Radiographic and Clinical Changes of the Tibial Tuberosity after Tibial Plateau Leveling Osteotomy
Presented at the 38th Annual Scientific Meeting of the American College of Veterinary Surgeons, Washington, DC, October 2003 (Vet Surg 32:488, 2003 [abstr]).
Abstract
Objective— To investigate radiographic changes of the tibial tuberosity after tibial plateau leveling osteotomy (TPLO) surgery and identify clinical findings and risk factors associated with such changes.
Study Design— Retrospective study.
Sample Population— Hundred and eighty-six client-owned dogs (219 stifles) that had TPLO surgery.
Methods— Patient data retrieved included radiographic changes of the tibial tuberosity during re-examination, age, body weight, whether unilateral or single-session bilateral surgery had been performed, location of the anti-rotational pin, approximate tibial tuberosity area, and approximate average tibial tuberosity width.
Results— Fracture with resulting caudal displacement of the proximal tibial tuberosity (1.4%; 3 of 219) occurred less frequently than non-displaced tibial tuberosity fractures (7.3%; 16 of 219). Age, weight, average tibial tuberosity width, location of the anti-rotational pin, and single session bilateral surgery were identified as risks factors for non-displaced fracture. Weight divided by the square of the average tibial tuberosity width may be a stronger risk factor than either weight or average tibial tuberosity width alone.
Conclusions— Dogs undergoing single session bilateral TPLO surgery are at greater risk for developing non-displaced tibial tuberosity fractures. The non-displaced tibial tuberosity fracture does not appear to adversely affect outcome or lead to tibial tuberosity avulsion. Significant risk factors for fracture of the proximal tibial tuberosity with caudal displacement were not identified.
Clinical Relevance— Factors including age, weight, tibial tuberosity thickness, and conditions that may enhance strain on the tibial tuberosity, such as single-session bilateral procedures, may increase risk of fracture.
Introduction
CRANIAL CRUCIATE LIGAMENT (CrCL) rupture is a common orthopedic problem in dogs. Tibial plateau leveling osteotomy (TPLO) is a surgical technique that treats CrCL disease by providing functional stability to the stifle joint during weight bearing by decreasing cranial tibial thrust.1–3 Although the TPLO technique has increased in popularity, few reports document postoperative findings or expectations.
One reported postoperative complication of TPLO surgery is avulsion of the tibial tuberosity.4–6 Tibial tuberosities cut thin during the osteotomy or tibial plateaus that require a large amount of rotation for leveling may produce a greater risk for avulsion.4 There have been few reports describing the frequency of tibial tuberosity avulsion and none that specify risk factors that may predispose to fracture and avulsion.
In 2 recent retrospective studies of TPLO surgery, minimally displaced fractures of the tibial tuberosity occurred in 4% and 3.1% of the patients.5,6 Nine of the 14 cases in the first report had no acute exacerbation of lameness, and the fracture was considered an incidental finding.5 Six cases reported in the second study healed without specific treatment.6
Observation of a radiographic lucency or fracture of the tibial tuberosity in some patients after TPLO surgery has resulted in questions about what changes are clinically significant. Our purpose was to investigate radiographic changes of the tibial tuberosity after TPLO surgery and to identify clinical findings and risk factors associated with such changes. Our hypothesis was that TPLO surgery alters the mechanical characteristics of the tibial tuberosity leading to increased bone stresses that are associated with tibial tuberosity fractures.
Materials and Methods
Inclusion Criteria
Medical records of 243 dogs that had TPLO surgery (March 2000–February 2002) were reviewed; 57 dogs were excluded because re-evaluation radiographs were unavailable. Of the resulting group of 186 dogs, 219 stifles had TPLO. Twenty-two of the dogs had single session bilateral TPLO surgery and 11 dogs had unilateral TPLO surgery at different times for treatment of sequential CrCL.
Data Retrieved
TPLO osteotomy was performed on every stifle reviewed, by 1 surgeon according to the patented technique described by Slocum.4 Data retrieved included age (years), body weight at surgery (kg), whether unilateral or single-session bilateral surgery had been performed, and the location of the anti-rotational pin. Re-evaluation radiographs were examined for evidence of radiographic changes of the tibial tuberosity.
Radiographic Measurements
Immediate postoperative lateral radiographic projections of each hind limb from the hock to the stifle were examined to derive relevant structural characteristics of the tibial tuberosity. One surgeon identified each of the anatomic landmarks of the tibia used to define the long axis of the tibia (x-axis). The x-axis was the line drawn along the tibial long axis, proximally passing through a point that divided the medial and lateral intercondylar tubercles and a point distally passing through the center of the talus.
The boundaries of the tibial tuberosity were then defined (Fig 1). First, a line representing the y-axis at the distal-most point of the osteotomy was drawn perpendicular to the tibial long axis. Next, the cranial boundary of the tibial tuberosity was defined as the cranial surface of the tibial tuberosity (line f, Fig 1) and the caudal boundary of the tibial tuberosity was defined as the radial osteotomy surface (line g, Fig 1). The tibial tuberosity area lies between curves f and g. To determine this area, we subtracted the area under the curve for line g from the area under the curve for line f (Fig 2). The trapezoid rule was used to approximate these areas. The length of the tibial tuberosity was defined as the distance between the proximal-most aspect of the tibial tuberosity and the distal-most aspect of the radial osteotomy. The approximate average tibial tuberosity width was determined by dividing the approximate tibial tuberosity area by the length of the tibial tuberosity (Fig 2).
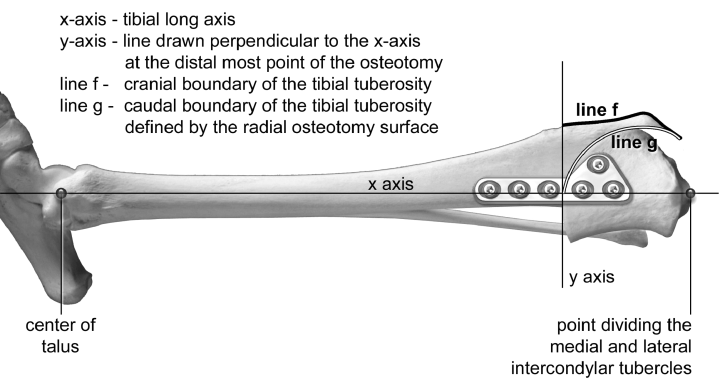
Defining the boundaries of the tibial tuberosity.
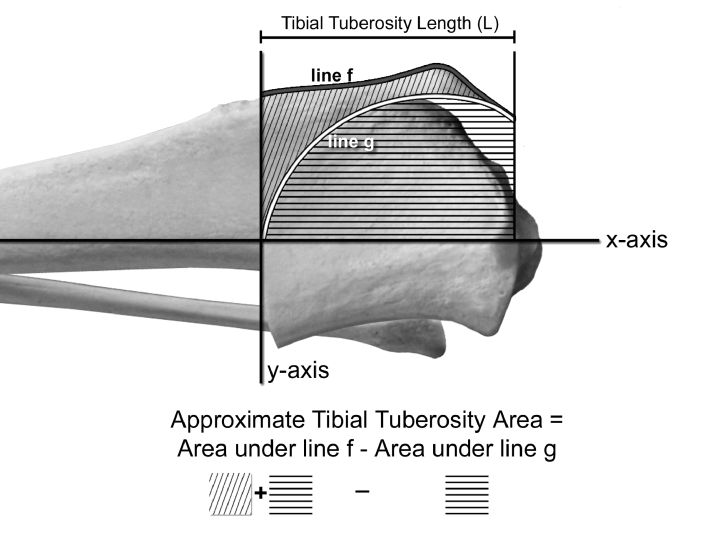
Measuring the tibial tuberosity.
The curves f and g were determined by superimposing graph paper with 1-mm intervals (National Brand Engineering Forms, 10 mm/cm) over the immediate postoperative radiographs. The tibial tuberosity was then traced using an ultra fine point 1 mm permanent marker. Graph points were recorded at 1-mm intervals for both the cranial boundary (line f) and the caudal boundary of the tibial tuberosity (line g).
Radiographs provide only 2-dimensional information about the tibial tuberosity. Given the stress on the tibial tuberosity generated by tension on the patellar ligament, the average cross-sectional area perpendicular to the area measured above (i.e. going into the radiograph) is a more relevant quantity. Intuitively, the average cross-sectional area is the average thickness of the tibial tuberosity. The only information in the radiograph on the cross-sectional area is the tibial tuberosity width. Assuming the tibial tuberosity to be some part of the solid of revolution with radius equal to the tibial tuberosity width, a simple approximation to the average cross-sectional area is C width2, where C is some constant incorporating π and the fraction of the solid of revolution constituting the tibial tuberosity, assumed to be roughly the same for all dogs.
The true stress on the tibial tuberosity at any moment would clearly be complex to measure or model. A reasonable indicator of this stress would be (D weight)/(C width2), where D is some fraction of the gravitational acceleration. Hence, in addition to the structural features measured directly from the radiographs (length, area, and average width) we will also consider the derived stress indicator weight/width2. The reason being, if the hypothesis that bone stresses are responsible for the non-displaced fracture is correct, the directly measured features may have little significance when considered individually. Put more simply, the thinness of the tibial tuberosity, if it is a factor, clearly needs to be measured relative to the size of the dog.
Sources of uncertainty in the geometric quantities describing the tibial tuberosity include inconsistency in radiographic technique, small errors in the x and y coordinates used in the trapezoidal rule, approximation error of the trapezoidal rule, and non-uniformity of the tibial tuberosity (i.e. the extent to which it is not a solid of revolution with radius=width). None of these sources of uncertainty can be quantified precisely, however, a reasonable attempt at minimizing these errors was made by including a large number of points in the trapezoid rule, using a fine point marker on finely graded graph paper, and positioning the dog's leg consistently.
Statistical Analysis
The end points of this study were whether radiographic changes (fracture and caudal rotation of the proximal tibial tuberosity or non-displaced fracture of the tibial tuberosity) were seen in the re-evaluation radiographs before starting rehabilitation. Logistic regression was applied to analyze the data set and assess the risk factors for these end points using PROC LOGISTIC (SAS, SAS Institute Inc., Cary, NC). Initially, the following criteria were identified as possible risk factors for the fracture of the tibial tuberosity: age, body weight, whether unilateral or single-session bilateral surgery had been performed, location of the anti-rotational pin, approximate tibial tuberosity area, and approximate average tibial tuberosity width.
Model selection was performed using a stepwise selection technique and validated using backward selection as well as forward selection with an alternative test of statistical significance. Two-way interactions were checked with the resulting models, with the hierarchy principle enforced except as discussed below. In stepwise and backward selection mode, the Wald statistic (square of the ratio of the regression coefficient to the standard error of the coefficient) was used to measure statistical significance; risk factors with a Wald statistic P-value >.05 were removed from the model with the exception of the pin placement risk factor, as discussed below. In forward selection, the score statistic was used with a score statistic P-value <.05 required for entry into the model. In a separate analysis, we replaced weight and width with the stress indicator derived above, weight/width2, and repeated the selection procedures above.
Results
Two radiographic changes were seen in the tibial tuberosity after TPLO surgery. Fracture and the subsequent caudal displacement of the proximal tibial tuberosity with the tibial plateau (3 of 219; 1.4%) occurred less frequently than non-displaced tibial tuberosity fractures (16 of 219; 7.3%). The clinical findings associated with each differed greatly.
Dogs with caudal displacement of the fractured tibial tuberosity were readmitted to the hospital within the first 2 weeks after surgery for an acute exacerbation of lameness. Physical examination findings included marked lameness, joint effusion, soft-tissue swelling, and bruising of the proximal tibia. Of the 3 stifles with this complication, 2 were in a grossly obese dog that had a single-session bilateral TPLO surgery.
Caudal Displacement of the Fractured Proximal Tibial Tuberosity (3 Cases)
Radiographic findings in these dogs included caudal displacement of the fractured proximal tibial tuberosity and caudal rotation of the proximal osteotomy segment resulting in an increased tibial plateau angle. The osteotomy gap between the fractured proximal tibial tuberosity and the proximal osteotomy segment was unchanged, hence there was no displacement of the proximal tibial tuberosity relative to the proximal osteotomy segment (Fig 3). Each TPLO had anti-rotational pins placed through the mid-section of the tibial tuberosity, at the same level that the fracture occurred. Because of the small number of joints with this complication, statistically significant risks factors could not be determined.
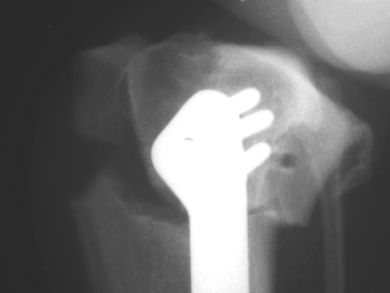
Caudally displaced tibial tuberosity fracture with concurrent caudal rotation of the proximal osteotomy segment.
Non-Displaced Tibial Tuberosity Fractures (16 Cases)
Non-displaced tibial tuberosity fractures occurred in 16 cases. Radiographs were taken during a scheduled 6- or 8-week re-evaluation appointment and none of these dogs presented for an acute exacerbation of lameness. The fracture site was approximately half-way between the proximal most aspect of the tibial tuberosity and the distal most aspect of the osteotomy. Slight obliquity of the fracture was seen with a craniodistal to caudoproximal orientation (Fig 4). The length of the fracture spanned the entire tibial tuberosity width on lateral radiographic projection. The width of the fracture line ranged from 1 to 5 mm, but no displacement of the tibial tuberosity or patella alta was observed. No clinical signs were associated with these findings. In all cases, findings were considered incidental and normal postoperative rehabilitation was begun.
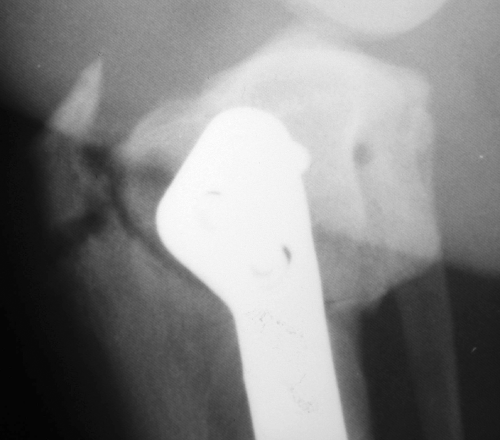
Non-displaced tibial tuberosity fracture.
Statistical Analysis
The stepwise selection procedure identified single-session bilateral TPLO surgery, age, weight, and the approximate average tibial tuberosity width as risk factors for the non-displaced tibial tuberosity fracture occurring. Backward selection identified the same set of risk factors whereas forward selection procedure also identified pin placement as a statistically significant risk factor. We chose to include pin placement in the model as it appeared to have some effect (P-value for the Wald statistic was .064). No statistically significant interactions were identified by any of the selection procedures while enforcing the model hierarchy. The model parameters, Wald statistic P-values, standard errors, and 95% confidence intervals (CIs) are presented in Table 1.
| Term | Estimate | Standard Error | Wald P-Value | 95% Confidence Interval |
|---|---|---|---|---|
| Intercept | –3.60 | 1.80 | 0.046 | −7.13, −0.06 |
| Pin placed in middle of tuberosity | 1.17 | 0.63 | 0.064 | −0.07, 2.40 |
| Age | 0.24 | 0.12 | 0.046 | 0.004, 0.48 |
| Width | −0.43 | 0.18 | 0.014 | −0.78, −0.09 |
| Weight | 0.063 | 0.024 | 0.009 | 0.016, 0.11 |
| Bilateral surgery | 2.45 | 0.71 | 0.000 | 1.26, 3.63 |
Some sample odds ratios representing the increase in risk predicted by the model for several different changes in risk factors are provided along with 95% CI for these odds ratios given in Table 2. For example, the model predicts a 2.63-fold increase in the risk of tibial tuberosity fracture for a 4-year increase in age.
| Effect | Unit Change | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|
| Width (mm) | −2 | 2.38 | 1.25, 5.05 |
| Width | −4 | 5.66 | 1.55, 25.48 |
| Width | −6 | 13.47 | 1.93, 128.6 |
| Age (years) | 2 | 1.62 | 1.02, 2.66 |
| Age | 4 | 2.63 | 1.03, 7.07 |
| Age | 8 | 6.94 | 1.06, 50.04 |
| Bilateral surgery | Yes | 11.58 | 3.67, 41.43 |
| Weight (kg) | 5 | 1.37 | 1.09, 1.77 |
| Weight | 10 | 1.87 | 1.19, 3.12 |
| Weight | 20 | 3.51 | 1.42, 9.72 |
| Weight | 40 | 12.35 | 2.00, 94.58 |
| Pin placed in middle of tuberosity | Yes | 3.22 | 0.93, 11.07 |
In light of the significance of weight and the tibial tuberosity width as risk factors, one possible physiologic cause of the non-displaced tibial tuberosity fracture is stress on the tibial tuberosity via the patellar ligament. As discussed above, a more appropriate risk factor might then be an approximation of the average and/or peak stress on the tibial tuberosity. The approximation of this stress discussed above would be proportional to weight/width2. Replacing weight and width by weight/width2 and running the selection procedure above yields the risk factors weight/width2, pin placement, and bilateral surgery as risk factors. Tables 3 and 4 provide the same information for the stress-based model as given previously.
| Term | Estimate | Standard Error | Wald Statistic P-Value | 95% Confidence Interval |
|---|---|---|---|---|
| Intercept | −4.80 | 0.80 | 0.0001 | −6.59, −3.39 |
| Weight/width2 | 2.50 | 1.15 | 0.029 | 0.43, 5.06 |
| Pin placed in middle of tuberosity | 1.39 | 0.57 | 0.015 | 0.27, 2.52 |
| Bilateral surgery | 2.19 | 0.56 | 0.0001 | 1.08, 3.30 |
| Effect | Unit Change | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|
| Weight/width2 (kg/mm2) | 0.2 | 1.65 | 1.05, 2.58 |
| Weight/width2 | 0.4 | 2.72 | 1.11, 6.68 |
| Weight/width2 | 0.8 | 7.39 | 1.23, 27.08 |
| Pin placed in middle of tuberosity | Yes | 4.03 | 1.30, 12.47 |
| Bilateral surgery | Yes | 8.93 | 2.95, 27.08 |
The approximate tibial tuberosity area was not a statistically significant risk factor for the non-displaced tibial tuberosity fracture. Tibial tuberosity avulsion, as classically described, appears to be a rare complication of TPLO surgery but was not reported in the study.
Discussion
Caudal Displacement of the Fractured Proximal Tibial Tuberosity
A caudally displaced fracture of the proximal tibial tuberosity with concurrent caudal rotation of the proximal osteotomy segment after TPLO has not been reported before. In each case the clinical findings were typical of bone fracture: acute exacerbation of lameness, pain, soft-tissue swelling, and bruising.
Placement of the anti-rotational pin at the site of the fracture may weaken the bone allowing fracture to occur. Implant loosening was confirmed during revision of each TPLO. These cases were repaired with open reduction and internal fixation after the removal and replacement of the TPLO plate and screws and the placement of a tension band wire in the tibial tuberosity. Each fracture healed and limb function after rehabilitation was good.
It is unclear whether the fracture of the tibial tuberosity precedes caudal rotation of the plateau or not. We feel that it is more likely that fracture fixation failure of the osteotomy resulted in caudal rotation of the tibial plateau, and that the proximal tibial tuberosity fractured as a result. Two of the 3 tibial plateaus having this complication belonged to the same patient who was grossly obese and had single-session bilateral TPLO surgery. Placement of the anti-rotational pin proximal to Sharpey's fibers of the straight patella ligament, the addition of a second TPLO bone plate, and/or staged unilateral TPLO surgery may prevent such complications.
Non-displaced Tibial Tuberosity Fracture
The second type of fracture observed, a non-displaced tibial tuberosity fracture, should not be confused with an avulsion fracture. Whether this radiographic finding is a true fracture or an osseous lucency created by bony remodeling is unknown. A true fracture would be expected to displace proximally because of patellar ligament tension and be associated with clinical signs. An alternative explanation may be osseous resorption of the tibial tuberosity resulting in a focal lucency without an actual fracture occurring. Osseous resorption may be caused by thermal necrosis during the osteotomy, stress remodeling because of changes in normal strain on the tibial tuberosity, or vascular compromise secondary to soft-tissue dissection. It is unknown why proximal displacement of the tibial tuberosity did not occur in these cases. An incomplete fracture and/or strong soft-tissue attachments may prevent proximal displacement.
Weight bearing was determined by physical examination and was deemed appropriate for the stage of recovery. It is possible that a more objective assessment of weight bearing such as force plate analysis may identify decreased weight bearing in these patients, or that clinical signs associated with fracture had resolved by the time of our re-evaluation.
Possible causes of the non-displaced tibial tuberosity fracture include excessive pull from the quadriceps mechanism, vascular compromise secondary to soft-tissue dissection, osteotomy gap stress, and thermal damage that occurred during the osteotomy. The data and statistical models in this study provide evidence that the non-displaced fracture may be because of stress on the tibial tuberosity during recovery. Dogs undergoing single-session bilateral TPLO surgery must bear weight on the affected limbs after surgery, and the statistical models predicted 8.5- and 9.6-fold increases in risk for these dogs. Indeed 8 of 16 cases with non-displaced fracture had bilateral surgery, whereas there were only 44 bilateral cases among the 219 cases in this study. One dog that had bilateral single-session TPLO surgery developed lucency on 1 tibial tuberosity but not the other. The tibial tuberosity with the lucency had a significantly smaller approximate average tibial tuberosity width.
Of the remaining 8 non-displaced fracture cases with unilateral surgeries, 6 had weight/width2 ratios greater than the mean weight/width2 of 0.47 (SD: 0.18) with a mean weight/width2 for these 6 cases of 0.70 (SD: 0.14). Of the remaining 2 cases, 1 occurred in a dog aged 11 years. Likewise, the logistic regression analysis suggested a correlation between the non-displaced fracture and weight, tibial tuberosity width (or weight/width2) and age. Other contributing factors to a stress-related fracture could be age-related degradation of bone strength, faster healing in younger dogs, and weakness because of thermal damage. Immature animals are known to have faster bone healing when compared with a mature dog, but the differences in healing between mature and geriatric patients is unknown. Thermal damage and/or vascular compromise could also have been a direct mechanism for the non-displaced fracture but none of the data collected contain any information on such processes.
In 5 of 16 cases with the lucency, the anti-rotational pin was placed in the mid-region of the tibial tuberosity. The radiographic lucency occurred proximal to the pin tract and a clear distinction between the 2 was present. Only in the backward selection procedure was the location of the pin identified as a risk factor for fracture, but the authors recommend placement of the anti-rotational pin proximal to the insertion of the patellar ligament on the tibial tuberosity (Sharpey's fibers).
Variations in tibial tuberosity length could explain why tibial tuberosity area was not a risk factor. Based on the technique that we used to approximate area, it was possible for a long thin tibial tuberosity to have an equal area to that of a short thick tibial tuberosity. Attempts to identify a “minimally acceptable tibial tuberosity width” were unsuccessful; however, the data support the perception that a thicker tuberosity is more resistant to fracture.
Factors including age, weight, tibial tuberosity width, and conditions, which may enhance strain on the tibial tuberosity, such as single-session bilateral procedures and pin placement, may increase the risk of fractures. Non-displaced tibial tuberosity fractures do not appear to adversely affect outcome and do not require surgical fixation.
Acknowledgments
The authors thank Tim Vojt for preparation of the figures, the SAS consulting group in the Department of Statistics at North Carolina State University for help with the SAS software system, and Dennis Boos in the Department of Statistics for his suggestions on the statistical analysis.




