) and spermine (37:00), in addition to N1-acetylspermine (35:30) and N1-acetylspermidine [29:10: (1, 12)]. This system utilized UV/visible detection (254 nm) and a flowthrough radiodetector (IN/US Systems, Miami, FL), using a (β-RAM integration program (IN/US Systems).
Protein determinations were by the method of Bradford [5] using bovine serum albumen as standard.
RESULTS AND DISCUSSION
Gradients of RK-13 preparations have been examined by electron microscopy and found to contain: light fraction (d =1.018–1.035) sporoblasts, empty spore casings and debris; heavy fraction (d=1.102–1.119)-pre-emergent mature spores and immature spores [3]. 1-[14C] Spermine was taken up by pellet suspensions of both fractions (Table 1) but uptake by the lower fraction was 4.1 times that of the upper fraction (255 vs 62 nmol/mg protein/2 h). No metabolism of spermine was detected in either of these incubations. Duplicate preparations incubated with 10 μM SL-11158 had reduced uptake: 33% for the upper preparation and 48% for the lower. Supernatant fractions from these preparations reflected this inhibition: each had ∼3 times the amount of label remaining as the respective supernatants from the full activity controls.
| nmol/mg protein/2 h | ||||
|---|---|---|---|---|
| Light Fraction | Heavy Fraction | |||
| Substrate | Pellet | Supernatant | Pellet | Supernatant |
| 14C-Spermine | 62 | 21 | 255 | 20 |
| + SL-11158 | 32 (−48%) | 64 | 171 (−33%) | 66 |
- aLight (d=1.018–1.035 g/ml) and heavy (d=1.102–1.119 g/ml) gradient fractions containing intact pre-spores were incubated for 2 h in 0.15 M KPO4 buffer + 0.9% NaC;, pH 7.4 with 0.25 μCi (2.1 nmol) [l-14C]spermine, with or without 10 μM SL-11158. After incubation, preparations were pelleted, washed and lysed with 10% TCA. The TCA extracts and incubation supernatants were analyzed for polyamines by HPLC methodology. Results of duplicate determinations.
Metabolism of [3H] spermidine was far more apparent than that of spermine (Table 2). Pellets of both gradient fractions metabolized spermidine to putrescine and an unknown peak at an RT of ∼ 4:00. Tlis peak was not Nl-acetylspermidine or N1-acetylspermine, whose RT's were 29:10 and 35:30, respectively, but may be acetamidopro-prionaldehyde, as it appeared to be nearly equimolar with putrescine in both pellet preparations (e.g., 1.1 and 0.8 for the light pellet, and 11.6 and 10.8 for the heavy pellet). Overall, the heavy pellet preparation took up ∼ 5.3 times more label than the light (36.6 vs 6.8 nmol/mg protein/2 h). Pellet preparations incubated with SL-11158 differed in their response: total uptake for the light pellet increased slightly, but the heavy pellet had an approximately 50% reduction in total uptake, and a ∼60% reduction in the product at RT 4:00. Putrescine production also decreased ∼ 40% in the presence of SL-11158 from 11.6 to 4.6 nmol/mg protein/2 h.
| nmol/mg protein/2 h | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Light Pellet | Heavy Pellet | |||||||||
| Substrate | Spermine | Spermidine | Putrescine | Unknown | Total | Spermine | Spermidine | Putrescine | Unknown | Total |
| 3H-Spermidine | 1.8 | 3.1 | 1.1 | 6.8 | 6.8 | — | 14.2 | 11.6 | 10.8 | 36.6 |
| + SL11158 | 0.02 | 4.2 | 1.6 | 1.7 | 7.52 | 0.1 | 10.2 | 4.6 | 3.9 | 18.8 |
| Light Supernatant | Heavy Supernatant | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Spermine | Spermidine | Putrescine | Unknown | Total | Spermine | Spermidine | Putrescine | Unknown | Total | |
| 3H-Spermidine | 0.4 | 1.0 | 1.2 | 4.0 | 6.5 | 1.0 | 7.7 | 28.9 | 112.6 | 150.2 |
| +SL11158 | 0.5 | 5.8 | 2.9 | 5.6 | 14.8 | 0.6 | 12.0 | 4.7 | 39.7 | 57 |
- aMethods as in Table 1 except incubation containing 0.5 μCi (0.2 nmol) [terminal methylenes 3H (N)] spermidine. with or without 10 μM SL-11158. After incubation, preparations were pelleted, washed and lysed with 10% TCA. Results of duplicate determinations.
Supernatant fractions from light and heavy pellet incubations differed considerably in their contents (Table 2). While the super-natants from the light pellet incubations contained small amounts of spermidine and the unknown, the supernatants from the heavy pellet had large amounts of the unknown-about 10 times more than found in the heavy pellet. The putrescine level in this supernatant was about 2.5 times that of the pellet. These findings indicate that the spermidine is taken up, and metabolized with the metabolites excreted into the medium. Mammalian cells interconvert polyamines, and can excrete the acetylated derivatives as well as acetamidopropionaldehyde [9]. HPLC traces of the [3H]spermidine used in the experiment did not contain any of the unknown peak at RT 4:00, and < 1% of the total label was present as putrescine.
The inhibition by SL-11158 of the RT 4:00 peak found in the heavy pellet is reflected in the supernatant: the level of this peak is reduced 64% from that of the control. Likewise, the level of putrescine in the treated supernatant is 84% lower than the control. Lysates of mature spores of Enc. cuniculi were demonstrated to have SSAT activity [3] which is inhibitable with SL-11158 (not shown).
This study reinforces our previous findings that Enc. cuniculi assimilates and interconverts polyamines [Fig. 1: (3)] but also indicates that spermidine is taken up far more readily than spermine. Moreover a large proportion of the metabolites are excreted as in mammalian cells The identity of the radiolabeled metabolite at RT 4:00 is presently unknown, but in light of the extensive interconversion of spermidine to putrescine seen in the heavy fraction, it is most likely an acetylated aminopropyl product of these reactions (spermidine/spermine N1-acetyltransferase and polyamine oxidase). Further studies are underway to identify this metabolite.
The primary effects of polyamine analogs on cells may include competition for uptake through polyamine transporters, upregulation of SSAT, and excretion of polyamines, resulting in reduction of polyamine content [6,7]. The inhibition of metabolite production and excretion by a low concentration of SL-11158 [IC50 value, 8 μM: (2)] indicates that this polyamine analog targets, in part, polyamine inter-conversion in this parasite.
ACKNOWLEDGMENTS
Supported by NIAID grants 141398 (M.W.) and AI43094 (B.F.).




