Expression of Relaxin Receptor LRG7, Canine Relaxin, and Relaxin-Like Factor in the Pelvic Diaphragm Musculature of Dogs with and Without Perineal Hernia
Abstract
Objectives— To compare the expression of canine relaxin, relaxin-like factor (RLF), and relaxin receptors within the muscles of the pelvic diaphragm of dogs with perineal hernia (PH) and clinically normal dogs.
Study Design— In vivo comparative study.
Animals— Fifteen client-owned intact male dogs with PH were studied. Four mature intact male dogs with no evidence of perineal pathology served as controls.
Methods— Biopsy samples from the levator ani, coccygeus, and internal obturator muscles were obtained. RNA samples were reverse transcribed and analyzed by real-time PCR for the expression of canine relaxin receptor LRG7, relaxin, and RLF.
Results— Significantly higher expression levels of canine relaxin receptors occurred in the musculature of the pelvic diaphragm and internal obturator muscle in dogs with PH compared with normal dogs. Expression of canine RLF revealed no significant difference between dogs with PH and controls. The difference in the expression of canine relaxin between groups was not statistically significant.
Conclusions— Relaxin receptor up-regulation occurs in the coccygeus, levator ani, and internal obturator muscles of dogs with PH.
Clinical Relevance— The higher expression of relaxin receptors within the muscles of the pelvic diaphragm in dogs with PH suggests that relaxin might play a role in the pathogenesis of PH. Atrophy of these muscles, which predisposes to PH, may be attributable to increased relaxin activity.
INTRODUCTION
PERINEAL HERNIA (PH) has been described as a failure of the supporting structures of the perineum that results in an inability of the pelvic diaphragm to contain the pelvic organs. It is recognized as an acquired disorder. Failure of the pelvic diaphragm allows the rectal wall to stretch and deviate. Atrophy of the muscles of the pelvic diaphragm results in a variety of clinical manifestations. These include herniation of abdominal contents, including the bladder, prostate, colon, rectum, and periprostatic fat, lateral bulging of the rectum, or a combination of both.1–4 PH occurs almost exclusively in intact male dogs, although it has rarely been described in female dogs. This fact suggests that there is an anatomic or hormonal basis for the syndrome.1,5 In many cases, PH occurs concurrently with prostatic hypertrophy and prostatic fluid-filled cysts, which form part of the hernial content. It is widely believed that when castration is combined with surgical repair, recurrence rates significantly decrease;1,6,7 however, this claim has been questioned.8,9
Many theories have been advanced regarding the pathogenesis of PH, but none have been proven. It was suggested that short-tail breeds were predisposed because of underdeveloped levator ani and coccygeal muscles.1–4,10 Other proposed theories include chronic constipation leading to straining and herniation, neurologic atrophy of the muscles of the pelvic diaphragm, and hormonal imbalances. The very strong predisposition of male intact dogs to PH suggests that sex hormones are involved in the pathogenesis of PH. Suggested hormonal mechanisms include imbalance between estrogens and androgens, or excess of androgens.10 However, significant differences in testosterone or estradiol 17-β serum concentrations between dogs with PH and non-affected dogs have not been demonstrated.10,11
Another theory is based on the suspected link between PH and prostatic hypertrophy, since both conditions occur in older intact male dogs;1 yet, a direct causal relationship has not been shown. Both mechanical and hormonal influences were speculated as a basis for such a link. For instance, a recent case report suggested that paraprostatic cysts, forming part of an enlarged prostate, can directly or indirectly lead to the development of PH.5 Prolonged, intense tenesmus caused by the presence of a large paraprostatic cyst was also thought to be a contributing factor in the development of the PH. Other factors related to the enlarged prostate and fluid-filled cyst were not ruled out.5
Relaxin was first indirectly described in 1926, when Hisaw13 reported on the relaxation of the interpubic ligament of female guinea-pigs after injection of serum from pregnant guinea pigs or rabbits. Four years later, the actual active substance was first obtained and identified as a new hormone, named relaxin.14
Relaxin is a polypeptide hormone belonging to the insulin and insulin-like growth factor family. Like insulin, it is synthesized in a precursor form, termed pre-prorelaxin. Studies in several species have shown that the highest production of relaxin occurs in the female reproductive organs during pregnancy, with the corpus luteum, deciduas, and placenta being the primary sources. Other, less prominent sources of relaxin include the endometrial glands, thecal cells, and mammary gland parenchymal cells.15,16 In the male, the primary site of relaxin synthesis is the prostate gland, from which the hormone is secreted in the seminal plasma.17 There is no evidence that in males relaxin is released into the circulation.12,13,18
During the last few years, evidence has been accumulating on the role that relaxin plays in modulating the function of cells, tissues, and organs other than those of the reproductive system. It is now recognized that relaxin is a pleiotropic hormone, with multiple, inter-related activities affecting several systems, such as the cardiovascular, respiratory, hemostatic, connective tissue, and urogenital systems. Relaxin is thought to affect connective tissue components (e.g., relaxation of the interpubic ligament and softening of the tissues of the birth canal) through an effect on collagen metabolism, but the exact mechanism of action is unclear.15,16
Several attempts were made to link relaxin to hernia formation. In a retrospective study reported by Uden and Lindhagen19 in 1988, it was shown that children suffering from congenital hip dysplasia (CHD) also had a higher than normal incidence of inguinal herniation. The authors speculated that relaxin, which enhances collagenase activity, could alter the connective tissues and could be an important factor in the development of both CHD and inguinal hernias. Niebauer et al12 suggested that in dogs, relaxin may be leaking from the hypertrophied prostate, and causing local muscle atrophy and softening of connective tissue, which in turn can lead to PH formation. The authors used immunohistochemistry to screen prostatic tissue of dogs with PH for relaxin reactivity. Intense immunostaining for relaxin was detected in 7 out of 10 dogs, while only weak immunostaining was found in normal dogs. Based upon these findings, it was suggested that relaxin may have a causative function in the pathogenesis of canine PH. The close anatomic proximity between the hyperplastic prostate and the pelvic diaphragm and inguinal region could also explain the increased incidence of concurrent PH and inguinal hernia in intact males.20 These hypotheses were not investigated further.
Relaxin-like factor (RLF), also known as Leydig cell-derived insulin-like factor (INSL-3), is structurally closely related to relaxin, and also belongs to the insulin and insulin-like growth factor family. Research on RLF is still in its infancy; however, it is known that RLF is a specific product of testicular Leydig cells in males and appears to be responsible for testicular descent in fetal life.21,22 Its function in the adult male is unknown. RLF cross-reacts with relaxin receptors.21–24
Our objective was to compare the expression of canine relaxin, RLF, and relaxin receptors within the muscles of the pelvic diaphragm between dogs with PH and clinically normal dogs. We hypothesized that increased relaxin production and/or up-regulation of relaxin receptors within the target tissues of the pelvic diaphragm (levator ani and coccygeus muscles) and the internal obturator muscle occurred in dogs with PH.
MATERIALS AND METHODS
Case Selection
Fifteen intact male dogs (all >7 years) diagnosed with PH between August 2002 and June 2004 were studied. Diagnosis of PH was made on the basis of history and a complete physical examination, which included digital rectal palpation. Other diagnostic tests, such as abdominal survey and contrast radiographs and abdominal ultrasonographic examination, were performed as necessary, particularly when concurrent pathology was suspected (e.g., herniation of abdominal organs into the PH, abdominal paraprostatic cysts, or prostatic neoplasia). Complete blood count and serum chemistry were obtained in all dogs before surgery, and were found to be within the normal range in all dogs, so that the dogs were considered to be suitable for surgical hernia repair.
Perineal herniorrhaphy was performed by the internal obturator muscle flap transposition technique.25 During herniorraphy, biopsy specimens were obtained from the levator ani, coccygeus, and internal obturator muscles. Small (3–5 mm) wedge biopsies were obtained from the center of the muscle mass, ensuring that the source of the biopsy was muscle tissue that did not appear fibrosed. Specimens were stored in an RNA-Later (RNA-later®, Ambion, Inc., Austin, TX) solution. Castration was performed after herniorrhaphy.
Four mature intact male dogs with no evidence of perineal pathology were included as controls. One dog had acute paraplegia from a traumatic spinal fracture, and samples were obtained before euthanasia, with the consent of the owners. The other three dogs were admitted by the local humane society for routine elective castration. The age of these dogs was not known precisely; however, they were judged to be mature dogs. Specimens were obtained during castration, with the owner's consent. Biopsies were obtained from the levator ani, coccygeus, and internal obturator muscles. Samples were stored immediately in an RNA-Later (RNA-later®, Ambion, Inc.) solution.
Total RNA Isolation
Total RNA was isolated from the biopsy samples using peqGOLD TRIFast™ (10 mL/g tissue) according to the manufacturer's protocol (Peqlab Biotecnologe GmbH, Enlangen, Germany).
Reverse Transcription (RT)
Two micrograms of total RNA were reverse transcribed in a final volume of 20 μL 50 mM Tris–HCl (pH 8.3), 75 mM KCl, 5 mM MgCl2, 10 mM dithiothreitol, 0.5 mM deoxy-NTP (dNTP), 0.5 μg oligo dT/hexamer primers (Promega Corporation, Madison, WI), and 1 U avian myeloblastosis virus (AMV) reverse transcriptase (Promega Corporation, Madison). Reaction temperatures were 42°C for 1 hour and 95°C for 10 minutes.
Real-time PCR (rt-PCR)
RNA samples were reverse transcribed as described above. Samples were analyzed by rt-PCR with the ABI Prism® 7000 system (Applied Biosystems, Foster City, CA). A 2-μL sample of cDNA was amplified for 40 cycles with 4 μL of the following primers at a final concentration of 20 μM: LRG7 primers, relaxin primers, RLF primers, and canine glyceraldehyde 3-phosphate dehydrogenase (GA3PDH) primers, as normalizing controls. The reaction was carried out in a final volume of 50 μL: 25 μL of SYBR® Green (Applied Biosystems), 8 μL of 5 μM primers mix, 2 μL of template (cDNA) and 15 μL of deionized water, for 40 cycles of 1 minute of denaturation at 95°C, 1 minute of annealing at 60°C, and 1 minute of extension at 72°C. The amplified PCR product was analyzed by means of ABI Prism® 7000 software (Applied Biosystems). At the end of the rt-PCR run, a melting curve was performed to verify the presence of a single amplicon (see Table 1).
| Gene | Sequence | Direction | Concentration (nM) |
|---|---|---|---|
| GA3PDH | 5′-GGAGAAAGCTGCCAAATATG-3′ | Forward | 20 |
| 5′- ACCAGGAAATGAGCTTGACA-3′ | Reverse | ||
| LRG7 | 5′-AGAACCGACCAAGCATTCAG-3′ | Forward | 20 |
| 5′-CTACTTCCCCTGTGGGAACA-3′ | Reverse | ||
| Relaxin | 5′-TCGGCTAGCAAGCTCTCTTC-3′ | Forward | 20 |
| 5′-AGGATAGCAACACGGTCTGG-3′ | Reverse | ||
| RLF | 5′- GTGTGGCCACCACTTCGT-3′ | Forward | 20 |
| 5′-CAGTAGTGTGCGGGATTGGT-3′ | Reverse |
The rt-PCR products were separated by electrophoresis on 1.5% agarose gel, and stained with ethidium bromide, to verify single amplicon acceptance.
Statistical Analysis of rt-PCR
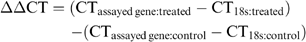
Statistical analysis of the rt-PCR was performed as described by Livake and Schmittgen.26 Quantified data (expression levels of canine relaxin, relaxin receptors, and RLF) were compared between clinically affected dogs and control dogs, using Student's t-test. Significance was set at P=.05.
RESULTS
Biopsy samples harvested from the pelvic diaphragm musculature (levator ani, coccygeus muscles) and internal obturator muscle of dogs with PH and normal unaffected control dogs were analyzed by rt-PCR for the expression of canine relaxin receptor LRG7, canine relaxin, and RLF. Significantly higher expression levels (P=.0004) of canine relaxin receptor occurred in muscles from dogs with PH compared with controls (Fig 1). The differences in the expression of canine relaxin were not found to be statistically significant (P=.069; Fig 2). Similarly, differences in the expression of canine RLF were not statistically significant (P=.63; Fig 3).
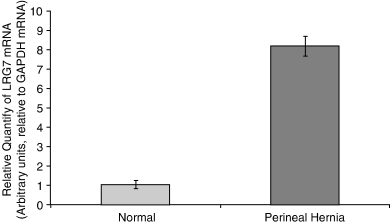
Mean (±SEM) real-time PCR (rt-PCR) expression of relaxin receptor mRNA in canine pelvic diaphragm musculature in 4 normal dogs and 15 dogs with perineal hernia. Significant difference was found (P=.0004).
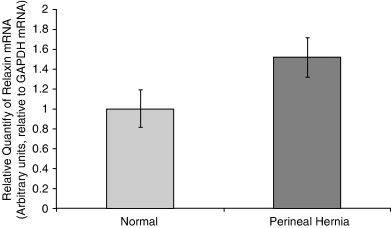
Mean (±SE) real-time PCR (rt-PCR) expression of relaxin mRNA in canine pelvic diaphragm musculature in 4 normal dogs and 15 dogs with perineal hernia. No significant difference was found (P=.069).
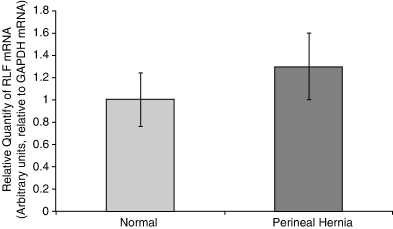
Mean (±SE) real-time PCR (rt-PCR) expression of relaxin-like factor mRNA in canine pelvic diaphragm musculature in 4 normal dogs and 15 dogs with perineal hernia. No significant difference was found (P=.63).
DISCUSSION
Ironically, a receptor for relaxin, one of the first reproductive hormones to be identified, has remained elusive;22 however, 2 years ago, 2 relaxin receptors, named LRG7 and LRG8, were reported.23 With identification of these relaxin-specific receptors, it became possible to investigate the specific cells and tissues that function as target organs for relaxin in diverse physiologic and pathologic circumstances. This included an opportunity to investigate a possible role of relaxin in the pathogenesis of PH, as had been speculated previously.12
LRG7 and LRG8 are present in different tissues, mainly the reproductive tract. We quantified the presence of LRG7 in the levator ani, coccygeus, and internal obturator muscles, in dogs with PH and in control dogs. rt-PCR analysis indicated significantly higher levels of expression of LRG7 receptor in the pelvic diaphragm muscles of dogs with PH, compared with normal dogs (P=.0004). This result lends support to a hypothesized hormonal cause of PH in dogs in general,1–5 and focuses it specifically on relaxin. LRG7 is more responsive to relaxin than LRG8;16 therefore, we investigated the LRG7 receptor. Future studies should also investigate expression of LRG8 in the same tissues.
We were unable to show that relaxin concentrations were significantly elevated in the muscles of the pelvic diaphragm in dogs with PH compared with normal dogs. It has been proposed that weakening of the pelvic diaphragm musculature, resulting in PH, could be attributed to excess secretion of relaxin from the hypertrophied prostate.12 Using immunohistochemistry techniques, increased relaxin reactivity was detected within the prostatic tissue of dogs with PH compared with normal dogs, suggesting that relaxin produced in higher quantities with prostatic hypertrophy was the causative link to PH. This hypothesis was based on a common finding of prostatic hypertrophy in dogs with PH. Furthermore, the close anatomic relationship between an enlarged prostate and/or relaxin-rich fluid-filled cysts within the hernia could contribute to this pathogenetic mechanism. Such cysts are often found adjacent to the prostate, or even communicate with it in the form of paraprostatic cysts.5 In our study, 12 of 15 dogs had prostatic enlargement, consistent with hypertrophy identified by rectal exam or surgery. We examined biopsy samples from muscles within the pelvic diaphragm, and not prostatic tissue per se, which may be one reason why significantly elevated relaxin levels were not identified. In fact, both excess relaxin secretion within close anatomic proximity of the pelvic diaphragm, by prostatic or para-prostatic tissue, and the up-regulation of relaxin receptors in the muscles of the pelvic diaphragm could possibly act in concert in the pathogenesis of PH.
To our knowledge, there are no reports of the action of relaxin on striated muscles. The significantly higher expression of relaxin receptors within the muscles of the pelvic diaphragm in dogs with PH is suggestive of a potential action of this hormone in the muscular system.
Sjollema et al27 provided electromyographic evidence for atrophy of the levator ani and coccygeus muscles in dogs with PH and suggested that atrophy was neurogenic in nature, because of the high incidence of spontaneous potentials. Although spontaneous electromyographic activity is more characteristic of neurogenic atrophy, it can also be associated with non-neourogenic myopathies.11 Therefore, the possibility that the observed atrophy of pelvic diaphragm muscles was caused by an effect of relaxin on muscles either because of relaxin receptor up-regulation or excess production cannot be ruled out because of the high incidence of spontaneous potentials.
Our goal was to examine a possible role for relaxin as a potential cause in the development of PH. The optimal design would have been a case–control study where control dogs were age matched with PH dogs. Although we planned the study accordingly, we encountered substantial resistance from owners for permission to obtain muscle biopsies from their normal dogs. We therefore used any mature dog for which permission could be obtained, regardless of its exact age. A future case-controlled study with age-matched controls is being planned. Differentiation of relaxin receptor modulation in each of the muscles of the pelvic diaphragm should also be studied to elucidate the role of relaxin in the pathogenesis of PH.
We did not study the role of RLF in the pathogenesis of PH. To our knowledge, almost no data exist on the physiologic activity of RLF, and it is unknown whether it affects connective tissue in a manner similar to relaxin. However, it could be argued that testicular descent, one of the few known effects of RLF, has certain similarities to hernia formation. Furthermore, it has been shown that RLF cross-reacts with relaxin-specific receptors, and could therefore have affected our results where it was expressed in higher levels in dogs with PH. The fact that differences in expression of RLF between normal and affected dogs were not significant decreases the likelihood that the findings were biased. It also suggests that even though much is still unknown about RLF and its activities within different target tissues, PH development is probably not associated with it.
We did not individually compare expressions of LRG7, relaxin, and relaxin-like factor in the different muscles of the pelvic diaphragm and we incorporated the internal obturator muscle even though it is not a part of the pelvic diaphragm per se. If, as hypothesized, excess relaxin secretion within close anatomic proximity of the pelvic diaphragm, by prostatic or para-prostatic tissue, is part of the pathogenesis of PH, then examination of other muscles in close proximity should be of interest.
We concluded that relaxin receptor up-regulation occurred in the muscles of the pelvic area of dogs with PH. This finding suggests that atrophy of these muscular structures, which predisposes to PH formation, may be caused by increased relaxin activity.




