Transurethral Resection in the Management of Urethral and Prostatic Neoplasia in 6 Dogs
No reprints available.
Dr. Liptak's current address is Department of Clinical Studies, Ontario Veterinary College, University of Guelph, Guelph, ON, Canada N1G 2W1.
Dr. Kazmierski's current address is Garden State Veterinary Specialists, Tinton Falls, NJ.
Abstract
Objective— To assess cystoscopic transurethral resection (TUR) for the palliative management of dogs with neoplastic infiltration of the urethra.
Study Design— Prospective clinical trial.
Animals— Six client-owned dogs.
Methods— Cystoscopic examination and electrosurgical TUR were performed in dogs with urination difficulties caused by prostatic or urethral neoplasia. TUR was performed in a retrograde manner in female dogs and antegrade in male dogs via exploratory celiotomy and ventral cystotomy. Cystoscopic examination was used to determine the extent of neoplastic involvement of the urethra. TUR involved piecemeal removal of neoplastic tissue from the urethral lumen using an electrocautery cutting loop. Hemorrhage was controlled with a cystoscopic cauterized roller-ball. In 2 male dogs, intraoperative radiation therapy (IORT) was used to treat both prostatic neoplasia and the sublumbar lymph node bed. Surgical technique, complications, adjuvant treatment, and outcome were recorded.
Results— TUR was performed in 3 male dogs with prostatic carcinoma and 2 female dogs with urethral transitional cell carcinoma (TCC). In 1 female dog, TUR was attempted but not successful because of cystoscope diameter. Iatrogenic urethral perforation occurred during TUR in 3 dogs. In 2 dogs, prolonged exposure to lavage fluid resulted in clinical and biochemical abnormalities consistent with TUR syndrome. Dysuria resolved in 5 dogs within 10 days of TUR. Treatment-related complications included urinary tract infection and tumor seeding. Local tumor progression and metastasis occurred in all dogs.
Conclusions— TUR (in combination with chemotherapy±IORT) resulted in rapid palliation of urination difficulties in male dogs with prostatic carcinoma. In female dogs with urethral TCC, however, electrosurgical TUR cannot be recommended because of a high intra- and postoperative complication rate with no improvement in postoperative management compared with historical reports of tube cystostomy.
Clinical Relevance— TUR is a novel alternative for the palliation of male dogs with prostatic carcinoma. In female dogs with urethral TCC, electrosurgical TUR does not provide any advantages compared with tube cystostomy.
Introduction
GENITOURINARY MALIGNANCIES are a common cause of urine outflow obstruction in dogs.1 In general, urothelial and prostatic tumors can be managed by palliative or curative-intent techniques. Surgery and radiation therapy have been used for curative-intent although the complication rate is high with minimal survival benefit.2–11 Accordingly, palliative techniques, like urethral catheterization and cystostomy tube placement, are commonly used to manage dogs with urinary outflow obstruction caused by urogenital neoplasia.12–14 Postoperative management for both techniques is time consuming, owner compliance limits the acceptability of these procedures, and both are associated with complications, including ascending bacterial urinary tract infection (UTI) and neoplastic dissemination.12–15
Transurethral resection (TUR) is a minimally invasive surgical technique commonly used in humans for the management of carcinoma in situ lesions in the bladder and benign prostatic hyperplasia (BPH).16–18 TUR is performed through a rigid cystoscope and uses a cutting loop to remove obliterative tissue from the urethral lumen. Electrosurgical, laser, and vaporization TUR techniques have been reported.18,19 Complications associated with TUR in humans include hemorrhage, TUR syndrome, UTI, and urethral perforation.17–22
We hypothesized that TUR could be used to remove neoplastic tissue from the urethral lumen and re-establish urine flow in dogs with urinary difficulties caused by neoplastic involvement of the urethra. TUR was only performed for palliative purposes to minimize the severity and frequency of disease-related clinical signs and improve quality of life. Curative-intent resection with the removal of all neoplastic tissue was not attempted. Furthermore, we hypothesized the postoperative management of dogs treated with TUR would be simplified compared with the urine collection requirements and complications associated with cystostomy tubes in dogs. Our purpose was to report our experience with TUR for the palliative management of 6 dogs with urinary difficulties caused by neoplastic infiltration of the urethra.
Materials and Methods
Dogs were prospectively included for evaluation of TUR in the palliation of urinary difficulties associated with genitourinary neoplasia after confirmation of obstructive urethral disease. Diagnosis and staging of neoplasia were performed before TUR. Neoplastic disease was confirmed by cystoscopic or open urethral biopsy. Staging procedures included hematology, serum biochemistry, urinalysis and urine culture, abdominal ultrasonography, and 3-projection thoracic radiographs. Owners consented to TUR after being offered cystostomy tube placement and advised that TUR was an investigational procedure in dogs, a urologist experienced in performing TUR in humans (SPB) would be involved in the surgical procedure, and of the potential advantages and complications associated with TUR.
For TUR, dogs were anesthetized and positioned in dorsal recumbency. Antegrade TUR was performed in males through a median celiotomy and ventral cystotomy, whereas a retrograde approach was used in females.
Male Dogs
An open approach was used so that TUR and intraoperative radiation therapy (IORT) could be performed in a single procedure. Incisional biopsy of the prostate and resection of sublumbar lymph nodes were performed, if required, after abdominal exploration. The bladder and prostate were isolated with moistened laparotomy sponges. Ventral cystotomy was performed and the bladder neck and prostatic urethra examined using a rigid 2.9 mm, 12° oblique cystoscope (Karl Storz Endoscopy America Inc., Culver City, CA; Fig 1A). The gross appearance of the urethra was noted and the circumferential and linear extent of urethral disease determined. A 1.5% solution of glycine or sterile water was used to lavage and dilate the bladder and urethra during cystoscopy and TUR.
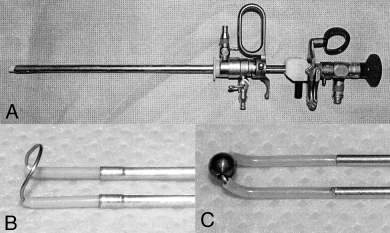
(A) Rigid cystoscope used for examination of the proximal urethra and transurethral resection. (B) Close-up view of the cutting loop (6-mm diameter) used for transurethral resection (TUR). (C) Close-up view of the coagulation roller-ball (3-mm diameter) used to control hemorrhage after TUR.
TUR of the prostatic urethra was performed with a specialized cystoscopic cauterized cutting loop (Cutting Loop, Angled, Karl Storz Endoscopy America Inc., Fig 1B). Piecemeal removal of neoplastic tissue was performed by advancing the cutting loop under direct cystoscopic observation, engaging a portion of abnormal tissue with the loop, and retracting the cutting loop toward the cystoscope. Electrocautery was either activated (hot cutting) or inactivated (cold cutting) as the cutting loop was retracted. The aim of TUR was to increase the internal urethral diameter to approximately 12 mm to palliate urinary difficulties associated with neoplastic infiltration of the urethra and permit free urine flow. Curative-intent resection was not performed in any dog. Hemorrhage from TUR was controlled with a cystoscopic coagulation roller-ball (Electrode, Ball End, Karl Storz Endoscopy America Inc.; Fig 1C).
After TUR, IORT was performed with the intent to control local prostatic neoplasia and regional disease in the sublumbar lymph node bed. After cystotomy closure, the abdominal skin was closed temporarily with towel clamps and protected by disposable sterile drapes, so the dog could be transported to the radiation therapy suite.
In the radiation suite, the dog was re-draped using aseptic technique and the towel clamps were removed to expose the abdominal cavity. A stay suture placed through the bladder apex facilitated cranial retraction and exposure of the prostate, which was isolated with laparotomy sponges ensuring adequate retraction of the ureters and colon. Secondary shielding of normal adjacent tissue was provided with a 6 mm thick lead sheet covered with saline-soaked gauze sponges, placed dorsal to the prostate, which attenuates 90–95% of the radiation beam.23 A 50 mm, sterile, circular plexiglas cone was positioned over the isolated prostate and 1 cm bolus of isotonic saline solution added to the cone (Fig 2). The cone was docked to a 6 MV linear accelerator, which was used to deliver 14 Gy radiation, using 12 MeV electrons, to the prostate. Radiation dose was calculated using skin-to-surface distance geometry with an 85–90% isodose of reference. The skin was temporarily closed with towel clamps to transport the dog to the surgery theater for abdominal lavage and celiotomy closure.
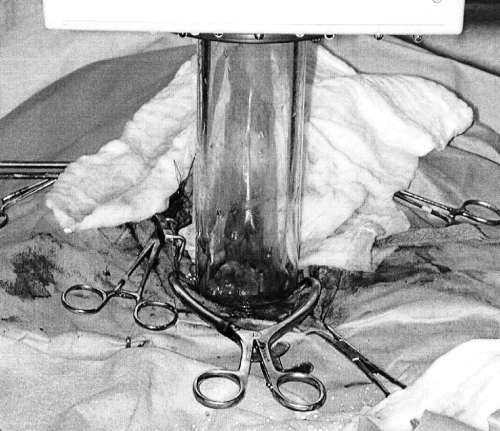
Intraoperative view of the sterile plexiglas cone positioned over the isolated prostate, with a 1 cm bolus of isotonic saline, docked to the linear accelerator.
Female Dogs
Open abdominal surgery and IORT were not used in females. Cystoscopy and TUR were performed through a retrograde approach. The urethral papilla was identified using a combination of digital palpation and cystoscopy, and then the cystoscope was inserted into the urethral papilla and advanced cranially for examination of the urethra, bladder neck, and urinary bladder. A 1.5% solution of glycine or sterile water was used to lavage and dilate the bladder and urethra during cystoscopic examination and TUR, which were performed as described for males.
The surgical technique, complications, and postoperative urination, recovery, and adjuvant treatment were assessed and recorded after TUR.
Results
Dog 1—Prostatic Transitional Cell Carcinoma
A 10-year-old male castrated Samoyed was admitted with a history of tenesmus for 8 weeks and dysuria for 7 days. A smooth, enlarged prostate was palpated on rectal examination. Hematologic and serum biochemical abnormalities were mild and non-specific. On urinalysis, there was proteinuria (1+), hematuria [4+ blood; >200 red blood cells (RBC)/high-power field (hpf)], pyuria [40–80 white blood cells (WBC)/hpf], and numerous atypical transitional cells. Prostatic transitional cell carcinoma (TCC) was diagnosed by histologic examination of an ultrasound-guided needle-core biopsy of the prostate. There was no evidence of metastatic disease.
On exploratory ventral median celiotomy, the prostate was enlarged with a smooth, uniform capsule. No other abnormalities were detected. During cystourethroscopy, the prostatic urethra appeared poorly distensible with multifocal areas of soft-tissue proliferation and ulceration. TUR and IORT was performed as described and without complications. A 1.5% solution of glycine was used to lavage and dilate the bladder and urethra during cystoscopy and TUR. After IORT, a cystostomy tube was placed because the efficacy and rapidity of TUR in ameliorating clinical signs of lower urinary tract obstruction was unknown.
Urination and defecation abnormalities continued for 5 days postoperatively. However, despite dysuria and stranguria, the dog could urinate without assistance and the cystostomy tube was not used. The sutures and cystostomy tube were removed at 10 days. Urination and defecation were normal despite a positive urine culture with Escherichia coli. UTI was treated, based on susceptibility results, with trimethoprim-sulfonamide (15 mg/kg orally every 12 hours); however, the dog remained refractory to antimicrobial management for 8 months. Adjunctive chemotherapy, initiated at 10 days, consisted of piroxicam (0.3 mg/kg orally every 24 hours) and mitoxantrone (5.5 mg/m2 intravenously [IV] every 3 weeks for 5 treatments). Sublumbar lymphadenomegaly was detected by ultrasound 6 months after TUR; the left and right medial iliac nodes measured 1.7 × 2.8 and 2.0 × 3.3 cm, respectively. Sublumbar lymph node size increased despite administration of carboplatin (300 mg/m2 IV) and doxycycline (5 mg/kg orally every 12 hours). A 2.0 × 3.5 cm ventral bladder neck mass was detected by abdominal ultrasonography at 7 months, and implantation of prostatic TCC into the cystotomy incision was suspected.
Serum biochemical abnormalities were azotemia [blood urea nitrogen (BUN) 84 g/dL, reference range (RR) 7–32 g/dL; creatinine 3.1 g/dL, RR 0.7–1.8 g/dL] and hyperphosphatemia (6.5 g/dL, RR 2.1–6.0 g/dL). Azotemia may have occurred because of urine flow obstruction but excretory urography was not performed to confirm obstructive disease and dilation of the renal pelves and ureters were not noted during abdominal ultrasonography. There was persistent proteinuria (3+), hematuria (3+ blood; packed RBC/hpf), pyuria (10–12 WBC/hpf; 3+ bacterial rods), and an E. coli UTI.
Celiotomy was performed to assess the bladder, prostate, and sublumbar lymph nodes. TUR was performed, and the left and right medial iliac lymph nodes were resected using a combination of digital and blunt dissection. Histologic examination of biopsies from the bladder, prostatic urethra, and sublumbar lymph nodes confirmed tumor cell implantation into the cystotomy incision, local recurrence of prostatic TCC, and metastasis to sublumbar lymph nodes.
The dog had a poor recovery and removed an indwelling urethral catheter on 2 occasions. The catheter was reinserted using fluoroscopic guidance after the initial removal and then surgically replaced with a cystostomy tube the second time. Hypoproteinemia (1.3 × 103/μL) and dependent edema of the submandibular region and right pelvic limb were evident within 24 hours. Hydroxyethyl starch and fresh frozen plasma were administered in addition to a balanced polyionic solution. Ventricular premature contractions, with occasional R-on-T phenomenon, were also noted and treated by continuous rate infusion of lidocaine (50 μg/kg/h IV). Rupture of the proximal urethra was diagnosed by positive-contrast retrograde urethrogram. The dog was euthanatized 264 days after the initial TUR procedure. Necropsy was not performed.
Dog 2—Undifferentiated Prostatic Carcinoma
A 6-year-old male castrated Miniature Schnauzer was admitted with a 12-month history of dysuria. The dog was in a thin condition with a firm, enlarged and painful prostate palpable during rectal examination. Hematologic and serum biochemical abnormalities were mild and non-specific. Hematuria (2+ blood; 30–40 RBC/hpf), proteinuria (2+), pyuria (10–15 WBC/hpf), 10–15 transitional cells/hpf, and 1+ calcium oxalate crystals were noted on analysis of urine collected by cystocentesis. Urine culture was negative. Prostatic neoplasia was suspected. There was no evidence of metastatic disease on thoracic radiographs. The prostate was enlarged and mineralized on abdominal radiographs with enlarged sublumbar lymph nodes displacing the colon ventrally. On abdominal ultrasonography, there were irregular and asymmetric prostatic margins with multiple hyperechoic shadowing foci and medial iliac lymphadenomegaly, consistent with metastatic prostatic neoplasia.
During exploratory celiotomy, a 10-mm diameter mass in the greater omentum was resected. Three palpably enlarged sublumbar lymph nodes were resected by digital and blunt dissection. An incisional biopsy of the prostate was submitted for histopathologic examination. During cystourethroscopy, the proximal urethra appeared non-distensible with diffuse soft-tissue proliferation and multifocal areas of soft-tissue mineralization (Fig 3). TUR and IORT were performed as described and without complications. A 1.5% solution of glycine was used to lavage and dilate the bladder and urethra during cystoscopic examination and TUR. Anaplastic and metastatic prostatic carcinoma was diagnosed in prostatic and sublumbar lymph nodes.
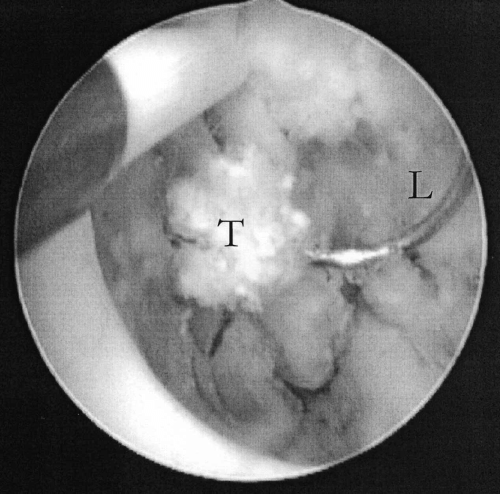
An intraoperative view through the cystoscope during transurethral resection. A segment of prostatic carcinoma (T) is being removed piecemeal by the electrosurgical cutting loop (L). The diameter of the electrosurgical cutting loop is 6 mm.
Urination and defecation returned to normal within 24 and 72 hours, respectively. The dog was discharged with instructions to administer piroxicam for analgesic and antineoplastic effects, famotidine (1 mg/kg orally every 12 hours for 5 days) as prophylaxis against piroxicam-induced gastric ulceration, docusate sodium (2 mg/kg orally every 24 hours for 7 days) as a fecal softener to minimize discomfort associated with tenesmus, and enrofloxacin (5 mg/kg orally every 24 hours for 7 days).
The dog was euthanatized at 32 days because of caudal abdominal pain and dyschezia. Urination remained normal. A necropsy was not performed although speculative causes of caudal abdominal pain and dyschezia included colonic perforation secondary to IORT, or colonic obstruction secondary to local recurrence, regional extension into the colon, or sublumbar node metastasis.
Dog 3—Urethral TCC
A 10-year-old female spayed Belgian Teruvien presented to the referring veterinarian with a 12-month history of polyuria and an acute onset of dysuria and urinary obstruction. Exploratory celiotomy, ventral cystotomy, and cystostomy tube placement were performed. TCC was confirmed by biopsy of a mass involving the trigone and urethra. The dog was referred for further treatment. No abnormalities were detected during physical examination or hematologic and serum biochemical evaluation. There was mild hematuria (2+ blood; 15–20 RBC/hpf) and pyuria (3–5 WBC/hpf). Urine culture yielded no bacterial growth. There was no evidence of metastatic disease.
The dog was anesthetized and retrograde cystoscopy revealed diffuse neoplastic involvement extending from the trigone and bladder neck caudally to the distal aspect of the urethra. Sterile water was used to lavage and dilate the bladder and urethra during cystoscopy and TUR. Iatrogenic distal urethral perforation occurred during TUR resulting in extravasation of lavage fluid into the abdomen. An indwelling urethral catheter was inserted to divert urine from the urethral rent.
A second celiotomy was performed approximately 6 hours after TUR because of poor postoperative recovery, abdominal distension, and evidence of active hemorrhage. Serosanguineous fluid (4 L) was suctioned from the peritoneal and retroperitoneal cavities, a large hematoma was noted in the right body wall between the transversus abdominus and oblique muscles, and the cystostomy tube was replaced.
Postoperative recovery was complicated by cardiac, electrolyte, and hematologic abnormalities. The dog was tachycardic (heart rate 190–210 b.p.m.) with premature ventricular contractions. There was neutrophilia (13.1 × 103/μL, RR 2.6–11 × 103/μL) with a left shift (band neutrophils, 0.4 × 103/μL, RR <0.2 × 103/μL), anemia (35%, RR 42–58%), and thrombocytopenia (147 × 103/μL, RR 200–500 × 103/μL). Coagulation times were prolonged (activated partial thromboplastin time 162 seconds, RR 59–87 seconds; and prothrombin time 17 seconds, RR 9–12 seconds). Biochemical abnormalities included hypocalcemia (7.9 mEq/L, RR 9.2–11.7 mEq/L), hypomagnesemia (1.3 mEq/L, RR 1.8–2.5 mEq/L), hypoproteinemia (2.8 g/dL, RR 5.3–7.2 g/dL), hypoalbuminemia (1.8 g/dL, RR 3.0–4.5 g/dL), and hypoglobulinemia (1.0 g/dL, RR 2.0–3.8 g/dL). IV crystalloid and colloidal fluids were administered for 3 days. Heart rate stabilized after 36 hours without medical management of ventricular premature contractions. Other treatments included cephazolin (22 mg/kg IV every 8 hours), IV calcium gluconate for correction of hypocalcemia, piroxicam for analgesia and management of TCC, misoprostol (50 μg/kg orally every 12 hours) for prophylaxis against gastric ulceration, and fentanyl (2–6 μg/kg/h IV) for 3 days followed by a 5-day course of morphine (1 mg/kg orally every 12 hours) for analgesia. Antibiotics were changed from cephazolin to cephalexin (22 mg/kg orally every 8 hours) 5 days postoperatively and continued for 28 days.
Hematologic and electrolyte abnormalities resolved over 10 days. The indwelling urethral catheter was removed at 10 days when urethral patency was confirmed by positive-contrast retrograde vaginourethrogram. Mitoxantrone (4 cycles) was started on day 10 after TUR. The cystostomy tube was removed at 33 days when the second dose of mitoxantrone was administered and hematologic variables were within normal reference limits. Urine collected by cystocentesis yielded Enterococcus and Pseudomonas spp. and, based on susceptibility testing, cephalexin was discontinued and enrofloxacin administered for 10 days. Doxycycline was administered for antineoplastic effects.
Stranguria occurred 95 days after TUR. The abdominal wall in the paralumbar area was thickened, firm, and mildly painful. Magnetic resonance imaging of the abdominal cavity revealed diffuse thickening of the right abdominal wall with abnormal tissue extending into the pelvic inlet and surrounding the urethra and colon. Cytologic examination of fine-needle aspirates from the abdominal wall was consistent with anaplastic carcinoma. Tumor extension was most likely caused by implantation of tumor cells into the cystostomy tube stoma±pelvic inlet after urethral perforation. There was no evidence of pulmonary metastasis on thoracic radiographs. Urine culture revealed persistent UTI with multiple bacterial isolates, including E. coli, Actinomyces spp., and Enterococcus spp. Based on susceptibility results, imipenem (5 mg/kg subcutaneously every 8 hours) was administered for 7 days. Stranguria resolved with antimicrobial treatment although persistent and ascending UTI resulted in pyelonephritis and azotemia. The dog was euthanatized at 245 days because of progressive renal failure and peritoneal tumor seeding.
Dog 4—Urethral Transitional Cell Carcinoma
A 11-year-old female spayed mix breed dog was admitted with a 6-month history of chronic and recurrent UTI, and sonographic evidence of small, irregular kidneys with mild hydronephrosis. No hematologic abnormalities were detected although creatinine concentrations were increased (2.1 g/dL) on serum biochemistry. Urine was collected by cystocentesis and analysis revealed hematuria (2+ blood; 40–50 RBC/hpf) and 4–6 transitional cells/hpf. There was no bacterial growth on urine culture.
On ultrasonography, the kidneys were small and irregular with decreased corticomedullary differentiation and no evidence of hydronephrosis, consistent with primary renal insufficiency. There was a small thickened and hypoechoic area in the trigone of the bladder. During cystourethroscopy, the urothelial mucosa was obliterated by multiple proliferative and friable mass-like structures from the trigone of the bladder to the cranial third of the urethra. TCC was diagnosed from biopsies of the urethral masses.
Stranguria and pollakiuria worsened over the next 7 days. The dog was administered 2 doses of mitoxantrone, 3 weeks apart, and doxycycline. However, after a 2-month period during which urination abnormalities resolved, the dog became listless with progressively worsening polyuria, dysuria, and stranguria. There was moderate azotemia with increased creatinine (2.2 g/dL) and BUN (39 g/dL) concentrations. Bladder and renal abnormalities persisted unchanged on abdominal ultrasonography. No evidence of pulmonary metastasis was detected on thoracic radiographs.
The dog was anesthetized and, during retrograde cystourethroscopy, diffuse neoplasia extended from the proximal third of the urethra to the trigone of the bladder. A 1.5% solution of glycine was used to lavage and dilate the bladder and urethra during cystoscopy and TUR. Iatrogenic perforation (2 mm) of the dorsal aspect of the proximal segment of urethra occurred during TUR. Lavage fluid leaked into the abdominal cavity and dilation of the urethra for TUR could not be maintained. Caudal ventral median celiotomy was used for access to suction lavage fluid from the abdominal cavity and an indwelling urethral catheter was placed for urinary diversion.
Recovery was uneventful. Azotemia resolved with creatinine (1.3 g/dL) and BUN (17 g/dL) returning to normal levels by 2 days. No urethral defect could be detected by positive-contrast retrograde vaginourethrogram at 3 days, so the indwelling urethral catheter was removed and the dog urinated voluntarily without difficulty.
The dog was discharged with instructions to administer piroxicam and amoxicillin-clavulanate (15 mg/kg orally every 12 hours for 10 days). Adjuvant chemotherapy with doxorubicin (27 mg/m2 IV every 3 weeks for 4 treatments) was started at 10 days. Stranguria and urinary incontinence developed 7 days after the first postoperative chemotherapy treatment. Hematologic and serum biochemical abnormalities were anemia (PCV 38%) and worsening azotemia (creatinine 2.9 mg/dL and BUN 64 mg/dL). Hyposthenuria (USG 1.019), hematuria (1+ blood; 1–3 RBC/hpf), and pyuria (0–1 WBC/hpf) were evident on analysis of urine collected by cystocentesis. Piroxicam was discontinued at 14 days post-TUR because of worsening azotemia (creatinine 3.3 mg/dL and BUN 70 mg/dL) and replaced with deracoxib (2 mg/kg orally every 24 hours) because of its potential renal-sparing effects and antineoplastic efficacy through inhibition of cyclooxygenase-2-mediated tumor growth.24 The dog was alive at 284 days with persistent but stable renal insufficiency, intermittent urinary incontinence, and mild dysuria caused by progression of urethral TCC growth.
Dog 5—Urethral Transitional Cell Carcinoma
A 14-year-old female spayed Kerry Blue Terrier was admitted with a history of medically managed pituitary-dependent hyperadrenocorticism and 4 weeks of urinary incontinence, pollakiuria, stranguria, and hematuria. Mild thickening of the urethra was detected on digital palpation per rectum and a grade II/VI systolic heart murmur noted during thoracic auscultation. There was mild thrombocytopenia (184 × 103/μL) and all liver enzymes were elevated (alkaline phosphatase [ALP] 234 IU/L (RR 20–142 IU/L); alanine transferase [ALT] 311 IU/L (RR 10–110 IU/L); aspartate transferase [AST] 83 IU/L (RR 16–50 IU/L); and γ-glutamyltransferase [GGT] 23 IU/L (RR 0–9 IU/L)). Hematuria (3+ blood; 20–30 RBC/hpf) and pyuria (10–20 WBC/hpf) were noted on urinalysis. During cystoscopy, there was a diffuse mass causing extensive and irregular narrowing of the urethral lumen and trigone of the bladder. Urethral and vesical TCC were diagnosed from biopsies.
The dog was administered piroxicam and mitoxantrone (4.95–5.5 mg/m2 IV every 3 weeks for 9 treatments) and had an initial response with improved urination and decrease in the size of the urethral and trigonal masses as determined by ultrasonography. Clinical signs worsened 5 months after starting chemotherapy with recurrence of pollakiuria, stranguria, dysuria, and hematuria. TUR was recommended to palliate the clinical signs and minimize the risk of lower urinary tract obstruction.
The dog was anesthetized and retrograde cystoscopic examination of the urethra and bladder was attempted. The cystoscope was too large to insert into the urethra. Ventral median celiotomy and ventral cystotomy were performed for normograde insertion of the cystoscope. The bladder neck and proximal urethra were partially occluded and non-distensible because of diffuse neoplastic involvement, so normograde insertion of the cystoscope was not possible. No further surgical treatment was attempted and the cystotomy and celiotomy incisions were closed. The dog was discharged with instructions to continue with piroxicam and administer oral enrofloxacin for 10 days.
After surgery, the dog developed polydipsia and polyuria with worsening of urinary incontinence. Renal failure secondary to progressive disease was suspected with evidence of uremia (BUN 104 mg/dL, creatinine 2.3 mg/dL, phosphorus 6.6 mg/dL, and USG 1.018) and increased tumor burden on abdominal ultrasonography. Recurrent hyperadrenocorticism could not be excluded with persistent elevation of all liver enzymes (ALP 294 IU/L, ALT 272 IU/L, AST 91 IU/L, and GGT 10 IU/L). An ACTH stimulation test was recommended but not performed. Piroxicam was discontinued because of concerns about renal function. The dog was euthanatized 142 days after cystoscopy (331 days after initiating chemotherapy) because of persistent hematuria and dysuria.
Dog 6—Prostatic Transitional Cell Carcinoma
An 8-year-old male castrated Golden Retriever was admitted with a 4-month history of dysuria and recurrent urinary tract obstruction requiring intermittent urethral catheterization. A prostatic or urethral mass was palpable on digital rectal examination. Percutaneous needle-core biopsy of this mass was performed and histologically diagnosed as a prostatic TCC. The dog was treated with piroxicam and enrofloxacin for 7 days before referral.
The only abnormality detected during physical examination was a firm, enlarged, and asymmetrical prostate. Hematologic and serum biochemical variables were within normal reference ranges. There was mild hematuria (1+ blood; 6–10 RBC/hpf) and urine culture was negative. There was no evidence of metastatic disease.
On celiotomy, sublumbar lymph nodes were not palpable and there was no further evidence of intra-abdominal metastasis. Initially, the cystoscope could not be inserted into the lumen of the proximal urethra because of obstruction of the bladder neck by a proliferative mass extending distally from the trigone region of the bladder. After partial resection of the obstructive tissue, the cystoscope was inserted and TCC extended from the trigone to the prostatic urethra. The proximal urethra appeared non-distensible with diffuse soft-tissue proliferation. TUR was performed as previously described and without complications, although IORT was not used. A 1.5% solution of glycine was used to lavage and dilate the bladder and urethra during cystoscopy and TUR. Prostatic TCC was confirmed on histologic examination of the resected urethral tissue.
Recovery was uneventful with urination returning to normal within 24 hours. The dog was discharged with instructions to administer piroxicam for analgesic and antineoplastic effects. Two doses of mitoxantrone were administered starting at 10 days and repeated after 3 weeks. Mild stranguria was noted at 24 days presumably as a result of UTI as stranguria resolved after empiric treatment with enrofloxacin. The dog was euthanatized 74 days post-TUR when metastatic disease to the lungs and femur was detected.
Discussion
Neoplasia, particularly prostatic carcinoma and TCC of the bladder and urethra, is a common cause of lower urinary tract obstruction in dogs.1 Urothelial TCC has an aggressive biologic behavior. Local disease is usually advanced with diffuse urothelial involvement and invasion into the muscular layers of the wall of the urinary system.25,26 Metastasis to the sublumbar lymph nodes, bone, and lungs is reported in approximately 50% of cases.26 In humans, muscle-invasive TCC is managed by radiation therapy or total cystectomy and reconstruction of the bladder with segments of the gastrointestinal tract.16 In dogs, total cystectomy with urinary diversion, such as ureterocolonic or trigonal-colonic anastomosis, is associated with a high rate of complications, including UTI, pyelonephritis, hypochloremic acidosis, and poor survival.2–4,27–31 Radiation therapy has improved median survival time of dogs with TCC although radiation is limited by acute and late effects, such as bladder fibrosis, ureteral stenosis, colitis and colonic perforation, and radiation-induced bone tumors.8–10 Median survival for dogs with TCC varies from 3 months if untreated to 15 months with radiation therapy.2,3,8–10,25,26,32–39
Similarly, prostatic carcinoma has a very aggressive biologic behavior in dogs. Metastasis to the sublumbar lymph nodes, bone, and lungs is reported in 63–89% of dogs at the time of diagnosis.40–44 Total prostatectomy and radiation therapy are used in men with prostatic neoplasia.45 However, in dogs, total prostatectomy is associated with a high intraoperative mortality rate and frequent postoperative complications, especially urinary incontinence.5–7 Intraoperative and external beam radiation therapy have been used with some success in dogs with prostatic carcinoma although its use is restricted by acute and late radiation-induced effects.9–11 Median survival time varies from 0 days if untreated to 214 days with external beam radiation therapy.5–7,9–11,40–44
Dogs with lower urinary tract neoplasia are often managed with palliative techniques because of complications and poor survival benefit associated with curative-intent surgery and radiation therapy. Cystostomy tubes and urethral catheters have been used for urine diversion from the site of obstruction without attempting to address the obstructive lesion.12–14 They are well tolerated but associated with complications, such as ascending UTI, and are limited to select cases because of postoperative management and owner compliance.12–15 We describe palliative management of 6 dogs with neoplastic obstruction of the lower urinary tract by use of TUR and adjunctive techniques (IORT and chemotherapy).
TUR was effective in reducing tumor volume within the urethral lumen and alleviating signs of urinary difficulties. In female dogs, TUR was attempted by retrograde insertion of a cystoscope through the urethral papilla. However, this was not possible in 2 dogs because of small dog size (<15 kg) and tumor-associated decrease in urethral size and distensibility. Retrograde TUR was not possible in male dogs because of the necessity of performing TUR through a rigid cystoscope. Percutaneous cystoscopy has been described in dogs46 and could be used to perform minimally invasive TUR in male dogs. However, we performed TUR through a celiotomy and ventral cystotomy in male dogs because an open approach permitted further staging, palpation and resection of sublumbar lymph nodes, and use of IORT.
Urethral observation was excellent during cystoscopic examination and TUR because of the magnification provided by the cystoscope. TUR was performed with an electrosurgical cutting loop and neoplastic tissue was removed piecemeal with most tissue segments <5 mm in diameter. In men with BPH, the amount of hyperplastic tissue removed is controversial as there are no significant differences in postoperative function between minimal and extensive TUR.20 Extensive TUR can result in substantial hemorrhage and even exsanguination.17–20 Extensive TUR was required in these dogs to reduce the volume of tumor burden and relieve urinary obstruction. However, hemorrhage from the urethral wall and prostatic parenchyma was mild to moderate and easily controlled by the coagulation roller-ball. After TUR, the internal urethral diameter was increased by 100% to approximately 12 mm, or twice the diameter of the cutting loop. Extensive TUR may also be indicated in dogs with neoplastic infiltration of the urethra as cytoreductive surgery significantly improves survival time in dogs with urethral TCC.47 Conversely, aggressive TUR may increase the risk of other complications, such as urethral perforation.
Urethral perforation occurred during TUR in 3 dogs (dogs 1, 3, 4). Perforation is relatively common in men, especially with complicated resections using the cutting loop.17 In humans, TUR is usually performed for curative intent with diseases confined to the urethral mucosa or protected from the prostatic parenchyma.16–19 In contrast, the intended purpose of TUR in the present series was to remove sufficient neoplastic tissue from the urethral lumen to widen the internal urethral diameter and permit normal urination. However, as a result of full-thickness neoplastic invasion of the urethral wall in all dogs, it was difficult to determine the depth of resection, as there was no normal urethral tissue to differentiate from neoplastic tissue. Furthermore, the canine bladder is much thinner than the human bladder, which increases the likelihood of perforation during TUR.48 The cystoscope, rather than TUR, may have been responsible for urethral perforation in dog 3 as perforation occurred at the urethral papilla, which was distant to the region of resection. The 2.9 mm diameter cystoscope may have been too large for this dog, which was the smallest with a body weight of 10 kg. Moreover, retrograde and normograde insertion of the cystoscope with attached cutting loop was not possible in a 14.5 kg dog (dog 5) because of body size and partial obstruction and decreased distensibility of the urethra secondary to urethral TCC. There are no published guidelines correlating cystoscope diameter to body weight and caution should be used when performing cystoscopy in dogs <15 kg. The prostatic parenchyma provided some level of protection during TUR in male dogs although perforation occurred in dog 1 during a second TUR and this procedure was complicated by scarring from the previous TUR, irradiation, and extension of the tumor beyond the caudal limit of the prostate. As our experience with TUR increased, we were able to diagnose urethral perforation intraoperatively when lavage fluid no longer dilated the urethra because of extravasation through the urethral rent into the abdomen.
Urethral perforation can result in leakage of lavage fluid and urine into either the peritoneal or retroperitoneal space. In humans, perforation often results in extraperitoneal leakage.17 In contrast, lavage fluid leaked into the peritoneal space in all 3 dogs. The major problem associated with urethral perforation is TUR syndrome.17,21 TUR syndrome is caused by the excessive absorption of lavage fluids during TUR and is dependent on the hydrostatic pressure of the fluid, vascularity of the prostate and urethra, type of lavage fluid, and duration of exposure to the lavage fluid.17,21 A variety of lavage fluids can be used, including glycine, with or without additional ethanol, mannitol, glucose, and balanced isotonic electrolyte solutions.17,21 Glycine was used in 4 dogs because of its ability to conduct electrical current during electrosurgical TUR. Excessive absorption of lavage fluid can cause circulatory overload, hyponatremia, glycine and ammonia toxicity, hypothermia, and neurological signs secondary to cerebral edema.17,21 TUR syndrome is more likely after urethral perforation because of absorption of lavage fluid across the large surface area of the peritoneum. Lavage fluid had prolonged peritoneal contact in the first 2 dogs with urethral perforation (dogs 1 and 3) and both had complicated postoperative recoveries consistent with TUR syndrome. As a result of our experiences with these dogs, lavage fluid was immediately removed from the third dog with urethral perforation (dog 4) and recovery was uncomplicated. A caudal celiotomy is recommended to suction lavage fluid from the abdominal cavity after iatrogenic urethral perforation to limit absorption of lavage fluid and minimize the risk of TUR syndrome.
Urethral perforation was managed by urine diversion. In dogs 1 and 3, a combination of cystostomy tube and indwelling urethral catheter were used, whereas only an indwelling urethral catheter was used in dog 4. Primary repair was not attempted because of the small size of the urethral defect (approximately 3 mm) and the rapid healing capacity of urothelium.49 Full-thickness urethral mucosa defects will heal within 7 days.49 Urine diversion is recommended in the management of urethral defects as urine will delay urethral healing, damage subepithelial tissue, cause severe cellulitis, pain and edema, and increase fibrosis of periurethral tissue resulting in stricture formation.49 Urethral stents, such as an indwelling catheter, should only be used for 3–5 days as prolonged stenting will cause mechanical irritation and predispose to UTI and stricture formation.49 In these dogs, urinary catheters, whether indwelling or cystostomy tubes, were maintained for 3 days (dog 4), 10 days (dog 1), and 33 days (dog 3).
Cystostomy tubes are commonly used for urinary diversion and palliation of clinical signs associated with obstructive diseases of the lower urinary tract. Cystostomy tube placement is relatively easy. However, drainage of urine is required 3–4 times daily and this level of management can limit the willingness of an owner to pursue permanent cystostomy tube placement for palliation of obstructive neoplastic diseases. Our hypothesis that TUR would minimize this requirement for postoperative management by the owner was proven with durable palliation of urinary difficulties in all dogs. When the times for indwelling urinary catheter placement were excluded, the period for which clinical signs resolved or improved compared with preoperative signs that ranged from 32 to >281 days. The median survival time for all dogs was 245 days (range, 32 to >284 days), which is numerically superior to the median survival time of 106 days (range, 28–148 days) reported in 6 dogs managed with tube cystostomy.14 However, this result should be interpreted with caution as chemotherapy was administered to all our dogs and the intent of TUR, as with tube cystostomy, was to palliate clinical signs and improve quality of life but not necessarily improve survival time. Furthermore, TUR was associated with a high complication rate, particularly in female dogs, which did not represent an improvement in quality of life when compared with historical reports of dogs managed with cystostomy tubes.14,15
Bacteriuria is a common after TUR in men with BPH.22 The risk of developing a bacterial UTI increases with prolonged operative time and postoperative catheterization.17,22 UTIs were diagnosed in dogs 1 and 3, both of which had cystostomy tubes in situ for ≥10 days. The incidence of nosocomial UTI in dogs with indwelling urinary catheters is reported between 32% and 52% and, in humans, increases by 5–7% for each day of catheterization.50 The prevalence of UTI in dogs with cystostomy tubes ranges from 57%14 to 100%,15 which is comparable to the 40% rate of UTIs in our dogs. Furthermore, UTI is common in dogs with genitourinary cancers, because of bacterial colonization of compromised urothelial mucosa,16 and may account for many postoperative UTIs in dogs managed with cystostomy tubes and TUR. Both dogs with refractory UTIs (dogs 1 and 3) were diagnosed with UTI before TUR, whereas the suspected UTI in the remaining dog (dog 6) resolved with a short course of antibiotic therapy. Urinary catheterization is not recommended after uncomplicated TUR because TUR provided rapid relief of urinary difficulties in most dogs with a return to normal urination within 5 days. However, catheterization is indicated for urinary diversion in dogs with iatrogenic urethral perforation, although the duration of catheterization should be minimized to decrease the risk of developing nosocomial UTI.
Tumor seeding occurred in the ventral cystotomy wound of dog 1 and in the pelvic canal and cystostomy tube stoma of dog 3. In the latter dog, seeding was directly attributed to TUR as tumor growth was apparent adjacent to the site of iatrogenic urethral perforation. However, TUR was unlikely to be the cause of tumor seeding of the ventral cystotomy and tube cystostomy sites in dogs 1 and 3, respectively, because tumor seeding is a relatively common event after diagnostic and therapeutic procedures in both dogs and humans with TCC.51–54 Extravesical implantation of TCC is a concern in humans after rupture of the bladder during TUR however, this risk is not significantly increased when compared with carcinoma in situ lesions treated with TUR where the bladder is not ruptured.54
Adjunctive techniques, such as IORT and chemotherapy, have been investigated in the management of neoplastic disease of the lower urinary tract. Intraoperative and fractionated external beam radiation have been described for the treatment of canine bladder and prostatic neoplasia.8–11 External beam radiation therapy is not commonly used to treat prostatic tumors in dogs because of acute and late radiation-induced effects.8–10 In contrast, IORT is associated with minimal radiation-induced adverse effects and provides effective palliation of local and regional metastatic disease.11,23 However, radiation-induced colonic perforation cannot be excluded as a cause of caudal abdominal pain and dyschezia in dog 2. The biologic effect of a single intraoperative dose of radiation is equivalent to 2–5 times a conventional fractionated dose while minimizing radiation-induced damage to normal adjacent tissue.23 The dose of radiation we used was 14 Gy. In humans, the dose of IORT is dependent on the degree and volume of residual disease.23 An overall median survival of 114 days (range, 41–750 days) has been reported after IORT for canine prostatic tumors.11 These survival times are not different than the 32 to 264 days for the dogs with prostatic carcinomas in the present series and it can be argued that the addition of TUR to the management protocol may not have provided a survival benefit. However, radiation-induced responses in prostatic size are slow55 and, in the dogs described herein, TUR provided rapid relief of urinary obstruction and improvement in quality of life.
Chemotherapy is an important consideration in the management of dogs with lower urinary tract neoplasia because of their high metastatic rate. However, an effective chemotherapy protocol has not been identified. Non-steroidal anti-inflammatory drugs, platinum-based agents, and anthracyclines have been investigated in canine bladder TCC with moderate success, although complete responses are rare with most dogs having either a partial response or stable disease.30–35 We used mitoxantrone and piroxicam based on results of a Veterinary Comparative Oncology Group trial where the response rate to both agents was better than single-agent chemotherapy.56
We investigated TUR for palliative management of 6 dogs with urinary difficulties caused by neoplastic infiltration of the urethra. In male dogs, TUR, in combination with chemotherapy±IORT, provided rapid and durable palliation of urination abnormalities. However, electrosurgical TUR is difficult to recommend in female dogs because of the high intra- and postoperative complication rate. Urethral perforation was common and may be an inherent risk of TUR in female dogs with TCC because neoplastic disease is often advanced at diagnosis with full-thickness invasion of the urethral wall. The high incidence of urethral perforation potentially limits the usefulness of TUR in female dogs as it complicated postoperative management and predisposed to other conditions, such as TUR syndrome and tumor seeding. Laser TUR may reduce the incidence of urethral perforation as the fiberoptics are smaller in diameter and the depth of resection can be controlled (Dr. Christopher J. Chamness, Karl Storz Veterinary Endoscopy America Inc., 2004, personal communication). TUR syndrome was the most morbid of the immediate postoperative complications and can be avoided by intraoperative recognition of urethral perforation and immediate removal of lavage fluid from the abdominal cavity. Other complications, such as UTI and tumor seeding, are common in dogs with genitourinary cancers and were not necessarily associated with TUR. TUR is a novel technique for the palliation of male dogs with urination difficulties caused by prostatic carcinoma, however, TUR should be used with caution in female dogs with urethral TCC because of a high complication rate and no apparent advantages compared with tube cystostomy.
Acknowledgments
The authors would like to thank Karl Storz (Veterinary Endoscopy America Inc.) for providing cystoscopic and TUR equipment and technical assistance; the staff at Poudre Valley Hospital, Fort Collins, Colorado; and the assistance and expertise provided by Maura Green, Joann Harper and Kris Obssuth during cystoscopy; Billie Arceneaux, Frank Conway and Chana Fuller during IORT; and the anesthesia, critical care unit, and technical surgery staff at Colorado State University Veterinary Teaching Hospital.




