Inflammation and hemostasis biomarkers and cardiovascular risk in the elderly: the Cardiovascular Health Study
Abstract
Summary. Background: There are few studies of inflammation and hemostasis biomarkers and cardiovascular disease risk (CVD) in older adults. Objectives: To assess multiple biomarkers simultaneously and in combinations for CVD risk assessment in older individuals. Patients/methods: Thirteen biomarkers, interleukin-6 (IL-6), C-reactive protein (CRP), D-dimer, fibrinogen, factor VII, factor VIII, leukocyte count (WBC), platelet count, lipoprotein(a), soluble intercellular adhesion molecule-1 (sICAM-1), albumin, homocysteine and uric acid, were correlated with incident CVD in 4510 individuals in the Cardiovascular Health Study. Baseline biomarkers were analyzed as gender-specific SD increments and quintiles in proportional hazards models adjusted for demographics, CVD risk factors and medications. Results: Over 9 years with 1700 CVD events, seven biomarkers were associated with CVD. Adjusted hazard ratios (HRs, 95% CI) per SD increment were 1.16 (1.09, 1.23) for IL-6, 1.16 (1.09, 1.23) for CRP, 1.13 (1.05, 1.21) for D-dimer, 1.17 (1.09, 1.25) for homocysteine, 1.06 (1.00, 1.12) for WBC, 1.06 (1.00, 1.12) for factor VIII, and 1.07 (1.00, 1.13) for lipoprotein(a). Fibrinogen was associated with CVD in men only (HR 1.12, 95% CI 1.04, 1.22) and sICAM-1 in women only (HR 1.16, 95% CI 1.05, 1.27). IL-6 and CRP remained associated with CVD when modeled with WBC. Participants were classified by all combinations of two biomarkers being high or low (IL-6, CRP, WBC, factor VIII, cholesterol/HDL). All were associated with CVD when cholesterol/HDL was low and none when CRP was low. Conclusions: Seven biomarkers were associated with CVD in older adults, with CRP having some advantages compared with others. Even larger studies are needed to better characterize these associations.
Introduction
Many inflammation and hemostasis biomarkers are associated with cardiovascular disease (CVD) risk; however, only C-reactive protein (CRP) is clinically used [1,2]. Data on biomarkers and cardiovascular risk come from studies of middle-aged individuals, with fewer data available for older adults [1,3]. In the elderly, increased incidence of inflammatory conditions and potential differences in cardiovascular disease phenotypes may affect associations of biomarkers, including those representing multiple related domains such as hemostasis and endothelial cell function, with CVD [2,4,5]. As traditional CVD risk factors are less predictive and biomarkers less studied in the elderly, these biomarkers must be examined, compared qualitatively, and evaluated in combination and with lipid measurements to address their use in CVD risk stratification [1,2,6].
The Cardiovascular Health Study (CHS) provides a unique opportunity to simultaneously investigate the associations of multiple biomarkers with CVD alone and in combinations in older adults [7]. Previous CHS papers have reported individual associations of interleukin-6 (IL-6), CRP, D-dimer, factor (F) VIIc, FVIIIc, lipoprotein(a) (Lp(a)), and soluble intracellular adhesion molecule-1 (sICAM-1), with various vascular outcomes and shorter follow-up in nested case–control subsets or the whole cohort [8–13]. Here we evaluated the associations of 13 biomarkers (IL-6, CRP, D-dimer, fibrinogen, FVII, FVIII, leukocyte count (WBC), platelet count, Lp(a), sICAM-1, albumin, homocysteine, and uric acid), alone and in combinations with cardiovascular events over 9.2 years of follow-up. While some of these biomarkers are not traditionally thought of as inflammation markers, they are all to a greater or lesser degree correlated with inflammation status.
Methods
Subjects
The CHS is a prospective, observational cohort study of risk factors for and consequences of CVD in community-dwelling adults aged 65 years and older [7]. In 1989–1990, 5201 participants (original cohort) were recruited from four US communities (Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Allegheny County, Pennsylvania). An additional 687 primarily black participants were recruited in 1992–1993 (minority cohort) for a total cohort of 5888. Medicare eligibility lists were used to identify potential participants, and 57.3% of those eligible enrolled [14]. Informed consent was obtained using methods approved by institutional review committees at each study site [7]. Standardized enrollment procedures assessed height, weight, blood pressure, health history and non-invasive measures of vascular disease (ankle-arm index and duplex ultrasonography of the carotid arteries) [7].
Laboratory analysis
Phlebotomy was performed on the morning of enrollment after an 8–12-h fast [15]. WBC, platelet count, fibrinogen (von Clauss method), uric acid, FVII and VIII coagulant activity (VIIc, VIIIc), total cholesterol, high density lipoprotein cholesterol (HDL), and albumin were analyzed on enrollment [15]. IL-6, CRP, D-dimer, homocysteine, Lp(a), and sICAM-1 were measured using frozen samples. IL-6 and CRP were measured in the entire cohort using commercial (Quantikine IL-6, R&D Systems, Minneapolis, MN, USA) and validated in-house high-sensitivity enzyme-linked-immunosorbent-assays (ELISA), respectively [16,17]. D-dimer, homocysteine and sICAM-1 were measured in nested case–control studies of incident CVD [8–10]. D-dimer was measured by ELISA [18], homocysteine by high performance liquid chromatography [19], and sICAM-1 by ELISA (Parameter Human sICAM-1 Assay, R&D systems) [20]. Lp(a) (measured by ELISA [13]) and FVIIIc were not measured in the minority cohort.
Definitions
Ethnicity was assessed by participant self-report from a list. Baseline CVD was confirmed by medical record review [defined as previous myocardial infarction (MI), angina, angioplasty, coronary artery bypass grafting, stroke, or transient ischemic attack (TIA)] [21]. Family history of MI was defined as history of MI in a sibling. Hypertension was defined as blood pressure greater than 140/90 mmHg or use of antihypertensive medications with a physician diagnosis of hypertension. Diabetes was defined as fasting glucose greater than 126 mg dL−1, taking insulin or oral hypoglycemic medications.
Outcomes and follow-up
Those with baseline CVD were excluded from analysis (n = 1378). Participants were contacted biannually, alternating between telephone interviews and clinic examinations with periodic searches of Medicare files to ascertain events. Medications were assessed annually. The study endpoint was time to first cardiovascular event, including MI (non-exclusive of other events, n = 339), coronary artery angioplasty or bypass grafting (with or without angina, n = 124), angina alone (n = 250), stroke alone (n = 468), TIA alone (n = 119), and cardiovascular death (n = 400; death because of atherosclerotic coronary heart disease, cerebrovascular disease, atherosclerotic disease or other CVD) [7]. Outcomes were validated by committee using standardized criteria and complete through to 30 June 2002 [22].
Statistical analysis
Biomarkers were analyzed by gender-specific quintiles and gender-specific one-SD difference in value. For the SD analysis, IL-6, CRP, D-dimer and Lp(a) were log transformed. For all biomarkers except albumin (inversely associated with events) [23], the first quintile represented the reference group.
Three Cox-proportional hazard models assessed risk of incident CVD associated with baseline biomarker levels. The first model evaluated quintiles and SD increments adjusting for demographic characteristics (age, sex, and race), CVD risk factors [hypertension, diabetes, smoking, weekly kilocalories of activity, body mass index, cholesterol, HDL, triglycerides, forced vital capacity, family history of MI, alcohol use (drinks/week)] and medication use (analyzed as time-varying covariates: aspirin, HMG co-A reductase inhibitors, beta-blockers, or angiotensin converting enzyme inhibitors). Hazard ratios and 95% confidence intervals (CI) (HRs, 95% CI) for biomarkers were compared qualitatively with that of the cholesterol/HDL ratio, and gender differences were tested using interaction terms. The second model evaluated confounding by subclinical diseases (SD analysis), further adjusting model one for renal function (1/creatinine) and subclinical CVD (ankle-arm index and carotid intima-media thickness). A third model included adjustment for the Framingham score risk factors (age, sex, race, cholesterol and HDL, systolic and diastolic blood pressure, diabetes mellitus, and current smoking) [3]. This model was fit using the quintile analysis to approximate potential clinical use of these biomarkers and facilitate comparisons with other studies. To assess added utility to lipid screening, the area under receiver operating characteristic curves (AUC) were compared for a model with gender and cholesterol/HDL, adding each inflammation biomarker individually.
We evaluated all combinations of cholesterol/HDL and each biomarker significant in the AUC analysis using cross-classification, with the reference group being neither biomarker in the top quintile compared with either or both in the top quintile adjusting for model one covariates.
Results
The median age of the 4510 participants without baseline CVD was 68 ± 3.8 years (39% men, 15% black). Participant characteristics have been described previously [7,14]. During 9.4 ± 3.9 years of follow-up in the original cohort (35 917 person-years) and 8.0 ± 3.2 in the minority cohort (5515 person-years), there were 1479 and 221 incident events, respectively (total 9.2 years median follow-up, 41 432 person-years, 1700 incident cardiovascular events). Table 1 shows the distributions of the 13 biomarkers and of cholesterol/HDL.
| Biomarker (n) | n (CVD events) | Quintile 1 | Quintile 5 | Mean (SD) | |||
|---|---|---|---|---|---|---|---|
| Men | Women | Men | Women | Men | Women | ||
| Interleukin-6 (pg mL–1)* | 4129 (1550) | ≤ 1.17 | ≤ 1.01 | ≥ 3.18 | ≥ 2.75 | 1.74 (1.94) | 1.57 (1.95) |
| C-Reactive Protein (mg L–1)* | 4450 (1679) | ≤ 1.02 | ≤ 1.09 | ≥ 4.87 | ≥ 6.31 | 2.21 (9.11) | 2.58 (6.83) |
| D-dimer (ng mL–1)*† | 2320 (1057) | ≤ 86 | ≤ 84 | ≥ 302 | ≥ 259 | 147 (597) | 145 (487) |
| Fibrinogen (mg dL–1) | 4439 (1670) | ≤ 267 | ≤ 270 | ≥ 367 | ≥ 368 | 318 (66) | 323 (65) |
| Factor VIIc (IU dL–1) | 4445 (1672) | ≤ 92 | ≤ 108 | ≥ 130 | ≥ 154 | 112 (24) | 132 (30) |
| Factor VIIIc (IU dL–1)‡ | 3933 (1517) | ≤ 89 | ≤ 93 | ≥ 146 | ≥ 155 | 117 (35) | 123 (38) |
| White cell count (×109 L–1) | 4446 (1676) | ≤ 4.9 | ≤ 4.8 | ≥ 7.7 | ≥ 7.5 | 6.3 (1.9) | 6.2 (1.9) |
| Platelet count (×109 L–1) | 4422 (1667) | ≤ 177 | ≤ 206 | ≥ 280 | ≥ 315 | 233 (75) | 264 (74) |
| Lipoprotein(a) (mg dL–1)*‡ | 3971 (1535) | ≤ 12 | ≤ 12 | ≥ 79 | ≥ 84 | 39 (48) | 44 (60) |
| sICAM-1 (ng mL–1)† | 2074 (994) | ≤ 259.77 | ≤ 263.83 | ≥ 379.28 | ≥ 392.66 | 321.70 (84.01) | 329.42 (91.63) |
| Albumin (g L–1) | 4456 (1682) | ≥ 43.0 | ≥ 42.4 | ≤ 37.9 | ≤ 37.3 | 40.2 (2.9) | 39.8 (2.8) |
| Homocysteine (μmol L–1)† | 1041 (627) | ≤ 8.61 | ≤ 7.57 | ≥ 14.60 | ≥ 13.00 | 11.92 (4.38) | 10.89 (8.19) |
| Uric acid (mol L–1) | 4456 (1682) | ≤ 301 | ≤ 243 | ≥ 440 | ≥ 385 | 365 (84) | 310 (84) |
| Cholesterol/HDL | 4468 (1689) | ≤ 3.29 | ≤ 2.97 | ≥ 5.40 | ≥ 4.95 | 4.30 (1.21) | 3.92 (1.18) |
- *Median reported, SD log transformed.
- †Measured in a case–control subset of CVD cases and non-cases.
- ‡Not measured in the minority cohort.
Seven biomarkers and cholesterol/HDL were associated with incident CVD when evaluated in the SD analysis in the multivariable model (Table 2). The largest associations were for cholesterol/HDL, IL-6, CRP, D-dimer and homocysteine, with smaller associations for WBC, FVIIIc and Lp(a). Fibrinogen, sICAM-1 and albumin had significant gender interactions (P-interaction 0.04, 0.03 and 0.03, respectively). Higher fibrinogen was associated with CVD risk in men but not women and higher sICAM-1 in women but not men. Albumin, uric acid, platelet count and FVIIc were not associated with CVD in men or women. When 1/creatinine, ankle-arm index and carotid intima-media thickness were added to the model, interpretations were unchanged (Table 2).
| Biomarker¶ | HR (95% CI) per 1-SD increment | HR (95% CI) 5th vs. 1st quintile | ||
|---|---|---|---|---|
| Multivariable model* | Subclinical disease model† | Multivariable model* | Framingham model‡ | |
| Interleukin-6 | 1.16 (1.09, 1.23) | 1.14 (1.08, 1.21) | 1.60 (1.32, 1.94) | 1.71 (1.44, 2.03) |
| C-reactive protein | 1.16 (1.09, 1.23) | 1.13 (1.07, 1.20) | 1.53 (1.29, 1.83) | 1.57 (1.34, 1.83) |
| D-dimer | 1.13 (1.05, 1.21) | 1.10 (1.02, 1.18) | 1.35 (1.08, 1.69) | 1.40 (1.14, 1.72) |
| Homocysteine | 1.17 (1.09, 1.25) | 1.16 (1.07, 1.25) | 1.34 (1.01, 1.78) | 1.21 (0.93, 1.56) |
| Fibrinogen | ||||
| Men | 1.12 (1.04, 1.22) | 1.11 (1.02, 1.20) | 1.64 (1.27, 2.13) | 1.61 (1.28, 2.03) |
| Women | 1.01 (0.93, 1.09) | 0.98 (0.91, 1.07) | 0.99 (0.78, 1.25) | 1.02 (0.83, 1.25) |
| White cell count | 1.06 (1.00, 1.12) | 1.05 (1.00, 1.12) | 1.21 (1.01, 1.44) | 1.35 (1.15, 1.58) |
| Uric acid | 1.04 (0.98, 1.11) | 1.01 (0.95, 1.08) | 1.18 (0.99, 1.42) | 1.28 (1.09, 1.50) |
| Factor VIIIc | 1.06 (1.00, 1.12) | 1.05 (0.99, 1.11) | 1.18 (0.99, 1.40) | 1.22 (1.04, 1.44) |
| Lipoprotein(a) | 1.07 (1.00, 1.13) | 1.05 (0.99, 1.12) | 1.16 (0.97, 1.37) | 1.16 (0.99, 1.36) |
| sICAM-1 | ||||
| Men | 0.94 (0.84, 1.06) | 0.93 (0.83, 1.05) | 0.71 (0.50, 1.01) | 0.80 (0.58, 1.09) |
| Women | 1.16 (1.05, 1.27) | 1.14 (1.04, 1.25) | 1.56 (1.12, 2.19) | 1.50 (1.13, 2.00) |
| Platelet count | 1.02 (0.97, 1.08) | 1.02 (0.97, 1.08) | 1.08 (0.90, 1.30) | 1.14 (0.98, 1.13) |
| Factor VIIc | 1.04 (0.97, 1.08) | 1.03 (0.97, 1.10) | 1.06 (0.88, 1.28) | 1.10 (0.94, 1.30) |
| Albumin | ||||
| Men | 1.07 (0.99, 1.16) | 1.05 (0.97, 1.14) | 1.17 (0.89, 1.53) | 1.14 (0.92, 1.42) |
| Women | 0.94 (0.87, 1.02) | 0.94 (0.87, 1.01) | 0.89 (0.70, 1,13) | 0.90 (0.71, 1.14) |
| Cholesterol/HDL§ | 1.24 (1.05, 1.46) | 1.19 (1.01, 1.40) | 1.39 (1.17, 1.65) | – |
- *Adjusted for age, sex, race, hypertension, diabetes, smoking, physical activity, body mass index, total cholesterol, HDL, triglycerides, forced vital capacity, family history of MI, alcohol use, and use of aspirin, HMG Co-A reductase inhibitors, beta-blockers or angiotensin converting enzyme inhibitors.
- †Adjusted for variables in multivariable model plus 1/ creatinine, ankle-arm index and carotid intima-medial thickness.
- ‡Adjusted for Framingham risk factors: age, sex, race, total cholesterol, HDL, systolic and diastolic blood pressure, diabetes and current smoking.
- §Not adjusted for total or HDL cholesterol.
- ¶See Table 1 for information on biomarkers.
Comparing the fifth with the first quintile in the multivariable model, cholesterol/HDL, IL-6, CRP, D-dimer, homocysteine and WBC were associated with CVD. Uric acid and FVIIIc had borderline significance (Table 2). Associations of fibrinogen, sICAM-1 and albumin differed by gender; fibrinogen was associated with CVD in men only, sICAM-1 in women only. Albumin, Lp(a), platelet count and FVIIc were not associated with CVD. In models including Framingham risk score components, associations were generally larger than in the multivariable models (including uric acid and FVIIIc), but diminished for homocysteine (Table 2). No biomarker had a significant non-linear association (all P ≥ 0.14), and for most, the risk of CVD increased only for the top quintile. However, the top three quintiles of IL-6 (≥1.57 pg mL−1 for men, ≥ 1.39 pg mL−1 for women), the top two quintiles of cholesterol/HDL (≥4.62 for men and ≥ 4.12 for women), and the top three quintiles of sICAM-1 in women (≥301.3 ng mL−1) were associated with incident CVD in multivariable models (Fig. 1A and B).
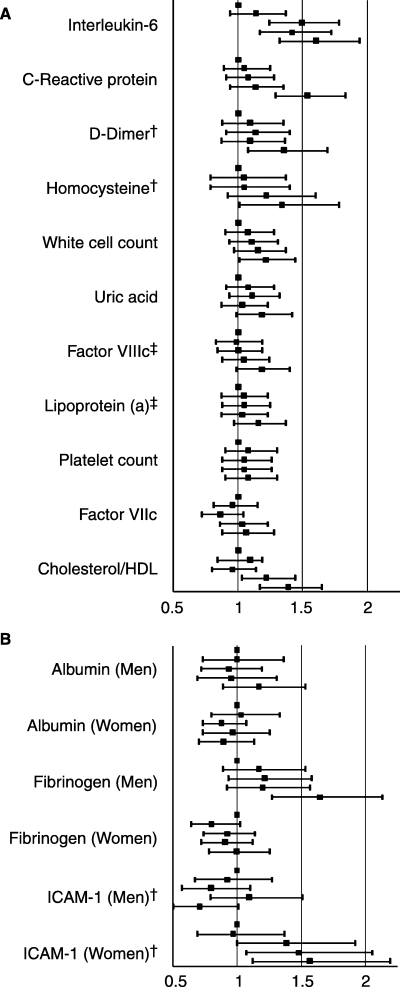
(A) Adjusted hazard ratio for cardiovascular events by biomarker quintile. *Adjusted for variables in multivariable model Table 2. HR for cholesterol/HDL not adjusted for cholesterol, HDL cholesterol or triglycerides. Top data point represents 1st quintile, bars represent 95% CI. †Measured in a case–control subset of multiple CVD outcomes (see Table 1). ‡Not measured in the minority cohort (see Table 1). (B) Gender stratified adjusted hazard ratio for cardiovascular events by biomarker quintile. *Adjusted for variables in multivariable model Table 2. Top data point represents 1st quintile, bars represent 95% CI. †Measured in a case–control subset of multiple CVD outcomes (see Table 1).
Adjusted HRs for various CVD endpoints for biomarkers in the top quintile are shown in Table 1 in the Supplementary Material. For incident stroke or TIA, associations were similar to the composite endpoint, except WBC, fibrinogen in men and sICAM-1 in women did not reach statistical significance and lower albumin among women was a risk factor (HR, 1.41; 95% CI, 1.00, 1.99). For MI and cardiovascular death, higher fibrinogen was associated with events in women (HR, 1.40; 95% CI, 1.06, 1.85).
Table 3 shows the impact of simultaneous adjustment for biomarkers associated with CVD measured in the entire cohort (IL-6, CRP and WBC). IL-6 and CRP each remained associated with events with the addition of WBC to the model. WBC was not associated with events after addition of IL-6 or CRP. When all three biomarkers were included in the model, IL-6 and CRP remained associated with CVD. Accounting for IL-6, WBC and CRP, fibrinogen was not associated with events either gender stratified (men, HR, 1.00; 95% CI, 0.90, 1.12; women, HR, 0.93; 95% CI, 0.85, 1.02) or modeled with a gender interaction term (HR, 0.93; 95% CI, 0.86, 1.02).
| Adjusted model* | Biomarker hazard ratio (95% CI) | ||
|---|---|---|---|
| Interleukin-6 | C-reactive protein | White cell count | |
| Each marker individually | 1.17 (1.11, 1.24) | 1.17 (1.10, 1.25) | 1.06 (1.01, 1.12) |
| IL-6 + CRP | 1.13 (1.06, 1.20) | 1.11 (1.03, 1.19) | ― |
| IL-6 + WBC | 1.17 (1.10, 1.23) | ― | 1.03 (0.97, 1.10) |
| CRP + WBC | ― | 1.17 (1.09, 1.24) | 1.04 (0.97, 1.10) |
| IL-6 + CRP + WBC | 1.12 (1.06, 1.20) | 1.10 (1.03, 1.18) | 1.02 (0.96, 1.09) |
- *Adjusted for variables in multivariable model in Table 2.
The potential improvement of biomarkers to risk prediction by lipid levels was studied by comparing the AUC of a model including gender and cholesterol/HDL with a model including these factors and each biomarker individually. In a gender-only model, addition of cholesterol/HDL increased the AUC from 0.537 to 0.566 (P < 0.0001). IL-6, CRP, WBC and FVIIIc significantly increased the AUC, with IL-6 having the largest increment (Table 4).
| Biomarker top Quintile | Area under the receiver-operating curve | P-value† | |
|---|---|---|---|
| Gender + Chol/HDL* | Gender + Chol/HDL + each biomarker | ||
| Interleukin-6 | 0.564 | 0.590 | 0.0001 |
| C-reactive protein | 0.565 | 0.576 | 0.016 |
| D-dimer | 0.586 | 0.592 | 0.066 |
| Homocysteine | 0.550 | 0.575 | 0.061 |
| Fibrinogen | 0.565 | 0.579 | 0.106 |
| White cell count | 0.566 | 0.581 | 0.001 |
| Uric acid | 0.566 | 0.570 | 0.142 |
| Factor VIIIc | 0.569 | 0.578 | 0.034 |
| Lipoprotein(a) | 0.569 | 0.569 | 0.821 |
| sICAM-1 | 0.580 | 0.583 | 0.217 |
| Platelet count | 0.569 | 0.572 | 0.195 |
| Factor VIIc | 0.567 | 0.572 | 0.147 |
| Albumin | 0.566 | 0.567 | 0.322 |
- *AUC differences in the basic model reflect different sample sizes with available biomarker data.
- † P-value comparing the two AUC values.
We compared all combinations of biomarkers significant in the AUC analysis using cross-classification by elevations in sets of two biomarkers (top quintile), the reference group being neither biomarker in the top quintile (Table 5). High CRP was associated with CVD risk regardless of whether other biomarkers were high. In contrast, high IL-6, WBC or FVIIIc were associated with CVD only with concurrent elevations of other biomarkers. Elevated IL-6, CRP, WBC and FVIIIc were associated with CVD when cholesterol/HDL ratio was low. High cholesterol/HDL ratio was associated with events when other biomarkers were low or high, except WBC (Table 5).
| Referent biomarker 5th quintile | Biomarker 5th quintile | Biomarker hazard ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| IL-6 | CRP | WBC | Factor VIIIc† | Chol/HDL | ||
| IL-6 | ||||||
| No | No | – | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| No | Yes | – | 1.41 (1.17, 1.70) | 1.01 (0.85, 1.21) | 1.14 (0.96, 1.34) | 1.21 (1.00, 1.47) |
| Yes | No | – | 1.14 (0.95, 1.37) | 1.16 (0.97, 1.37) | 1.16 (0.97, 1.38) | 1.28 (1.09, 1.50) |
| Yes | Yes | – | 1.51 (1.25, 1.83) | 1.40 (1.13, 1.74) | 1.27 (0.99, 1.63) | 1.31 (0.98, 1.76) |
| CRP | ||||||
| No | No | 1.00 (reference) | – | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| No | Yes | 1.14 (0.95, 1.37) | – | 1.06 (0.90, 1.24) | 1.15 (0.98, 1.34) | 1.23 (1.02, 1.49) |
| Yes | No | 1.41 (1.17, 1.70) | – | 1.40 (1.13, 1.74) | 1.42 (1.20, 1.67) | 1.44 (1.24, 1.68) |
| Yes | Yes | 1.51 (1.25, 1.83) | – | 1.45 (1.17, 1.80) | 1.41 (1.11, 1.78) | 1.50 (1.15, 1.95) |
| WBC | ||||||
| No | No | 1.00 (reference) | 1.00 (reference) | – | 1.00 (reference) | 1.00 (reference) |
| No | Yes | 1.16 (0.97, 1.37) | 1.40 (1.13, 1.74) | – | 1.15 (0.99, 1.35) | 1.32 (1.09, 1.59) |
| Yes | No | 1.01 (0.85, 1.21) | 1.06 (0.90, 1.24) | – | 1.13 (0.96, 1.32) | 1.20 (1.03, 1.40) |
| Yes | Yes | 1.40 (1.13, 1.74) | 1.45 (1.17, 1.80) | – | 1.26 (0.98, 1.61) | 1.09 (0.82, 1.44) |
| Factor VIIIc† | ||||||
| No | No | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | – | 1.00 (reference) |
| No | Yes | 1.16 (0.97, 1.38) | 1.42 (1.20, 1.67) | 1.13 (0.96, 1.32) | – | 1.28 (1.06, 1.55) |
| Yes | No | 1.14 (0.96, 1.34) | 1.15 (0.98, 1.34) | 1.15 (0.99, 1.35) | – | 1.22 (1.05, 1.43) |
| Yes | Yes | 1.27 (0.99, 1.63) | 1.41 (1.11, 1.78) | 1.26 (0.98, 1.61) | – | 1.21 (0.91, 1.61) |
- Adjusted for variables in multivariable model Table 2.
- †Not measured in the minority cohort.
Discussion
In an elderly cohort with 1700 incident cardiovascular events over 9 years of follow-up, IL-6, CRP, D-dimer, homocysteine, WBC, FVIIIc and Lp(a) (the latter two only in the SD analysis), were independently associated with future CVD. Fibrinogen was associated with incident CVD in men only and sICAM-1 in women only. Adjustment for subclinical CVD had little effect on associations and adjustment for Framingham risk factors tended to strengthen associations. Results were similar for individual vascular outcomes. When IL-6, CRP and WBC were evaluated together, CRP and IL-6 remained associated with CVD. In a model including gender and cholesterol/HDL, IL-6, CRP, WBC and FVIIIc each increased the AUC for predicting CVD. When CRP was low, only an elevated cholesterol/HDL was associated with CVD.
These data confirm associations of these biomarkers with CVD in the elderly and report simultaneous assessment of 13 biomarkers using the same covariates and cardiovascular outcome. The modest incremental value of the biomarkers to lipid assessment seen in the AUC analysis has been reported before [24]. The findings also suggest that these biomarkers are not surrogate markers for renal dysfunction or subclinical CVD [1,11].
Biomarker associations with CVD varied depending on covariates used (homocysteine, FVIIIc and uric acid) and CVD endpoints assessed (fibrinogen). We suggest that future research consistently presents models using Framingham risk score components in multivariable analysis and uses cardiovascular endpoints that reflect the desired clinical use of these biomarkers.
Data are limited and findings diverse on the simultaneous effects of multiple biomarkers on CVD risk. Here biomarkers such as fibrinogen and FVIIIc did not provide additional information to other biomarkers, but IL-6 and CRP were independently associated with CVD when modeled together. However, if CRP was elevated, high IL-6 only minimally increased risk. In the Physicians Health Study IL-6 was associated with MI in men adjusting for CRP and other cardiovascular risk factors, but independence of CRP from IL-6 was not reported [25]. In contrast, IL-6 attenuated the association of CRP with risk of cardiovascular death or revascularization in high-risk men in a nested case–control study of the West of Scotland Coronary Prevention Study [26]. In the Atherosclerosis Risk in Communities cohort, CRP was not independently associated with MI, cardiovascular death or coronary revascularization with simultaneous adjustment for fibrinogen, WBC and sICAM-1 [27]. In contrast to our findings, adjustment for CRP in a subset of 7011 participants with CRP data in the Fibrinogen Collaboration (not including CHS) reduced but did not eliminate the association of fibrinogen with CVD [28]. Further, adjusting only for age in the Women’s Health Study, simultaneous elevation of CRP and fibrinogen provided complementary information in CVD risk prediction [29]. It is difficult to generalize across these studies because of the diversity of populations, heterogeneity of follow-up times, differences in adjustment factors used, and the different cardiovascular endpoints assessed; however, in the elderly CHS population measuring other biomarkers in addition to CRP and cholesterol/HDL ratio did not provide meaningful incremental improvements in CVD risk stratification.
We observed no association of other inflammation and hemostasis biomarkers with CVD risk when CRP was low. Few studies have evaluated the effect of low biomarkers on the associations of other biomarkers with CVD. In the Quebec Cardiovascular Study, IL-6 below the median attenuated the association of high CRP and fibrinogen (defined as greater than the median) with coronary death or non-fatal MI [30].
In contrast to other reports, though consistent with previous CHS data, fibrinogen in women and sICAM-1 in men were not associated with CVD [10,12,28]. In the Fibrinogen Collaboration (154 211 participants from 31 studies, including CHS) [28], fibrinogen was associated with risk of coronary artery disease and stroke (fatal and non-fatal). While the association of fibrinogen with CVD diminished with age, this analysis was not gender stratified. In the Framingham Heart Study the association of fibrinogen with CVD diminished with age in women but not men, which may reconcile our findings with those of the Fibrinogen Collaboration [31]. Similar reasoning may explain our finding that sICAM-1 was associated with CVD in women but not men. sICAM-1 was associated with CVD events in middle-aged men (Physicians’ Health Study) and women (Women’s Health Study) but has not been extensively studied in the elderly apart from the CHS [10,32,33]. With shorter follow-up in CHS, sICAM-1 was associated with all-cause mortality in men and women, and fatal cardiovascular events in women, but not with non-fatal CVD in men or women [10]. While gender differences for fibrinogen and sICAM-1 seen here could represent chance findings, they may represent true gender and age differences in the expression of biomarkers in CVD or differences related to diverse phenotypes for CVD in the elderly [2,4].
Our study has several limitations. We cannot infer causal associations with observational data. Power was less for biomarkers measured in subsets of the cohort. With the large number of women but fewer blacks, we could assess gender but not racial differences. For fibrinogen, the assay used (activity level vs. mass assay) may have influenced our results, and biomarkers such as IL-6 have circadian variations (phlebotomy was performed in the morning but times varied by several hours). Despite these limitations, we simultaneously assessed the association of multiple biomarkers with CVD over many years in a large older cohort with a significant percentage of women, carefully ascertained and classified 1700 CVD events and presented a model with risk factors used in clinical practise (Framingham model).
In conclusion, biomarker assessment adds to lipid assessment in cardiovascular risk stratification in older adults, especially in the setting of normal lipid levels [3,11]. Here, biomarkers were associated with modest increases in CVD risk. Of the 13 biomarkers studied, CRP has characteristics that might best allow clinical use in the elderly because of the linearity of the association, lack of association of other biomarkers with CVD when CRP was low, its association with multiple CVD endpoints, and the widespread availability of standardized assays. Our data suggest little additional information is gained from measuring multiple biomarkers in addition to CRP. To determine a clinical role for biomarkers in the elderly more data are needed on how to best integrate the information in risk stratification. Future areas of research include intervention studies based on some of these biomarkers and epidemiologic work on other novel domains of vascular risk and their interactions with established CVD risk factors, particularly among women, the elderly, and non-Caucasian ethnic groups. Studies of larger data sets should better delineate potential clinical utility.
This research was supported by contracts N01-HC-85 079 through N01-HC-85 086, N01-HC-35 129 and N01-HC-15 103 and research project grant R01HL054711 from the National Heart, Lung, and Blood Institute, Bethesda, MD, USA. The sponsor was involved in the design and conduct of the study and approval of the manuscript. A full list of CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interests.




