INTRACELLULAR LOCALIZATION OF AN ENDOGENOUS CELLULOSE SYNTHASE OF MICRASTERIAS DENTICULATA (DESMIDIALES, CHLOROPHYTA) BY MEANS OF TRANSIENT GENETIC TRANSFORMATION1
Received 30 July 2009. Accepted 12 March 2010.
Abstract
The desmid Micrasterias denticulata Bréb. is useful for the study of streptophyte cell wall biology and morphology. However, no tools to analyze cell biological processes in vivo in this species are available. In the present study, transient gene expression under the control of the chl a/b–binding protein gene of the Closterium peracerosum–strigosum–littorale complex (CpCAB1) promotor was achieved for M. denticulata and illustrated by the intracellular localization of an endogenous cellulose synthase (MdCesA1). A transformation efficiency of 1/5,000 cells was achieved following microparticle bombardment. The free green fluorescent protein (GFP) signal was detected both in the nucleus and in the cytoplasm. The MdCesA1-GFP fusion protein, on the other hand, occurred at the plasma membrane in particles concentrated at the lobe indentations, the lobe tips, and, to a lesser extent, along the lobe sides. Hence, the multipolar growth mechanism of the cell is reflected. In addition, the margins of cytoplasmic compartments, most likely dictyosomes, were labeled, in accordance with the known secretory pathway of cellulose synthase complexes. Besides intracellular localization studies, the utility of the system for overexpression phenotyping is discussed.
Abbreviations:
-
- C. psl. complex
-
- Closterium peracerosum–strigosum–littorale complex
-
- CaMV
-
- cauliflower mosaic virus
-
- cDNA-AFLP
-
- cDNA-amplified fragment length polymorphism
-
- CesA
-
- cellulose synthase
-
- cgfp
-
- GFP gene adapted to codon usage of Chlamydomonas reinhardtii
-
- CLSM
-
- confocal laser scanning microscopy
-
- CpCAB1
-
- chl a/b–binding protein gene of the Closterium peracerosum–strigosum–littorale complex
-
- ER
-
- endoplasmic reticulum
-
- GFP
-
- green fluorescent protein
-
- ORF
-
- open reading frame
-
- pNOS
-
- promoter of the nopaline synthase gene of Agrobacterium tumefaciens
-
- YFP
-
- yellow fluorescent protein
In contrast to land plants, little is known about cell wall formation and cell morphogenesis in other streptophytes. These processes have been studied for decades in the unicellular green alga M. denticulata (Zygnemophyceae, Charophyta, Streptophyta) (reviewed in Meindl 1993, Kim et al. 1996, Lütz-Meindl and Brosch-Salomon 2000, Nakashima et al. 2006, Eder and Lütz-Meindl 2008, Eder et al. 2008). As in other placoderm desmids, vegetative cells of Micrasterias consist of two bilaterally symmetrical semicells. Upon cell division, the semicells are separated by ingrowth of a septum. From this septum, new semicells are modeled mirroring the parental semicells through a complex cytomorphogenetic process. The localized and stage-specific occurrence of alternating growing and nongrowing zones in the cell wall, mediated by the incorporation of distinct Golgi-derived vesicle populations (Meindl et al. 1992), brings about the multilobed cell pattern of a full grown Micrasterias cell, providing an excellent system to analyze cell wall properties in relation to growth and development.
Whereas the flexible primary cell wall mainly consists of pectin, a cellulose secondary cell wall gives the cell its rigidity. Concerning cellulose biosynthesis, Micrasterias occupies an interesting phylogenetic position. Its so-called multimeric rosettes, consisting of six cellulose synthase enzymes forming the functional cellulose biosynthetic terminal complexes, are organized in hexagonal arrays of linear rows during secondary cell wall synthesis (Kiermayer and Sleytr 1979, Giddings et al. 1980), whereas solitary rosettes deposit primary wall microfibrils. From an evolutionary point of view, this configuration follows the linear terminal complexes of Acetobacter sp., Dictyostelium sp., and the chlorophyte algae (for references, see Roberts et al. 2002). In contrast, the rosettes of more advanced charophycean algae and vascular plants involved in both primary and secondary wall synthesis are individual (Tsekos 1999). Moreover, as a result of this rosette configuration, the secondary wall cellulose microfibrils of Micrasterias are among the widest ever reported (Kim et al. 1996).
To date, studies have used classical cell biological methods, including freeze-etch replica techniques (Kiermayer and Sleytr 1979, Giddings et al. 1980, Schmid and Meindl 1992) and immunocytochemical labeling (Lütz-Meindl and Brosch-Salomon 2000, Nakashima et al. 2006, Eder and Lütz-Meindl 2008, Eder et al. 2008). However, no tools are available to analyze processes in vivo in this species. Therefore, the establishment of a transformation protocol would greatly enhance the possibilities to explore the molecular biology of Micrasterias. Recently, a transient expression system was established for the Closterium peracerosum–strigosum–littorale complex (C. psl. complex), another desmid. An expression vector was developed that puts a gene of interest under the control of the endogenous promoter of the chl a/b–binding protein encoding gene and allows carboxy-terminal fusion to cgfp, a GFP gene adapted to the codon usage of Chlamydomonas reinhardtii (Abe et al. 2008). In the present work, we adapted this protocol for Micrasterias and illustrate its usefulness for in vivo molecular studies on the basis of the intracellular localization of an endogenous cellulose synthase.
Culture conditions. M. denticulata cells (kindly provided by Prof. Dr. Ursula Lütz-Meindl, University of Salzburg, Austria) were grown in Desmidiaceae medium (Schlösser 1982), 2-fold diluted with distilled water, under a constant temperature of 23°C with an irradiation from above of 120–140 μmol photons · m−2 · s−1 (Philips TL-D 36W 54-765 lamps; Philips, Eindhoven, the Netherlands) at a photoperiod of 14:10 light:dark (L:D).
Identification and cloning of a CesA of M. denticulata. A clone containing the full-length cDNA encoding a CesA was identified during a random small-scale (100 clones) sequence analysis of a Nanoquantity Uncut cDNA library from synchronized samples of dividing M. denticulata cells, which was purchased as a service from Invitrogen (Paisley, UK). The pDONR222.1 sequencing primers, specific for the vector used to clone the cDNA library, were 5′-ACGACGGCCAGTCTTAAGCTCGG-3′ (forward) and 5′-ACGACGGCCAGTCTTAAGCTCGG-3′ (reverse). Additional sequencing primer pairs (forward and reverse, respectively) were designed with the eprimer3 program (Rice et al. 2000): 5′-ATGATGCA-AGGAGGGTCAAT-3′ and 5′-ACAGGAAACAGGTG-TGCAG-3′, 5′-GAGGTTCTATGCTGACTTTTGA-3′ and 5′-AAGAGATCACATGAATAGCTTCC-3′.
Homologous sequences were retrieved from GenBank (http://www.ncbi.nlm.nih.gov) using BlastX (Altschul et al. 1997) and aligned using ClustalX (Larkin et al. 2007) with default settings. Protein family identifiers and protein domains were found using Pfam 23.0 (http://pfam.sanger.ac.uk/) (Finn et al. 2008). The absence of an SpeI splicing site in the open reading frame (ORF) of MdCesA1 was controlled with the NEBCutter V2.0 (New England BioLabs Inc., Ipswich, MA, USA) program (http://tools.neb.com/NEBcutter2/index.php). The ORF was amplified from a 100 ng plasmid template by a PCR reaction (comprising 2 min preincubation at 94°C and five cycles at 94°C for 45 s, 45°C for 45 s, and 68°C for 3 min, followed by 25 cycles at 94°C for 45 s, 55°C for 45 s, and 68°C for 4 min, followed by one cycle at 72°C for 5 min), using the primer pair 5′-ATGACTAGTATGCCAACTTTGTACAAAAAA-3′ (SpeI site underlined, start codon italicized) and 5′-GGAACTAGTGCAGGAGACACCACACTG-3′ (stop codon omitted). The PCR product was cloned into the SpeI-site of the pSA405A vector (Abe et al. 2008) and sequenced with the pDONR222.1 sequencing primers and the gene-specific primers mentioned above.
Transient genetic transformation of M. denticulata. Midlogarithmic or early stationary phase cultures were transferred to a 90 mm plate containing culture medium with 1.5% (w/v) agar as follows. Culture flasks were shaken, and cells were allowed to settle and were then sucked up and pipetted into a 4 cm diameter plastic cylinder, which was placed on the center of the plate. About 70,000 cells were inoculated per plate this way, forming a dense monolayer after settling down on the agar and liquid removal. After preculturing the cells at 21°C for 2 d under continuous light at 55 μmol photons · m−2 · s−1, which turned out to be appropriate to drive the CpCAB promoter (Abe et al. 2008), the recombined plasmid was introduced into M. denticulata by microparticle bombardment using a Biolistic PDS-1000/He Particle Delivery System (Bio-Rad Laboratories S.A.-N.V., Nazareth Eke, Belgium). The following parameters were used: 0.6 μm diameter gold particles, a 28 in. Hg vacuum, a target distance of 6 cm, and a helium pressure of 1,350 psi. As described in Abe et al. (2008), 15 μg plasmid DNA and 0.25 mg of gold particles were used to perform two shots per plate. The plasmid DNA was coated onto the gold particles according to the protocol of BioRad, using 225 μL of 2.5 M CaCl2 and 90 μL of 0.1 M spermidine (from a 1 M stock solution) (Sigma-Aldrich, Vienna, Austria). Three plates were handled per experiment. After bombardment, 2 mL of liquid culture medium was added, and the cells were again incubated for 2 d under continuous light to further drive the CpCAB promoter prior to transfer to liquid medium and manual isolation of GFP-expressing cells under a fluorescence binocular microscope (Leica Microsysteme GmbH, Vienna, Austria).
Confocal laser scanning microscopy (CLSM). Transgenic cells were individually cultured and regularly observed by confocal microscopy using a Zeiss 100M, equipped with LSM510 software version 3.2 (Zeiss, Oberkochen, Germany). Samples were scanned with a ×20 and a ×63 water-corrected objective (numerical aperture of 1.2 and 0.5, respectively). Helium-neon laser illumination at 543 nm visualized chloroplast fluorescence; argon laser illumination at 488 nm and a 500–530 nm band emission filter were used for GFP fluorescence.
GFP fluorescence of transgenic cells was measured on confocal z-stack projections, taken under the same conditions, ensuring that no signal saturation occurred, using the open-source software ImageJ (http://rsbweb.nih.gov/ij/index.html). The mean intensity values were multiplied by the area of the cell to obtain a total, area corrected, qualitative intensity value.
A transient transformation system allowing overexpression of GFP-tagged proteins in M. denticulata. In this study, the transient transformation protocol using the expression vector pSA405A developed for the C. psl. complex (Abe et al. 2008) was adapted for M. denticulata. Following particle bombardment among ∼70,000 cells, 5–34 (mean 15.3, st. dev. 13, n = 6) cgfp-expressing cells could be observed, yielding a transformation efficiency of ∼1/5,000. It was found that to attain this transformation efficiency, the minimum rupture disk-pressure should be at least 1,100 psi and must not exceed 1,550 psi, while 1,350 psi is the optimal value in combination with the other parameters mentioned in the “Transient genetic transformation of M. denticulata” section and in Table 1. In contrast, using the promoters CaMV 35S and pNOS, only two GFP-expressing cells could be observed per ∼210,000 cells (Table 1); moreover, these transgenic cells were unable to divide. In addition to these promoters, other parameters were tested prior to the protocol of Abe et al. (2008) (Table 1). At first, tungsten particles were replaced by 0.6 μm gold particles, as the viability of the transgenic cells was bad, possibly related to tungsten toxicity (Russell et al. 1992). Next, the effect of the intracellular targeting of the GFP on cell viability was tested by comparing nucleus-targeted (NLS-GFP) and ER-targeted GFP (ER-GFP). At the same time, the amount of plasmid DNA was doubled. The outcome of those experiments showed no clear difference, however. Moreover, the possible negative effect of the vacuum during bombardment on cell viability was eliminated, but no transgenic cells could be obtained from these experiments at all.
| Test | Particles | Amount of plasmid DNA/plate (μg) | Construct | He pressure (psi) | Target level (cm) | Inches Hg | No. of cells expressing GFP (cells/trial) | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | n | |||||||
| Target level/He pressure | Tungsten | 1.7 | 35S-NLS-GFP | 1,550 | 6 | 28 | 5.3 | 3.9 | 4 |
| Tungsten | 1.7 | 35S-NLS-GFP | 1,550 | 9 | 28 | 1.5 | 0.7 | 2 | |
| Tungsten | 1.7 | 35S-NLS-GFP | 1,550 | 12 | 28 | 1.0 | 1.4 | 2 | |
| Tungsten | 1.7 | 35S-NLS-GFP | 1,100 | 6 | 28 | 1.0 | 1 | ||
| Tungsten | 1.7 | 35S-NLS-GFP | 1,100 | 9 | 28 | 0.0 | 1 | ||
| Tungsten | 1.7 | 35S-NLS-GFP | 1,100 | 12 | 28 | 0.0 | 1 | ||
| Particles | 0.6 μm gold | 1.7 | 35S-NLS-GFP | 1,550 | 6 | 28 | 0.7 | 0.6 | 3 |
| He pressure, GFP targeting | 0.6 μm gold | 3.4 | 35S-ER-egfp | 650 | 6 | 28 | 0.0 | 1 | |
| 0.6 μm gold | 3.4 | 35S-ER-egfp | 900 | 6 | 28 | 0.0 | 1 | ||
| 0.6 μm gold | 3.4 | 35S-ER-egfp | 1,100 | 6 | 28 | 1.5 | 1.0 | 4 | |
| 0.6 μm gold | 3.4 | 35S-ER-egfp | 1,350 | 6 | 28 | 1.5 | 0.7 | 2 | |
| 0.6 μm gold | 3.4 | 35S-ER-egfp | 1,550 | 6 | 28 | 2.0 | 1 | ||
| Construct | 0.6 μm gold | 3.4 | 35S-AtFM6-GFP | 1,100 | 6 | 28 | 0.8 | 1.0 | 4 |
| Promotor | 0.6 μm gold | 3.4 | pNOS-GFP | 1,100 | 6 | 28 | 2.0 | 1.4 | 6 |
| Vacuum | 0.6 μm gold | 3.4 | pNOS-GFP | 1,100 | 6 | 5 | 0.0 | 0.0 | 2 |
| 0.6 μm gold | 3.4 | pNOS-GFP | 1,100 | 6 | 15 | 0.0 | 0.0 | 2 | |
| 0.6 μm gold | 3.4 | pNOS-GFP | 1,350 | 6 | 5 | 0.0 | 1 | ||
| 0.6 μm gold | 3.4 | pNOS-GFP | 1,350 | 6 | 15 | 0.0 | 1 | ||
| 0.6 μm gold | 3.4 | pNOS-GFP | 2,200 | 6 | 5 | 0.0 | 0.0 | 5 | |
| Protocol, Abe et al. (2008) | 0.6 μm gold | 15 | pSA106 | 1,100 | 6 | 28 | 14.0 | 4.6 | 3 |
| 0.6 μm gold | 15 | pSA405A | 1,100 | 6 | 28 | 15.3 | 13.0 | 6 | |
- GFP, green fluorescent protein.
Upon introduction of the plasmid pSA405A, the intracellular localization of the cgfp protein was investigated by CLSM. In addition to a diffuse cytoplasmic signal, a clear fluorescence was observed in the nucleus (Fig. 1, Fig. S1 in the supplementary material). This is unlike the C. psl. complex, where it was primarily observed in the cytosol (Abe et al. 2008). However, it is known that free (untargeted) fluorescent proteins are not only cytoplasmic but also migrate into the nucleus due to their small size (Berg and Beachy 2008).
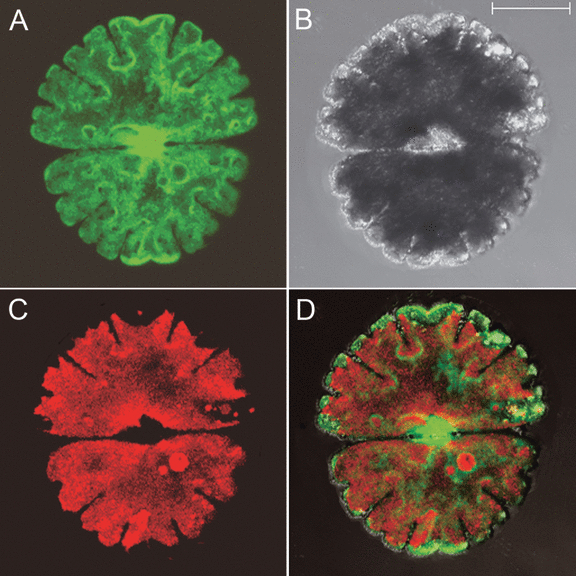
Intracellular localization of cgfp in Micrasterias denticulata following particle bombardment with pSA405A. The cgfp localizes in the nucleus and the cytoplasm. Images are confocal projections. (A) The cgfp signal. (B) Bright-field image. (C) Chl fluorescence. (D) Image obtained by merging images (A–C). Scale bar, 50 μm.
The nature of the transgenic cells obtained by the method described in this study is transient. Since Western blot or qPCR for the detection of the cgfp is not feasible on individual cells, the cgfp fluorescence intensity in different transgenic cells was determined as an indicative and qualitative, rather than a quantitative, measure for the level of cgfp expression.
As a result, three types of transgenic cells could be distinguished. A first type weakly expressed the cgfp and lost the signal after ∼4–7 d, followed by normal cell divisions. For others, especially, but not exclusively, those cells strongly expressing the cgfp, the transformation was lethal. These cells eventually died without having divided, although in rare cases viability could last up to 7 weeks. In intermediate cases, the signal could be clearly observed, even after a first and a second cell division. It took such transgenic cells more than the usual 3–4 d for division to occur, however, sometimes even up to 3 weeks. The morphology of newly formed semicells remained unaffected. Cloning a gene into the vector pSA405A and introducing it into M. denticulata cells will therefore be suitable not only for protein localization but also for overexpression phenotyping.
Intracellular localization of the MdCesA1-cgfp protein in M. denticulata. A cDNA-library clone was identified containing a 3,245 bp ORF expected to encode a protein of 1,081 amino acids with a molecular mass of 121.16 kDa. The deduced amino acid sequence of the ORF revealed high similarity with several CesA’s: 84% similarity with CesA1 of another desmid, Mesotaenium caldariorum (accession number: AF25360); 81% similarity with CesA4 of the moss Physcomitrella patens subsp. patens (accession number: XP_001767133); and 81% similarity with CesA6 of the angiosperm Populus tremuloides (accession number: AY196961) (Fig. S2 in supplementary material). Moreover, a zinc-binding domain near the N-terminus, characteristic for plant CesA’s (Kurek et al. 2002), and a protein family identifier for the cellulose synthase domain (PF03552) were found in the sequence (Fig. S2). The gene was therefore named MdCesA1. To illustrate the intracellular localization of MdCesA1, the full-length coding sequence was cloned into the expression vector pSA405A for transient overexpression following biolistic transformation. Dot-like MdCesA1-cgfp fluorescence was detected at the plasma membrane, concentrated at the lobe indentations, the lobe tips, and, to a lesser extent, along the sides of the lobes (Fig. 2, A–C). The signal was also observed in cytoplasmic compartments, as a ring of fluorescence possibly bordering organelles (Fig. 2D).
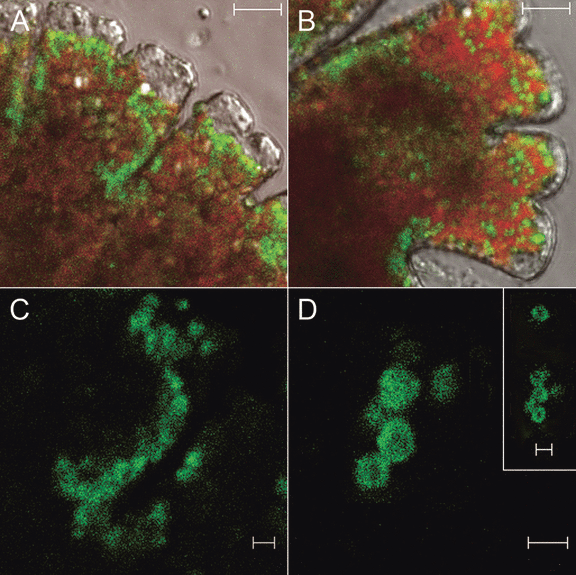
Intracellular localization of MdCesA1-cgfp in Micrasterias denticulata. (A, B) The MdCesA1-cgfp fusion protein is localized at the plasma membrane in particles concentrated at the lobe indentations, the lobe tips, and, to a lesser extent, along the lobe sides. Scale bars, 10 μm. (C) Detail of (A), occurrence of MdCesA1-cgfp at the splitting between two lobes. (D) Detail of ring-shaped labeling of cytoplasmic compartments. Scale bars (C, D), 2 μm. Merged green fluorescent protein (GFP), chl fluorescence, and bright-field confocal images (A, B), and GFP fluorescence confocal images (C, D).
The localization of MdCesA1-cgfp at the plasma membrane is consistent with immunolabeling of β-1,4-glucan synthase, which was detected in the plasma membrane along the convoluted edges of the lobes, and was more densely located near the lobe tips than in the basal regions of the lobes (Nakashima et al. 2006). The high density of MdCesA1-cgfp at the lobe tips reflects the polar growth mechanism by which the lobes elongated during cell growth (Lacalli 1975) as also seen in fern protonemata (Adiantum, Wada and Staehelin 1981) and moss caulonemata (Funaria, Reiss et al. 1984). By analogy with lobe-forming cells from a variety of land plant species, its dense occurrence at the indentations between the lobes, on the other hand, might be related to the formation of local, cellulosic wall thickenings. Alternating thinner and more extensible regions of the wall can bulge out under the force of turgor pressure to form lobes (Smith 2003). The targeted nonuniform localization of cellulose synthase to the plasma membrane in Micrasterias, as observed in full-grown cells, thus still reflects the multipolar growth mechanisms of the alga. The dot-like fluorescence signal occurring at the plasma membrane might correspond to a hexagonal array of linear rows of rosette terminal complexes of cellulose synthases during secondary cell wall formation as revealed by freeze-fracture EM (Giddings et al. 1980), a unique characteristic of the Klebsormidiophycean and Zygnematophycean algae (Tsekos 1999). Immuno-EM study could confirm this hypothesis. The organelles labeled by a ring of fluorescence are possibly Golgi bodies, since a very similar appearance of YFP-CesA6 in Arabidopsis transgenic plants was confirmed to be Golgi bodies by colocalization with a Golgi marker (Paredez et al. 2006) and since it is known that rosettes consisting of CesA’s are assembled in Micrasterias at the inner membrane surface of Golgi-derived flat vesicles that are transferred and incorporated in the plasma membrane during secondary cell wall formation (Dobberstein and Kiermayer 1972).
In the present work, a tool is provided for in vivo molecular analysis in M. denticulata, by adaptation of the transient genetic transformation protocol established for. C. psl. complex (Abe et al. 2008), another member of the charophyte order Desmidiales. Furthermore, it has been validated that the vector pSA405A is useful for the analysis of intracellular localization of the protein encoded by an endogenous gene of interest in M. denticulata. Moreover, the system is expected to allow phenotype characterization resulting from transient overexpression in a feasible way. Since each experiment results in transgenic cells expressing the recombinant protein in varying degrees, it can be expected that cell division in these cells will generate overexpression phenotypes accordingly. Aberrant morphological phenotypes will be easily detected in these peculiarly and regularly shaped cells. The large cell size (∼250 μm) of M. denticulata is not only convenient for localization studies, but also allows the rapid isolation of transgenic cells without the need for antibiotics. No efforts have been made yet to determine a suitable antibiotic and its concentration to be used in experiments that screen for stable transgenics, because of the ease of manual isolation and because of the particular low cell division rate in M. denticulata (every 3–4 d in optimal conditions, up to 3 weeks for transgenic cells), which would severely retard the appearance of stable colonies. In addition, none of the transgenic cells studied so far stably expressed the cgfp. The establishment of a stable transformation protocol for desmids thus remains an exciting challenge. Although other technologies, such as mutagenesis, gene silencing, gene knockout, and cotransformation will be required before gene function in desmids can be explored as it can be done in other eukaryotic model systems, our results demonstrate the utility of the molecular tool here presented in investigating in vivo biological processes in M. denticulata.
Acknowledgments
This work was supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (predoctoral fellowship to K. V.). This work was also supported in part by Grants-in-Aid for Scientific Research (Nos. 20570044 and 20247032) to H. S. from the Japan Society for the Promotion of Science, Japan, by a research grant (2009-2011) of the Institute for Fermentation, Osaka, Japan, to H. S. and by a grant from the New Technology Development Foundation to H. S. The authors acknowledge Wilson Ardilez-Diaz for the sequencing, Mansour Karimi and Jacob Pollier for cloning advice, and Marie Huysman for technical advice concerning the use of the Biolistic PDS-1000/He Particle Delivery System.