Glycine Receptors Involved in Acamprosate’s Modulation of Accumbal Dopamine Levels: An In Vivo Microdialysis Study
Abstract
Background: Glycine receptors (GlyRs) in the nucleus accumbens (nAc) and nicotinic acetylcholine receptors (nAChRs) in the ventral tegmental area (VTA) have been suggested to be involved in the positive reinforcing and dopamine elevating effects of ethanol. Recent studies have also shown that ethanol high-preferring rats substantially decrease their ethanol intake when treated with a glycine transporter 1 inhibitor (ORG 25935). Acamprosate, a drug used for relapse prevention in treatment of alcohol dependence, has also been demonstrated to elevate extracellular dopamine levels in the nAc. However, the underlying mechanism of action of acamprosate is not fully understood. Here we investigated whether acamprosate interferes with a neuronal circuitry that previously has been demonstrated to be involved in the dopamine elevating effects of ethanol and taurine.
Methods: In vivo microdialysis in freely moving rats was used to assess accumbal dopamine levels before and during local (nAc) or systemic administration of acamprosate.
Results: Perfusion of 0.5 mM acamprosate in the nAc significantly increased dopamine levels. Pretreatment either with 10 μM strychnine in the nAc or 100 μM mecamylamine in the VTA, completely antagonized the acamprosate-induced elevation of accumbal dopamine levels. Also, systemic acamprosate administration elevated accumbal dopamine output, an effect that was abolished by local (nAc) pretreatment with 10 μM strychnine.
Conclusions: These results suggest that both systemic and local application of acamprosate elevate extracellular dopamine levels in the nAc by activating accumbal GlyRs, and, secondarily, tegmental nAChRs.
The rewarding and positive reinforcing properties of ethanol (EtOH) are believed to be mediated, at least in part, by the mesolimbic dopamine system, consisting of dopaminergic neurons originating in the ventral tegmental area (VTA) and projecting primarily to the nucleus accumbens (nAc; Gonzales et al., 2004; Koob and Swerdlow, 1988). It is well known that most drugs of abuse, including EtOH, activate this dopamine system (Blomqvist et al., 1997; DiChiara and Imperato, 1985; Ericson et al., 1998), which results in elevated extracellular dopamine levels in the nAc. However, the exact mechanism underlying EtOH’s ability to increase dopamine neurotransmission is not fully understood. We have, in a series of experiments, demonstrated the importance of nAc glycine receptors (GlyRs) for EtOH’s ability to elevate dopamine in the same brain region (Molander and Söderpalm, 2005a,b) as well as for regulating voluntary EtOH intake (Molander et al., 2007). The importance of GlyRs in the mesolimbic system has also been demonstrated in the VTA where this receptor type was found to be involved in enhanced dopaminergic firing (Ye et al., 2004).
However, it does not appear that the interaction between EtOH and nAc GlyRs elevates dopamine by a local interference with dopamine terminals in the nAc but rather that this interaction engages a neuronal circuitry involving ventral tegmental nicotinic acetylcholine receptors (nAChRs) (Ericson et al., 2003, 2008). Thus, we have hypothesized that EtOH acts locally in the nAc by direct or indirect activation of GlyRs and that this activation secondarily involves ventral tegmental nAChRs which drive dopaminergic neurons and ultimately elevate accumbal dopamine levels (for review see Söderpalm et al., 2009).
A recent study suggested that the endogenous amino acid taurine, when applied in the nAc, engages the same neuronal circuitry and receptors as EtOH with respect to its ability to influence dopamine output in the nAc (Ericson et al., 2006). Taurine is the second most abundant excitatory amino acid (after glutamate) in the mammalian brain (Banay-Schwartz et al., 1993) and shows, just like glycine, affinity for GlyRs (Albrecht and Shousboe, 2005; Wang et al., 2005). Wang et al. also demonstrated the presence of functional glycinergic synapses in a subset of VTA neurons and it was suggested that these neurons were under tonic influence of taurine/glycine. Interestingly, EtOH increases accumbal taurine levels (Dahchour et al., 1994, 1995, 1996), indicating that EtOH may indirectly activate GlyRs via release of taurine. One of the studies revealed that also acamprosate (Campral®; a drug used clinically for treatment of alcohol dependence) elevates extracellular taurine levels (Dahchour et al., 1996). Since acamprosate structurally is a GABA (gamma-amino-butyric acid) analog, it was initially hypothesized that its mechanism of action was related to GABAergic neurotransmission (Boismare et al., 1984). More recently studies have indicated that acamprosate is a glutamatergic modulator, directly or indirectly modulating the N-methyl-d-aspartate (NMDA) receptor (Cano-Cebrián et al., 2003a; Harris et al., 2002; Naassila et al., 1998; Popp and Lovinger, 2000; Rammes et al., 2001; Spanagel et al., 2005). Interestingly, acamprosate is a synthetically acetylated homotaurinate and several studies have demonstrated that acamprosate and homotaurine increase striatal extracellular dopamine levels (Cano-Cebrián et al., 2003a,b; Ruotsalainen et al., 1998) and decrease EtOH intake in rats (Olive et al., 2002). However, even though several hypotheses have been forwarded, the precise molecular mechanism of action underlying the EtOH intake reducing effect of acamprosate remains unclear. In order to facilitate the development of new and more effective anti-alcohol drugs it appears important to unravel these mechanisms.
In the present study, we explored the possibility that acamprosate interferes with nAc GlyRs in vivo, and, more specifically, that this interference mediates the dopamine elevating effects of acamprosate.
Materials and Methods
Animals
Male Wistar rats (n = 111, B&K Universal AB Scanbur, Sollentuna, Sweden) weighing 290 to 330 g, were used for the experiments. Upon arrival the rats were housed in groups of 4 in a humidity (65%) and temperature (22°C) controlled room on a 12/12 h light/dark cycle (on 07:00 off 19:00), with free access to rat food (Harlan Teklad Europe, UK) and tap water. Animals were allowed to adapt for at least 7 days to the animal maintenance facilities of the department prior to the start of the experiments. All experiments were conducted according to the Declaration of Helsinki and were approved by the Ethics Committee for Animals Experiments (Göteborg, Sweden) with the diary number 5/04.
Drugs and Chemicals
Acamprosate (kindly provided by Merck, Lyon, France), strychnine, a competitive glycine receptor antagonist (Sigma-Aldrich, Stockholm, Sweden), and the unspecific nAChR antagonist mecamylamine (mecamylamine hydrochloride, Sigma-Aldrich), were dissolved in Ringer solution or 0.9% NaCl and administered by reversed dialysis (perfusion) either into the nAc and/or the VTA or administered systemically (i.p.). Ringer solution consisted of (in mmol/l): 140 NaCl, 1.2 CaCl2, 3.0 KCl, and 1.0 MgCl2.
Microdialysis Technique
Brain microdialysis experiments were performed in awake and freely moving animals. Rats were anesthetized by isoflurane (Apoteket AB, Stockholm, Sweden), mounted into a stereotaxic instrument (David Kopf Instruments) and put on a heating pad to prevent hypothermia during the surgery. Holes were superficially drilled for placement of 2 anchoring screws and 1 or 2 I-shaped dialysis probes, custom made in the laboratory. The dialysis probes were lowered unilaterally into the nAc (A/P: +1.85, M/L: −1.4, D/V: −7.8) and the VTA (A/P: −5.2, M/L: −0.7, D/V: −8.4 relative to bregma; Paxinos and Watson, 2007). The exposed length of the dialysis membrane was 2 mm. The dialysis probes were placed in the core-shell borderline region (suggesting that sampling was done in both core and shell of the nAc) and probes as well as anchoring screws were fixed to the scull with Harvard cement (DAB Dental AB, Gothenburg, Sweden). After surgery, the rats were allowed to recover for 2 days before the dialysis experiments were initiated. On the experimental day, the sealed inlet and outlet of the probes were cut open and connected to a micro-perfusion pump (U-864 Syringe Pump, AgnTho’s, Sweden) via a swivel allowing the animal to move around freely. The probes were perfused with Ringer solution at a rate of 2 μl/min and dialysate samples (40 μl) was collected every 20 minutes. The rats were perfused with Ringer solution for 1 hour in order to obtain a balanced fluid exchange before baseline sampling begun. Animals were killed directly after the experiment, brains were removed, and probe placement was verified using a vibroslicer (Campden Instruments, Leicester, UK; Fig. 1).
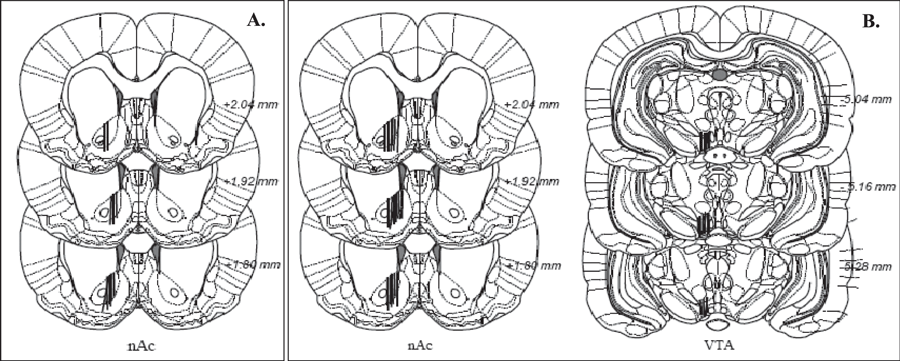
(A) Coronal sections of the rat brain indicating the placements of the microdialysis probes (black lines) in the nucleus accumbens; only correctly placed probes were included. Numbers besides each plate represent distance from bregma. (B) Coronal section of rat brain of probes in the nucleus accumbens and the ventral tegmental area in the 2-probe approach study.
Biochemical Assay
To analyze the dopamine content of the samples, a high-pressure liquid chromatography system was used for the separation and detection of dopamine as described by Waters and colleagues (1993). To identify the dopamine peak, an external standard was used containing 2.64 fmol/μl of dopamine. When at least 3 consecutive stable values of dopamine were obtained (±5%) the first drug was introduced.
Experimental procedure
In the first set of experiments, acamprosate (0.5 or 5 mM in the perfusate) or Ringer was perfused in the nAc for 1 hour after which the treatment was discontinued and the animal received Ringer solution for the remainder of the experiment. The second set of animals received local pretreatment with the competitive GlyR antagonist strychnine (10 μM in the perfusate) or Ringer in the nAc 40 minutes before and during acamprosate (0.5 mM)/Ringer perfusion. The third set of animals was equipped with 2 dialysis probes, 1 in the nAc for drug administration and sampling of dopamine and 1 in the VTA for local drug administration via reversed microdialysis. The nAChR antagonist mecamylamine (100 μM in the perfusate) was perfused in the VTA 40 minutes before perfusion of acamprosate (0.5 mM) was initiated in the nAc and was then maintained for the remainder of the experiment.
After the effects of local acamprosate application in the nAc had been studied, a new set of experiments was performed using systemic administration of acamprosate. Here animals were administered acamprosate (200 or 400 mg/kg i.p.) or vehicle (2 ml/kg, 0.9% NaCl) during continuous monitoring of extracellular accumbal dopamine levels. Finally, animals were pretreated with strychnine (10 μM in the perfusate) in the nAc 40 minutes prior to a systemic injection of either acamprosate (200 mg/kg i.p.) or vehicle.
Statistics
The dopamine content in each sample was expressed as the percentage of the average pretreatment baseline. Data correspond to the mean ± SEM values of the percentage. The overall drug effect was calculated as the average of dopamine content from dialysates collected after their administration, in most experiments between the 40–100 and 40–160 minutes. The microdialysis data were statistically analyzed using 2-way ANOVA (analysis of variance) with repeated measures followed by LSD (least significant difference) post hoc test, or independent t-test to allow adequate comparisons between treatment-groups. A probability (p) value less than 0.05 was considered statistically significant.
Results
Effect of Local Perfusion of Acamprosate on Extracellular Concentration of Dopamine in the Nucleus Accumbens
In acamprosate perfused rats (0.5 or 5 mM in the perfusate), a significant elevation of extracellular dopamine levels was observed in the nAc as compared to Ringer perfused animals (Fig. 2; ANOVA with repeated measures during the 60 minutes of drug administration, 40 to 100 minutes, followed by LSD, 0.5 mM Acamprosate vs. Ringer p = 0.006, acamprosate [5 mM] vs. Ringer p < 0.001). Time effect, 40 to 100 minutes, F(3,54) = 23.422, p < 0.001, treatment effect, F(2,18) = 21.542, p <0.001; interaction term, F(6,54) = 8.993, p < 0.001. After perfusion of acamprosate was discontinued (60 minutes), the elevated dopamine levels returned to predrug baseline.
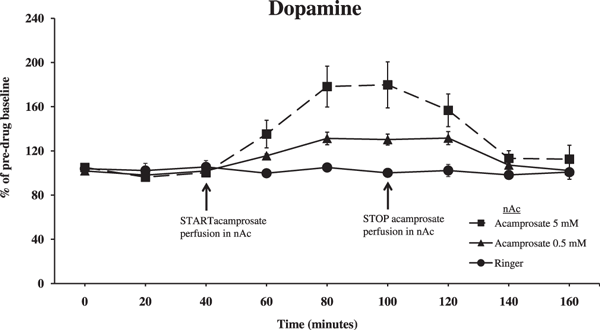
Extracellular dopamine levels in the nucleus accumbens (nAc) as measured by in vivo microdialysis in freely moving and awake male Wistar rats, after perfusion of acamprosate (0.5 or 5 mM) for 60 minutes or Ringer in the nAc. Acamprosate administration was initiated as indicated by the arrow. Shown are the means ± SEM; n = 4 to 9. Statistics: 2-way ANOVA with repeated measures over time points 40 to 100 minutes, followed by post hoc test LSD, Ringer vs. 0.5 mM Acamprosate p = 0.006, Ringer vs. 5 mM Acamprosate p < 0.001.
After establishing that acamprosate elevates accumbal dopamine levels, we pretreated animals with the GlyR antagonist strychnine (10 μM in the nAc). An ANOVA with repeated measures at time points 40 to 100 minutes revealed that perfusion of strychnine (40 minutes prior to and concomitant with acamprosate perfusion) completely abolished the previously observed acamprosate-induced (0.5 mM in the nAc) elevation of accumbal dopamine levels (Fig. 3; Strychnine/Acamprosate vs. Acamprosate p = 0.009, Strychnine/Acamprosate vs. Strychnine p = 0.836). Time effect, 40 to 100 minutes, F(3,69) = 3.900, p = 0.012; treatment effect, F(2,23) = 6.632, p = 0.005; and interaction term, F(6,69) = 3.736, p = 0.003.
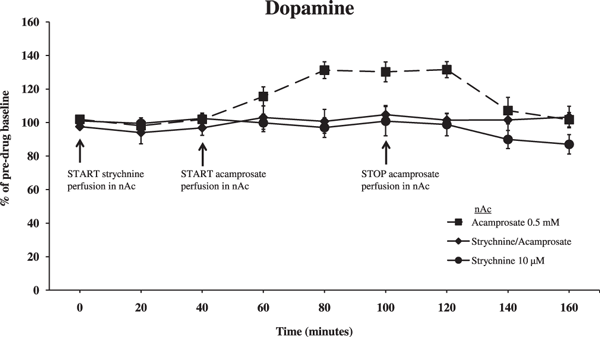
Effect of strychnine (competitive glycine receptor antagonist, 10 μM) perfused in the nAc 40 minutes before and during acamprosate (0.5 mM) or Ringer perfusion in the nAc on accumbal dopamine levels. Both start and end of perfusion of substances are indicated by arrows; strychnine perfusion was prolonged during the whole experiment. Shown are the means ± SEM; n = 7 to 10. Statistics: 2-way ANOVA with repeated measures over time points 40 to 100 minutes, followed by post hoc test LSD, Strychnine/Acamprosate vs. Acamprosate p = 0.009, Strychnine/Acamprosate vs. Strychnine p = 0.836.
In order to explore whether the acamprosate-induced elevation of nAc dopamine involves ventral tegmental nAChRs, as previously observed with both ethanol and taurine, we used dialysis probes in both the VTA and in the nAc. The nAChR antagonist mecamylamine (100 μM perfused in the VTA) significantly blocked the acamprosate-induced (0.5 mM perfused in the nAc) elevation of accumbal dopamine levels (Fig. 4; ANOVA with repeated measures at time points 40 to 100 minutes, followed by LSD, Mecamylamine/Acamprosate vs. Acamprosate p = 0.023, Mecamylamine/Acamprosate vs. Mecamylamine p = 0.967). Time effect, 40 to 100 minutes, F(3,75) = 6.730, p < 0.001; treatment effect, F(2,25) = 4.166, p = 0.027; and interaction term, F(6,75) = 2.834, p = 0.015.
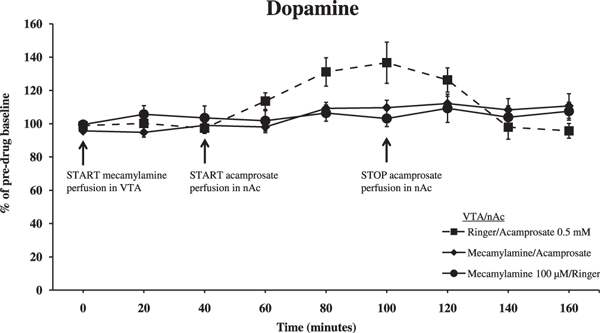
Dopamine levels in the nAc as measured by in vivo microdialysis after perfusion of mecamylamine (nicotinic acetylcholine receptor antagonist, 100 μM) or Ringer in the ventral tegmental area (VTA) and acamprosate (0.5 mM) or Ringer in the nAc. Drug application was initiated as indicated by the arrows. Shown are the means ± SEM; n = 8 to 11. Statistics: 2-way ANOVA with repeated measures over time points 40 to 100 minutes, followed by LSD, Mecamylamine/Acamprosate vs. Acamprosate p = 0.023, Mecamylamine/Acamprosate vs. Mecamylamine p = 0.967.
Effect of Systemic Administration of Acamprosate on Extracellular Concentration of Dopamine in the Nucleus Accumbens
Wistar rats implanted with a dialysis probe in the nAc received either an acamprosate (200 or 400 mg/kg i.p.) or saline injection after initial baseline dopamine levels had been established. The lower dose of acamprosate (200 mg/kg i.p.) elevated dopamine levels, even though this elevation was slightly less pronounced than the one previously observed after local application of acamprosate. The higher dose of acamprosate (400 mg/kg i.p.) did not significantly alter dopamine output in the nAc (Fig. 5; ANOVA with repeated measures over time points 40 to 160 minutes, followed by LSD, Acamprosate 200 mg/kg vs. NaCl p = 0.006, Acamprosate 400 mg/kg vs. NaCl p = 0.289). Time effect, 40 to 160 minutes, F(6,126) = 2.254, p = 0.042, treatment effect, F(2,21) = 4.679, p = 0.02; and interaction term, F(12,126) = 2.581, p = 0.004. Furthermore, independent t-tests at time points 80 and 100 minutes produced p-values of 0.006 and 0.035, respectively, between 400 mg/kg acamprosate and NaCl.
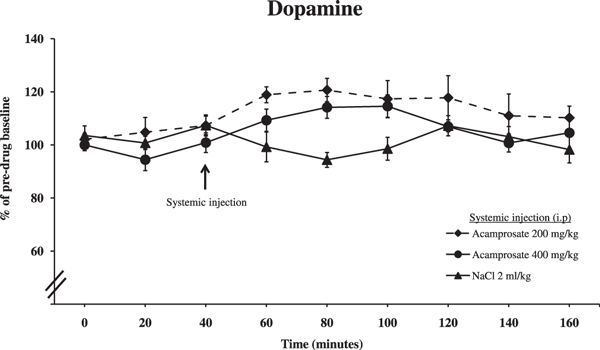
Effect of systemic application of acamprosate (200 or 400 mg/kg i.p.) or 0.9% NaCl on accumbal dopamine levels as measured by in vivo microdialysis. Shown are the means ± SEM; n = 7 to 10. Statistics: 2-way ANOVA with repeated measures over time points 40 to 160 minutes, followed by LSD, NaCl vs. Acamprosate (200 mg/kg) p = 0.006, NaCl vs. Acamprosate (400 mg/kg) p = 0.289.
In the final experiment, animals were pretreated with strychnine in the nAc before receiving a systemic injection of either acamprosate or vehicle. Blockade of accumbal GlyRs prevented the acamprosate-induced (200 mg/kg i.p.) elevation in dopamine levels in the nAc, in line with what we observed following local administration of the drug (Fig. 6; ANOVA with repeated measures over time points 40 to 160 minutes, followed by LSD, Strychnine/Acamprosate vs. Acamprosate p = 0.017, Strychnine/Acamprosate vs. Strychnine p = 0.999). Time effect, 40 to 160 minutes, F(6,114) = 3.887, p = 0.001; treatment effect, F(2,19) = 5.081, p = 0.017; and interaction term, F(6,114) = 1.405, p = 0.174.
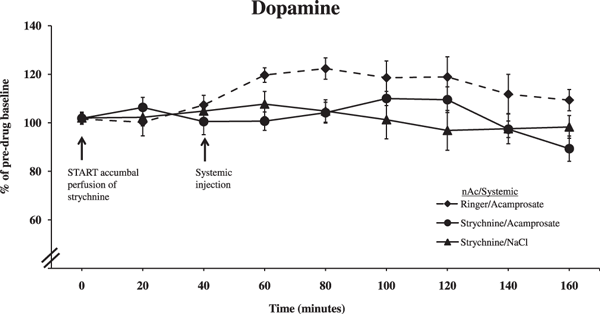
Effect of strychnine (10 μM) perfused in the nAc 40 minutes before systemic administration of either acamprosate (200 mg/kg i.p.) or 0.9% NaCl on accumbal dopamine levels as measured by in vivo microdialysis. Shown are the means ± SEM; n = 6 to 10. Statistics: 2-way ANOVA with repeated measures over time points 40 to 160 minutes, followed by LSD, Strychnine/Acamprosate vs. Acamprosate p = 0.017, Strychnine/Acamprosate vs. Strychnine p = 0.999.
Discussion
This study proposes a novel mechanism of action of acamprosate compared to what has been previously discussed. In line with previous results regarding ethanol and taurine (Ericson et al., 2006; Söderpalm et al., 2009), our results suggest that acamprosate modulates extracellular dopamine levels in the nAc primarily via GlyRs in the nAc and, secondarily, via nAChRs in the VTA. Local application of acamprosate in the nAc clearly implicated both GlyRs in the nAc and nAChRs in the VTA, since antagonism of either of these receptors prevented the extracellular accumbal dopamine elevations induced by acamprosate. In addition, systemic administration of acamprosate also implicated GlyRs in nAc whereas the tentative involvement of ventral tegmental nAChRs was not addressed. However, even though not tested here, it is hypothesized that nAChRs in the VTA are involved in the dopamine activating effect of acamprosate also after systemic administration. Altogether, it appears that acamprosate activates the mesolimbic dopamine system by interacting with GlyRs in the nAc and, indirectly, with nAChRs in the VTA, thus using the same hypothetical neuronal circuitry as previously proposed for ethanol and taurine.
The finding that local administration of acamprosate elevates dopamine levels in the nAc is in line with the findings of Cano-Cebrián and colleagues (2003a), whereas, to our knowledge, we are the first to demonstrate that also systemic administration of acamprosate elevates accumbal dopamine levels. While the lower dose of acamprosate (200 mg/kg) induced a dopamine response in a similar range as that observed after local administration of 0.5 mM, the higher dose (400 mg/kg) did not alter dopamine output significantly. Since the drug was applied systemically, in a rather high concentration, other counteracting mechanisms such as interaction with other receptor types or receptor desensitization may have interfered with the dopamine elevating effect mediated via nAc GlyR, thus preventing acamprosate from modulating dopamine levels in this brain region. Furthermore, in an accompanying paper we demonstrate that 200 mg/kg acamprosate sufficiently decreases ethanol intake in the rat.
Our conclusions drawn with respect to GlyRs and nAChRs rely on the specificity of strychnine and mecamylamine, respectively. Strychnine is a selective and potent competitive antagonist at GlyRs (Tokutomi et al., 1989), whereas it does not influence the modulatory glycine site on NMDA receptors. It should also be noted that Molander and Söderpalm (2005a) have demonstrated that strychnine-induced decrease (20 to 200 μM in the perfusion fluid) of accumbal dopamine is concentration-dependently reversed by glycine. Mecamylamine, used in the present study to demonstrate interactions with ventral tegmental nAChRs, is an unselective antagonist acting both at central and peripheral nAChRs of various subtypes. Mecamylamine also interacts with the NMDA receptor at the phencyclidine site (Snell and Johnson, 1989). However, the latter mechanism is unlikely to be involved in the present observations because applications of the noncompetitive NMDA antagonists phencyclidine and MK-801 increase dopamine turnover and release (Bristow et al., 1993), while mecamylamine applied locally in the present concentration or systemically, does not alter dopamine overflow (Blomqvist et al., 1997).
The prevailing view of the mechanism of action of acamprosate is that it interferes with the glutamate system. The acamprosate-glutamate interaction has been postulated to be mediated via interaction with ionotropic NMDA receptors but there appears to be contradicting opinions whether acamprosate acts as an antagonist (Rammes et al., 2001) or agonist (Berton et al., 1998) at the receptor. More specific NMDA receptor studies suggested that acamprosate interacts with the polyamine (spermidine) site of the NMDA receptor (Naassila et al., 1998; Popp and Lovinger, 2000). Convincing evidence that acamprosate inhibits the increase in extracellular glutamate during EtOH withdrawal has also been presented (Dahchour et al., 1998), but the molecular mechanism underlying the prevention of glutamate release has not been established. Other studies have demonstrated that acamprosate interacts with the metabotropic glutamate receptor 5, mGluR5 (Harris et al., 2002). However, when investigating acamprosate and the specific mGluR5 antagonist MPEP in the conditioned place preference model, the behavioral profiles of the 2 compounds differ (McGeehan and Olive, 2003a,b).
Evidence linking these proposed mechanisms of action of acamprosate to the acamprosate-induced dopamine elevation observed in this study is scarce (NMDA) or absent (mGluR5). Cano-Cebrián and colleagues (2003a) concluded that acamprosate modulates accumbal dopamine levels via the NMDA receptor, and that the effect is compatible with an NMDA receptor antagonist-like action. There are several arguments that these results could have been misinterpreted. Firstly, electrophysiological studies have not only given different results, but more recent studies indicate that there is no direct interaction between acamprosate and the NMDA receptor (Popp and Lovinger, 2000; Reilly et al., 2008). Secondly, the modulatory effects on dopamine levels mediated by NMDA receptors do not appear as straightforward as when mediated via GlyRs. Both agonists (NMDA, glutamate) and antagonists (AP5) elevate extracellular levels of dopamine (Cano-Cebrián et al., 2003a). This phenomenon may depend on whether the receptors are tonically active or not. Also with respect to acamprosate’s mechanism of action in reducing EtOH intake, current evidence is far from conclusive. Although several studies have shown that various NMDA receptor modulators may decrease EtOH intake (for review see Gass and Olive, 2008) it has not been clearly demonstrated that NMDA receptors are involved in acamprosate’s effect in this respect.
In the current study, we explored whether acamprosate has similar mechanisms of action in the mesolimbic dopamine system as previously demonstrated with taurine. Indeed, acamprosate was able to mimic the results previously obtained with taurine (Ericson et al., 2006) but it remains to be determined whether acamprosate acts via a direct interaction with GlyRs (which is most likely the case for taurine) or via its previously observed ability to increase extracellular taurine levels (Dahchour et al., 1996). Recently, Reilly and colleagues (2008) did not observe any direct interaction between acamprosate and the GlyR, or, for that matter, the NMDA receptor, the group I mGluR or the GABAA receptor. However, only a few subtypes were studied, i.e., the α1 homomeric and α1β heteromeric GlyR, leaving the possibility that acamprosate might interfere with other subtypes, and, of course, that the effect could be mediated via taurine. In vitro studies have demonstrated strychnine to be a specific antagonist for taurine-mediated GlyR activation (Choe and Bourque, 2007), and according to Wu and colleagues (2001), acamprosate produces its effects through a taurine system rather than the glutamatergic or GABAergic system. This conclusion was based on observations that acamprosate strongly inhibits binding of taurine to a specific taurine “receptor.” The common agonists or antagonists of major amino acid neurotransmitter receptors, among these also glycine and strychnine, did not show any affinity for this receptor. Neither did acamprosate have any influence on the binding of glutamate or muscimol to NMDA- and GABAA receptors, respectively. The fact that there exists a taurine-specific binding site is very interesting. This binding site could represent a specific taurine receptor but also a taurine transporter. Since acamprosate elevates extracellular taurine levels, it is a clear possibility that acamprosate interferes with a taurine transporter. Needless to say, only further experimentation can resolve these issues.
So far, only a few studies have investigated the interaction/relationship between acamprosate, EtOH and mesolimbic dopamine. Olive and colleagues (2002) showed that acamprosate dose-dependently reduces EtOH intake and preference, and, in a separate in vivo microdialysis experiment, that acamprosate delays or suppresses the EtOH-induced dopamine increase in the nAc. Another study performed by Cowen and colleagues (2005) also indicated that some effects of acamprosate may be mediated via modulation of the mesolimbic dopamine system, since subchronic acamprosate treatment altered both dopamine transporter and dopamine D2-like receptor density. It remains to be determined whether acamprosate’s interference with the mesolimbic dopamine system, and by inference the GlyRs, is related to its EtOH intake reducing effect. Given the extensive evidence linking the mesolimbic dopamine system to reward and positive reinforcement mechanisms and the high degree of similarity with respect to how EtOH and acamprosate modulate the mesolimbic dopamine system, such a relationship appears plausible. In fact, it appears that acamprosate in its interaction with this particular system, i.e., the mesolimbic dopamine system, may actually provide a pharmacokinetically slow substitution for EtOH.
In summary, our findings indicate that acamprosate, applied both intra-accumbally and intrapertioneally, modulates accumbal dopaminergic output via GlyRs in the nAc, and, indirectly, via ventral tegmental nAChRs. These receptors are located in a dopamine regulatory nAc-VTA-nAc pathway and we here demonstrate that acamprosate most likely interacts with this same neuronal circuitry as previously described for EtOH (Molander and Söderpalm, 2005a,b) and taurine (Ericson et al., 2006). Whether the EtOH intake reducing effects of acamprosate involves the same pathway remains to be elucidated.
Acknowledgments
The authors would like to acknowledge Dr. Chris Pickering for reading the manuscript and correcting linguistic errors. Financial support for this work was obtained from the Swedish Medical Research Council (Diary numbers 2006–4988 and 2006–6385) the Swedish Labor Market Insurance (AFA) support for biomedical alcohol research, governmental support under the LUA/ALF agreement, the Alcohol Research Council of the Swedish Alcohol Retailing Monopoly, the Council for Medical Tobacco Research-Swedish Match, Wilhelm and Martina Lundgrens Veternskapsfond, Fredrik och Ingrid Thurings Stiftelse, Magnus Bergvalls Stiftelse, Gunnar och Märta Bergendahls Stiftelse, Adlerbertska Forskningsfonden, Konrad och Helfrid Johanssons forskningsfond and Sigurd och Elsa Goljes minne.




