CELL-SPECIFIC EXTRACELLULAR PHOSPHATASE ACTIVITY OF DINOFLAGELLATE POPULATIONS IN ACIDIFIED MOUNTAIN LAKES1
Received 13 March 2009. Accepted 28 January 2010.
Abstract
High bulk extracellular phosphatase activity (PA) suggested severe phosphorus (P) deficiency in plankton of three acidified mountain lakes in the Bohemian Forest. Bioavailability of P substantially differed among the lakes due to differences in their P loading, as well as in concentrations of aluminum (Al) and its species, and was accompanied by species-specific responses of phytoplankton. We combined the fluorescently labeled enzyme activity (FLEA) assay with image cytometry to measure cell-specific PA in natural populations of three dinophyte species, occurring in all the lakes throughout May–September 2007. The mean cell-specific PA varied among the lakes within one order of magnitude: 188–1,831 fmol · cell−1 · h−1 for Gymnodinium uberrimum (G. F. Allman) Kof. et Swezy, 21–150 fmol · cell−1 · h−1 for Gymnodinium sp., and 22–365 fmol · cell−1 · h−1 for Peridinium umbonatum F. Stein. To better compare cell-specific PA among the species of different size, the values were normalized per unit of cell biovolume (amol · μm−3 · h−1) for further statistical analysis. A step-forward selection identified concentrations of total and ionic Al together with pH as significant factors (P < 0.05, Monte Carlo permutation test), explaining cumulatively 57% of the total variability in cell-specific PA. However, this cell-specific PA showed an unexpected reverse trend compared to an overall gradient in P deficiency of the lake plankton. The autecological insight into dinophyte cell-specific PA therefore suggested other factors, such as light availability, mixotrophy, and/or zooplankton grazing, causing further PA variations among the acidified lakes.
Abbreviations:
-
- Ald
-
- dissolved aluminum
-
- Ali
-
- ionic aluminum
-
- Alo
-
- organic aluminum
-
- Alp
-
- particulate aluminum
-
- Alt
-
- total aluminum
-
- CSPAB
-
- cell-specific phosphatase activity per biovolume
-
- DOC
-
- dissolved organic carbon
-
- DOP
-
- dissolved organic phosphorus
-
- DP
-
- dissolved phosphorus
-
- ELFA
-
- ELF®97 alcohol
-
- ELFP
-
- ELF®97 phosphate
-
- FLEA
-
- fluorescently labeled enzyme activity
-
- FU
-
- relative fluorescence unit
-
- MCV
-
- mean cell volume
-
- MUFP
-
- methylumbelliferyl phosphate
-
- PC
-
- particulate carbon
-
- PP
-
- particulate phosphorus
-
- SRP
-
- soluble reactive phosphorus
-
- TP
-
- total phosphorus
-
- Z eu
-
- euphotic depth
-
- Z mix
-
- mixed depth
In plankton ecology, production of extracellular phosphatases usually has been considered as a general response of plankton microorganisms to P depletion in the environment (Jansson et al. 1988, Chróst 1991). Extracellular phosphatases are usually associated with the cell membrane or are present as dissolved enzymes supplied by autolysis or excretion from various microorganisms (Jansson et al. 1988). These enzymes hydrolyze dissolved phosphate esters outside the cell, allowing the cell to absorb P. Bulk PA has been proposed as an indicator of P deficiency in phytoplankton (e.g., Healey and Hendzel 1980, Gage and Gorham 1985). However, recent applications of FLEA techniques have revealed large disproportions in cell-specific PA among particular phytoplankton species (Rengefors et al. 2001, Dyhrman et al. 2002, Štrojsová et al. 2003, Cao et al. 2005) or bacterial morphotypes (Nedoma and Vrba 2006) co-occurring in the same environment.
With the exception of phagotrophic protists, microbial cells are able to take up only orthophosphate (Pi) from the surrounding environment (Reynolds 1997). Nevertheless, some plankton species make use of several mechanisms, allowing them to overcome P starvation, such as high-affinity uptake of Pi, standby energy metabolism, luxury uptake, utilization of P from stored polyphosphates, and/or production or activation of extracellular phosphatases. The production of these enzymes has been believed to be regulated by Pi availability (Gage and Gorham 1985, Jansson et al. 1988), and PA thus indicating P stress or P limitation, both in marine and freshwater ecosystems (Healey and Hendzel 1980, Cembella et al. 1984, Dyhrman and Palenik 1999, Dyhrman et al. 2002, Dyhrman and Ruttenberg 2006).
For direct detection of PA at the single-cell level, the FLEA assay (in earlier papers also called ELF technique) has been used (González-Gil et al. 1998, Nedoma et al. 2003, Štrojsová and Vrba 2006). The fluorogenic substrate, ELF®97 phosphate (ELFP), reacts with cell surface-bound phosphatases and is cleaved into Pi and ELF alcohol (ELFA), which forms fluorescent precipitates at or near the site of PA (Huang et al. 1992). Its fluorescent signal is bright and photochemically stable, allowing a sensitive quantification either by image (Nedoma et al. 2003) or flow cytometry (Dignum et al. 2004). Application of the FLEA assay indicated differences in the presence and localization of phosphatases among the phytoplankton and both short-term and seasonal variations of PA (Rengefors et al. 2001, 2003, Štrojsová et al. 2003, 2005, 2008, Dignum et al. 2004, Cao et al. 2005, Štrojsová and Vrba 2006, 2009). Production of extracellular phosphatases has been detected in many freshwater and marine species of dinoflagellates (Dyhrman and Palenik 1999, Rengefors et al. 2001, 2003, Štrojsová et al. 2003, Dyhrman and Ruttenberg 2006, Ou et al. 2006). Dinoflagellates also occurred regularly in P-depleted phytoplankton in atmospherically acidified mountain lakes of the Bohemian Forest (Vrba et al. 2003a, Nedbalová et al. 2006). Due to high levels of sulfur and nitrogen deposition in the 20th century, the Bohemian Forest lakes became strongly acidified, resulting in a depletion of carbonate buffering system, pH decline, increase in aluminum (Al) concentrations, and a drastic reduction of biodiversity (Vrba et al. 2003a). Chemical reversal of the lakes and their biological recovery from acidic stress started in the late 1980s, after reduction in emission rates of atmospheric pollutants in central Europe (Kopáček et al. 2002). According to their current acidity and trophic status, the lakes under study can be characterized as follows: strongly acidified oligotrophic Čertovo Lake, slightly acidified oligotrophic Prášilské Lake, and strongly acidified mesotrophic Plešné Lake (Nedbalová et al. 2006). Yet all these lakes are fishless and, due to their acidity status and continuous P deficiency (Vrba et al. 2006), belong to pelagic ecosystems with the highest bulk PA worldwide (cf. table 1 in Bittl et al. 2001). Bioavailability of P is permanently reduced in the lakes by Al-P coprecipitation (Kopáček et al. 2000), and the resulting severe P limitation is indicated by excessive bulk extracellular PA and high seston C:P ratios (Vrba et al. 2006). Both particulate and ionic forms of Al were shown to affect P availability in the lakes through inhibition of extracellular phosphatases (at pH < 5, Bittl et al. 2001) and P inactivation (Vrba et al. 2006). In these lakes indeed, substantial extracellular PA used to be detected in the bacterioplankton, which are characterized by a massive occurrence of filamentous morphotypes (Vrba et al. 2003b, Nedoma and Vrba 2006). This fact compromises the bulk PA assay as the indicator of phytoplankton P deficiency in these lakes. Bacterial cells are apparently more efficient in P acquisition than the phytoplankton under P-limited conditions (Currie and Kalff 1984), which is reflected by their dominance in the plankton of oligotrophic Čertovo Lake (Vrba et al. 2003b, Nedbalová et al. 2006).
This study aimed to quantify extracellular PA in three dinoflagellate species, occurring in the plankton of the Bohemian Forest lakes during one ice-free season. Based on substantial differences in P loading and concentrations of individual Al species among the lakes, we hypothesized significant species-specific responses of phytoplankton to varying P bioavailability. Beyond the differences in P and Al, our present study suggested other factors probably affecting phytoplankton PA at the species or population level.
Materials and methods
Study site and sampling. We studied the three following small mountain lakes in the Bohemian Forest (Šumava Mts., Czech Republic): Čertovo Lake (13°12′ E, 49°10′ N), Prášilské Lake (13°24′ E, 49°05′ N), and Plešné Lake (13°52′ E, 48°47′ N). Their crystalline bedrock is geologically sensitive to acidic deposition; catchments are small (65–88 ha), with steep slopes covered with thin acidic soils; and the vegetation is dominated by Norway spruce (Veselý 1994). The lakes are situated at elevations of 1,027–1,087 m, their surface areas range between 4.2 and 10.7 ha, and the maximum depths are 17–35 m (Nedbalová et al. 2006).
The epilimnion of the lakes was sampled at the 0.5 m depth regularly in 3-week intervals from May to September 2007. The samples were immediately prefiltered with a 200 μm polyamide sieve (Silk & Progress s. r. o., Brněnec, Czech Republic) and transported to the laboratory. For the determination of phytoplankton species composition and biovolume, a subsample of ∼0.5 L was fixed with acid Lugol’s solution and stored until processing.
Chemical and plankton analyses. In the laboratory, samples were filtered with glass-fiber filters (pore size 0.4 μm, Macherey-Nagel GmbH, Düren, Germany), except for samples for pH and alkalinity (acid neutralizing capacity determined by Gran titration), and total concentrations of aluminum (Al), phosphorus (P), and carbon (C), which were not filtered beyond the field prefilter. Dissolved organic C (DOC) was analyzed in the filtrate, and particulate C (PC) was analyzed by combustion of the retained particulate organic matter on the glass-fiber filter (both with TOC 5000A analyzer; Shimadzu, Kyoto, Japan). Soluble reactive P (SRP) was determined by the molybdate method. When the SRP concentration was below the detection limit of 50 nmol · L−1, half of this value was used in subsequent data evaluation. Total and dissolved P (TP and DP) were determined by perchloric acid digestion and the molybdate method; samples were 4-fold concentrated by evaporation (at ∼100°C prior digestion) to obtain a detection limit of 15 nmol · L−1. No TP and DP concentration was below this detection limit. Particulate P (PP) was the difference between TP and DP. Fractionation of Al, that is, total reactive Al (Alt), dissolved Al (Ald), and organically bound Al (Alo), was analyzed in nonfiltered samples, filtered samples, and cation exchange treated samples after their filtration, respectively (Driscoll 1984). Ionic Al (Ali) was calculated as the difference between Ald and Alo, and particulate Al (Alp) as the difference between Alt and Ald. Details on analytical methods are given in Kopáček et al. (2006). All chemical analyses were finished within 24 h after the sampling.
Phytoplankton counting was done in Utermöhl’s sedimentation chambers on an inverted microscope (Nikon Diaphot; Nikon, Tokyo, Japan). A presedimentation of samples (5×) was necessary except for Plešné Lake samples. A minimum of 400 individuals were counted in each sample. Mean cell volume (MCV) of each species was estimated by shape assimilation to known geometric forms (Hillebrand et al. 1999), and total biovolume was expressed in mm3 · L−1. Total biovolume of filamentous cyanobacteria was estimated in sedimentation chambers using the line intercept method (Nedoma et al. 2001). All species’ biovolumes were summed as phytoplankton fresh mass. Concentration of chl a was determined spectrophotometrically on Whatman GF/C filters (Maidstone, Kent, UK) after acetone extraction (Lorenzen 1967); values were not corrected for phaeopigments.
Detection and quantification of PA. Samples were processed immediately after transportation to the laboratory (usually <2 h after sampling). Two distinct assays were used for detection of extracellular PA in the samples: (i) bulk activity and (ii) single-cell activity (FLEA assay).
Bulk PA was measured using common fluorogenic substrate, 4-methylumbelliferyl phosphate (MUFP; Hoppe 1983). Duplicate 4.5 mL subsamples were supplemented with 0.5 mL of MUFP solution (100 μmol · L−1 final concentration; Glycosynth, Warrington, Cheshire, UK), mixed, and incubated at in situ temperature for 6–15 min. Then, the enzymatic hydrolysis was stopped by addition of 100 μL of 2 M NaOH, followed by intensive stirring. Fluorescence was read on a Spekol 11 photometer with a fluorometric device (Carl Zeiss, Jena, Germany).
The protocol for FLEA (Nedoma et al. 2003) was followed for the detection and measurement of cell-specific PA in the phytoplankton. Duplicate 2 mL (Plešné Lake) or 5 mL (other lakes) subsamples were incubated with fluorogenic substrate ELFP (Molecular Probes; Invitrogen, Eugene, OR, USA). The substrate was split into small portions (100 μL, stored at −18°C) used up within one experiment. ELFP was always filtered through a spin filter (0.2 μm pore size, Ultrafree-MC; Millipore Corp., Bedford, MA, USA) before use. The incubation was started by the addition of the ELFP solution (20 μmol · L−1 final concentration) and lasted 60 min at 20°C. Each incubation was terminated by transferring the sample to a filter holder, and the sample was immediately supplemented with HgCl2 as a fixative (4 mmol · L−1 final concentration). The fixation of incubated samples with HgCl2 prevents the destruction of fragile algal species, especially flagellates, during filtration and microscope slide preparation (Štrojsová et al. 2003). Then samples were filtered over mild vacuum (<20 kPa) through polycarbonate filter (pore size 2 μm, filter diameter 16 mm; Osmonics, Minnetonka, MN, USA). The filter with retained plankton was placed on a microscopic slide, embedded with the antifading reagent Citifluor AF1 (Citifluor, London, UK), and covered with a coverslip.
The phytoplankton was examined for the presence of ELFA precipitates with an epifluorescence microscope (Nikon Eclipse 90i). For the detection of extracellular phosphatases, we used an image analysis system consisting of a monochromatic digital integrating camera Vosskühler COOL-1300Q (Vosskühler GmbH, Osnabrück, Germany), mounted onto the fluorescence microscope Nikon Eclipse 90i and connected to a PC. The image analysis software used for microscope control, image acquisition, and processing was NIS-Elements 2.30 (Laboratory Imaging, Praha, Czech Republic). Two kinds of images were obtained: (1) ELF-images of the ELFA deposits’ fluorescence for the detection and quantification of PA, using ELFA-specific microscope filter block (excitation/emission: 360–370 nm per 520–540 nm), and (2) CHL-images of chl a autofluorescence for identification of algae, using a chl-specific filter block (excitation/emission: 510–550 nm per >590 nm). Both ELF image and CHL image of the same field were stored in one image file of JPEG2000 image format.
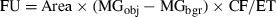 (1)
(1)Calibration factor was determined using a standard solution of 9.4% fluorescein (0.1 g · mL−1) as described in Model and Burkhardt (2001). FU was averaged per sample and converted to amount of ELFA precipitate by using a conversion factor of 0.021 fmol · FU−1, determined as described in Nedoma et al. (2003). Species-specific PA was expressed per cell in fmol · cell−1 · h−1. To compare cell-specific PA among the species of different size (i.e., MCV), the values were normalized per unit of cell biovolume and expressed as CSPAB in amol · μm−3 · h−1.
Statistics. Medians of seasonal data were tested using repeated measures nonparametric one-way analysis of variance (ANOVA; Friedman test with Dunns posttest) in the program Prism 4.0 (GraphPad, San Diego, CA, USA).
Multidimensional analysis was done in the program CANOCO 4.5 (ter Braak and Šmilauer 2002). Since the gradients calculated by the detrended correspondence analysis were short, we used linear gradient analysis (redundancy analysis). A step-forward regression was performed to explore the environmental parameters significantly correlated with the species-specific CSPAB (P < 0.05; Monte Carlo randomization test with 500 permutations).
Results
Table 1 summarizes selected surface lake water characteristics of the three Bohemian Forest lakes from May to September 2007. During the whole season, pH values varied between 4.54 and 5.11, and alkalinity did not reach positive values in any sample, having the lowest values in Čertovo Lake. Plešné Lake had the highest concentrations of TP (mean of 442 nmol · L−1) among the lakes, but SRP concentrations were below the detection limit (50 nmol · L−1) in all lakes at most sampling dates. Concentrations of dissolved organic P (DOP) in the lakes were roughly estimated as the difference between DP and SRP (Table 1). Considering the uncertainty in SRP determination, average DOP concentrations were as low as ∼10–40 nmol · L−1 and represented about a half of the DP concentrations in the lakes. The lowest seston C:P ratio (PC:PP mean of 488) and concentration of total Al (Alt, mean of 158 μg · L−1) were recorded in Prášilské Lake, while the highest values occurred in Plešné Lake, with averages of 854 and 522 μg · L−1, respectively. Due to the differences in pH, the lakes differed considerably in Al speciation, with the lowest proportion of Ali in the Alt observed in Prášilské Lake (Table 1). Relatively high chl a concentration was characteristic for mesotrophic Plešné Lake throughout the season (mean of 18.7 μg · L−1), whereas it averaged ∼3 μg · L−1 in the oligotrophic lakes. Similar differences were observed for total phytoplankton biomass among the lakes (Fig. 1). The mean bulk extracellular PA ranged from 1.3 μmol · L−1 · h−1 in Prášilské Lake to 8.7 μmol · L−1 · h−1 in Plešné Lake (Table 1). Using these values and mean half-saturation constant for each lake (2.0 and 1.9 μmol · L−1, Bittl et al. 2001), a turnover time of natural DOP pool could be estimated as low as 1.5 h to 0.2 h, respectively.
| Parameter | Prášilské Lake | Čertovo Lake | Plešné Lake |
|---|---|---|---|
| pH | 4.98 ± 0.08 | 4.61 ± 0.06 | 4.93 ± 0.11 |
| Alkalinity (μmol · L−1) | −11 ± 6 | −31 ± 5 | −12 ± 5 |
| TP (nmol · L−1) | 171 ± 29 | 142 ± 45 | 442 ± 81 |
| DP (nmol · L−1) | 74 ± 26 | 45 ± 16 | 61 ± 16 |
| SRP (nmol · L−1) | <32a | <32a | 39 ± 29 |
| PP (nmol · L−1) | 97 ± 39 | 97 ± 39 | 381 ± 77 |
| DOC (mg · L−1) | 4.50 ± 1.66 | 2.61 ± 1.26 | 3.49 ± 0.91 |
| Seston C:P (molar) | 488 ± 127 | 723 ± 237 | 854 ± 166 |
| Alt (μg · L−1) | 158 ± 21 | 331 ± 16 | 522 ± 54 |
| Ali (μg · L−1) | 43 ± 18 | 258 ± 31 | 228 ± 102 |
| Alo (μg · L−1) | 96 ± 36 | 51 ± 22 | 91 ± 25 |
| Alp (μg · L−1) | 19 ± 10 | 22 ± 10 | 202 ± 71 |
| Chl a (μg · L−1) | 3.5 ± 1.3 | 3.3 ± 0.8 | 18.7 ± 5.6 |
| Bulk PA (μmol · L−1 · h−1) | 1.33 ± 0.41 | 3.60 ± 1.72 | 8.67 ± 3.22 |
- Ali, ionic aluminum; Alo, organic aluminum; Alp, particulate aluminum; Alt, total aluminum; DOC, dissolved organic carbon; DP, dissolved phosphorus; PA, phosphatase activity; PP, particulate phosphorus; SRP, soluble reactive phosphorus; TP, total phosphorus.
- aSRP in most cases below detection limit.
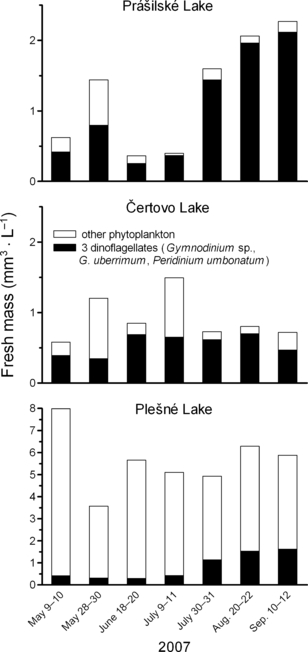
Seasonal development of phytoplankton biomass in the Bohemian Forest lakes in 2007. Stacked bars represent total fresh mass with proportions of the dinoflagellates in each sample.
Dinoflagellates G. uberrimum, Gymnodinium sp., and P. umbonatum were present in all lakes during the whole study. In term of abundance and biomass, they were among dominant species of the phytoplankton in two oligotrophic lakes, Čertovo and Prášilské, but not in mesotrophic Plešné Lake (Fig. 1). Each species showed remarkable differences in the cell size (MCV) among the lakes (Table 2).
| Species | MCV (μm3) | Cell-specific PA (fmol · cell−1 · h−1) |
|---|---|---|
| Gymnodinium uberrimum | ||
| Prášilské Lake | 17,654 ± 4,439a | 1,266 ± 592 |
| Čertovo Lake | 14,446 ± 1,002a,b | 1,831 ± 1,033 |
| Plešné Lake | 10,040 ± 6,163b | 188 ± 193 |
| Gymnodinium sp. | ||
| Prášilské Lake | 328 ± 98a | 150 ± 107 |
| Čertovo Lake | 242 ± 89a | 34 ± 35 |
| Plešné Lake | 313 ± 35a | 21 ± 26 |
| Peridinium umbonatum | ||
| Prášilské Lake | 2,678 ± 1,770a,b | 365 ± 51 |
| Čertovo Lake | 3,459 ± 1,325a | 52 ± 38 |
| Plešné Lake | 1,879 ± 347b | 22 ± 25 |
- MCV values with the same letter are not significantly different (P < 0.05) within a species.
In Čertovo Lake, the three dinoflagellates were dominant in most of the samples (>60% of total phytoplankton biomass), with the exception of the end of May and beginning of July, when the colonial chrysophyte, Dinobryon sp., prevailed and dinoflagellates represented 29% and 44% of total biomass, respectively (Fig. 1). Cell-specific PA was detected in the three dinoflagellates in almost all samples; P. umbonatum was not ELFA labeled at the beginning of the study (Fig. 2).
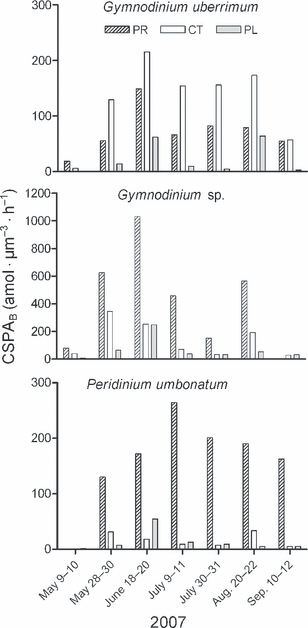
Seasonal development of cell-specific phosphatase activity per biovolume (CSPAB) of the dinoflagellates in Čertovo (CT), Prášilské (PR), and Plešné (PL) lakes in 2007.
In Prášilské Lake, biomass of the dinoflagellates varied from 55% in late May to 95% at the end of the study (Fig. 1). G. uberrimum formed >85% of total phytoplankton biomass during most of the summer stratification, whereas the other two dinoflagellates were present but less important. Chrysophyceae (mainly Dinobryon sp., Ochromonas sp., and Synura sp.) and Cryptophyceae were important components of phytoplankton biomass in May. Due to the high proportion of G. uberrimum, with virtually all the cells ELFA-labeled during the whole season, active species formed the major part of total biomass in Prášilské Lake (cf. Fig. 1).
Plešné Lake showed a distinct pattern with the dominance of the green coccal alga Monoraphidium dybowskii (Wolosz.) Hindák et Komárk.-Legn. and total phytoplankton biomass reaching up to 8 mm3 · L−1 (mean of 5.6 mm3 · L−1, in contrast to the means of 0.9 and 1.2 mm3 · L−1 in Čertovo and Prášilské lakes, respectively). Also, dinoflagellates, especially P. umbonatum and Gymnodinium sp., regularly occurred in Plešné Lake, but G. uberrimum reached quite low abundances. In the first half of the season, a clear prevalence of M. dybowskii was obvious, and dinoflagellates accounted for only <10% of total biomass. Since the end of July, their proportion had been slightly increasing to ∼25% (Fig. 1). Overall, the proportion of ELFA-labeled species was low in Plešné Lake; apart from the three dinoflagellates, extracellular phosphatases were also detected in Chlorogonium fusiforme Matv., Cryptomonas sp., filamentous cyanobacteria Limnothrix sp. and Pseudanabaena sp., but very exceptionally in the dominant M. dybowskii.
Mean cell-specific PA per cell varied in a wide range, which was within an order of magnitude for each species, due to the differences in cell size among the particular species and among the lakes (Table 2). The three dinoflagellates were ELFA labeled in almost all samples (Fig. 2); however, usually not all the cells in a given population were active, and both labeling pattern and intensity of fluorescence also varied. For example, G. uberrimum was mostly labeled on the whole cell surface in Čertovo Lake, but only on a part of it in Plešné and Prášilské lakes. A different pattern was recorded in P. umbonatum, whose entire cell surface was labeled in Prášilské Lake, in contrast to the other two lakes.
CSPAB of G. uberrimum varied from 5 to 215 amol · μm−3 · h−1 (Fig. 3), with maximum values occurring in June (Fig. 2). Active cells were recorded during the whole season in all lakes, with the exception of the early May sample from Plešné Lake. Low CSPAB values for G. uberrimum were characteristic in Plešné Lake. In contrast, its CSPAB in Čertovo Lake was relatively high, ranging from 130 to 215 amol ·μm−3 · h−1 (with the exception for the first and last samples, Fig. 2).
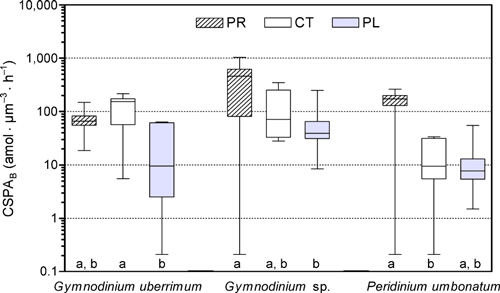
Seasonal range of cell-specific phosphatase activity per biovolume (CSPAB; note logarithmic scale) of the dinoflagellate populations of Čertovo (CT), Prášilské (PR), and Plešné (PL) lakes in 2007; median, 25% and 75% quartiles (box), minimum and maximum values (bars) are displayed. Medians with the same letter are not significantly different (P < 0.05) within a species.
Differences in CSPAB among the lakes and its variations in each lake were also found in Gymnodinium sp.; the lowest values were measured in Plešné Lake (similarly to G. uberrimum, Fig. 3). On the contrary, the highest values (460–1,030 amol · μm−3 · h−1) of Gymnodinium sp. were in Prášilské Lake, which represented the maximum dinoflagellate CSPAB in our study.
CSPAB of P. umbonatum was very low in Plešné Lake (1.5 amol · μm−3 · h−1), and it was not detected in Čertovo and Prášilské lakes at the beginning of the season (Fig. 2). Overall, the highest values in this species were characteristic for Prášilské Lake, where its CSPAB increased to 265 amol ·μm−3 · h−1 (Fig. 3).
The correlation of species-specific extracellular phosphatase production (i.e., CSPAB) with environmental parameters was explored using forward selection in redundancy analysis (Fig. 4). The analysis identified Alt and Ali concentrations together with pH as significant factors (P < 0.05, Monte Carlo permutation test), explaining cumulatively 57% of the total variability in the species-specific PA (Alt: 32%, F = 8.97, P = 0.002; Ali: 14%, F = 5.63, P = 0.018; pH: 11%, F = 3.50, P = 0.046). A negative correlation between CSPAB of all three species and Alt concentrations was significant, and the location of points representing particular samples illustrated the differences both in CSPAB and the significant factors among the lakes.
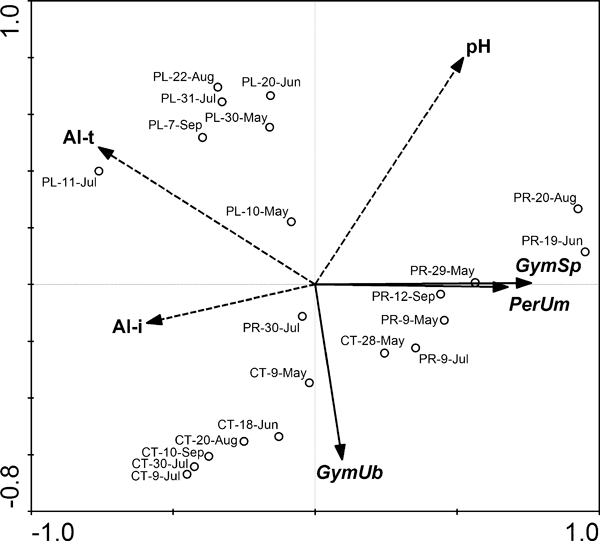
Direct gradient analysis (redundancy analysis)—relationship of significant environmental variables (P < 0.05) and species-specific phosphatase activity in the dinoflagellate species. Positions of particular samples with indication of date are also displayed. CT, Čertovo Lake; PR, Prášilské Lake; PL, Plešné Lake; GymUb, Gymnodinium uberrimum; GymSp, Gymnodinium sp.; PerUm, Peridinium umbonatum; Al-t, total reactive aluminum; Al-i, ionic aluminum.
As the Alt concentration was identified as the best parameter for explanation of variability in the data set, its relationships with CSPAB of particular species are shown in detail (Fig. 5). The lakes can be easily distinguished on the graph due to marked differences in their Alt concentrations, whereas the ranges of species-specific PA are widely overlapping among the lakes. Negative linear regressions were significant across all lakes for both Gymnodinium sp. (r2 = 0.41, P = 0.0025) and P. umbonatum (r2 = 0.62, P < 0.0001), but not for G. uberrimum. There were no significant regressions within each lake for any species.
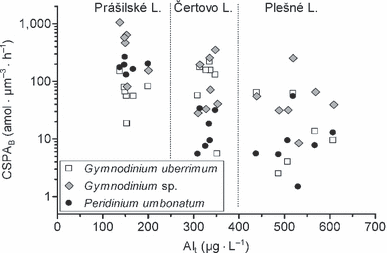
An overall relationship between cell-specific phosphatase activity per biovolume (CSPAB) of the dinoflagellates and concentration of total aluminum (Alt) in the Bohemian Forest lakes in 2007.
Discussion
This autecological study on three dinoflagellates presents, to our knowledge, the first survey of cell-specific PA in natural populations of the P-depleted phytoplankton under different environmental conditions. Our cell-specific PA per cell (Table 2) fits well into the range of the published values (for review, see Nedoma et al. 2003). At first look, however, CSPAB values seem to be rather contradictory to the particular environmental variables (Table 3). While seston C:P ratio, Alt concentrations, bulk PA, and turnover time of natural DOP pool consistently indicated an increasing gradient in P deficiency of the plankton (Prášilské L. < Čertovo L. < Plešné L.), CSPAB of the three dinoflagellates showed an unexpected reverse trend (Table 3). Moreover, CSPAB increased with the proportion of the three dinoflagellates in total biomass, in contrast to the commonly reported observation (Rengefors et al. 2001, Štrojsová et al. 2003) that dominant species do not tend to display PA. The lake chemistry and bulk PA indicate the highest P availability in Prášilské Lake; however, the summed CSPAB in this lake is 10-fold higher than that in Plešné Lake. Moreover, apparent low P availability is surprising in mesotrophic Plešné Lake with the highest P load (Table 3, cf. Vrba et al. 2006).
| Parameter | Prášilské Lake | Čertovo Lake | Plešné Lake |
|---|---|---|---|
| Trophic statusa | Oligotrophic | Oligotrophic | Mesotrophic |
| TP load (mg · m−2 · year−1)b | 103 | 58 | 313 |
| Median seston C:P (molar)c | 479 | 789 | 872 |
| Median Alt (μg · L−1)c | 152 | 334 | 518 |
| Median bulk PA (μmol · L−1 · h−1)c | 1.47 | 2.67 | 8.11 |
| Turnover time of natural DOP (h)d | ∼1.5 | ∼0.9 | ∼0.2 |
| Relative P availability | Higher | Lower | Impaired |
| Zooplankton grazing (dominant) | High (Cladocera) | Paltry (Rotifera) | Low (Copepoda) |
| Relative P regeneration | High to moderate | Low | Moderate |
| Median CSPAB (fmol · cell−1 · h−1)e | 696 | 235 | 56 |
| Mean proportion in biomassf | 81% | 65% | 15% |
| Competition efficiencyg | High | Moderate | Low |
| Median Zeu:Zmix ratioh (range) | (1.5) | 2.8 (1.6–6.0) | 0.8 (0.6–1.6) |
- Alt, total aluminum; PA, phosphatase activity; TP, total phosphorus.
- aAccording to Vrba et al. (2006) and this study (Table 1).
- bTP load is expressed on a lake area basis and represents sum of terrestrial P export by lake inlets and atmospheric P deposition on lake surface; data were measured for Čertovo and Plešné lakes in 2007, and estimated for Prášilské Lake on the basis of average P concentrations in its inlets (1997–2007), runoff, and average P deposition measured at Čertovo and Plešné lakes (J. Kopáček, unpublished data).
- cThis study (cf. Table 1).
- dTurnover time of natural DOP pool, hydrolyzable by extracellular phosphatases, was estimated according to Bittl et al. (2001) from the bulk PA (this study) and mean half-saturation constant for each lake (in Bittl et al. 2001).
- eSum of seasonal medians of the three dinoflagellates (from Fig. 3).
- fProportion of the three dinoflagellates in phytoplankton biomass (from Fig. 1).
- gEfficiency of the dinoflagellates in competition with other microorganisms in the plankton.
- hThe ratio of euphotic depth (Zeu, determined as the double Secchi depth, Zs) and mixed depth (Zmix, determined as the surface isothermal depth); only the September ratio is available for Prášilské Lake.
Why is the P status of dinoflagellates opposite the trend in general P deficiency of the lake plankton? This unexpected result apparently contradicts the widely accepted indicative value of PA and needs alternative explanations, such as (i) complex Al effects, (ii) top-down effects (grazing and P regeneration) of the distinct zooplankton, (iii) different light availability in the lakes (cf. Table 3), or (iv) an alternative strategy of P acquisition such as bacterivorous mixotrophy.
Complex aluminum effects under acidic stress. A high proportion of dinoflagellates in phytoplankton biomass is characteristic for acidified lakes (Almer et al. 1978, Findlay et al. 1999, Vrba et al. 2003a, Nedbalová et al. 2006), which used to be explained by their efficiency in competition for nutrients and tolerance to high Al concentrations (Hörnström 2002).
In this study, Al concentration together with pH were identified as the most important factors among tested parameters, which control—through P availability—CSPAB in the three dinoflagellates (Fig. 4). Yet there is, of course, natural autocorrelation of high Al concentrations with low pH. Although the Alt concentrations have decreased markedly in the study lakes since the peak of acidification in the 1980s, they still remain rather high compared to other lake districts (Vrba et al. 2003a, 2006). Jansson (1981) observed that high Al concentrations had an inhibitory effect on extracellular phosphatases at low pH. The inhibition effect on PA is dependent on Al speciation; Ali is considered to be a competitive inhibitor of extracellular phosphatases. Bittl et al. (2001) studied the impact of Al on bulk PA in Čertovo, Prášilské, and Plešné lakes and confirmed a direct inhibitory effect of Ali on extracellular phosphatases at diverse Ali concentrations (0–1,000 μg · L−1) in a laboratory experiment. They set a threshold value of pH < 5 for direct impact of Al on PA, which agreed well with the decrease in Ali proportion in Alt at pH > 5 (Driscoll 1985). Vrba et al. (2006) proposed that Al plays a key role in P availability in the acidified Bohemian Forest lakes, namely, through the inhibition of extracellular phosphatases and P inactivation due to the formation Al oxyhydroxides (Alp) strongly binding Pi (Kopáček et al. 2001). It was proposed that planktonic organisms produce more extracellular phosphatases to obtain a sufficient amount of P in order to overcome the inhibitory effect of Ali as well as the P inactivation by Alp (Vrba et al. 2006). This mechanism might take effect in the bacterioplankon that were largely ELFA labeled (e.g., in mesotrophic Plešné Lake). In fact, the plankton P demand causes such an excessive release of extracellular phosphatases that their natural substrate pool may turn over ∼120 times a day in Plešné Lake, while only eight times a day in Prášilské Lake (cf. turnover time in Table 3).
According to the above-mentioned assumption, one would expect a positive correlation between CSPAB and Al concentration at low pH, as it was in the case of bulk PA (Table 1). The analysis of particular phytoplankton populations, however, showed a negative correlation between CSPAB of the three species and both Alt and Ali concentration (Fig. 4). Moreover, the species-specific response to the analyzed factors was similar for P. umbonatum and Gymnodinium sp. Neither Al effect can satisfactorily explain the overall discrepancy of the dinoflagellates’ CSPAB. Vrba et al. (2006) also recognized Al as the key factor determining food web structure and plankton dynamics. Consequently, some indirect effect of Al is very likely superior to its direct interactions with the extracellular phosphatases, and the interlake differences in the plankton composition may better explain residual CSPAB variability.
Top-down effects of zooplankton. Unlike in Prášilské Lake, cladoceran zooplankton are entirely absent in the other two lakes under study (Vrba et al. 2003a,b), and these lakes further differ in both the composition and grazing efficiency of their zooplankton assemblages (Table 3, Nedbalová et al. 2006). While rotifers hardly can release much Pi in Čertovo Lake, abundant populations of both Cyclops abyssorum (recently reintroduced to the lake, see Kohout and Fott 2006) and indigenous Heterocope saliens likely may control the dinoflagellate populations as well as overall P regeneration in Plešné Lake. Therefore, lake internal P turnover might be higher than all indices implied (Table 3).
In Prášilské Lake, the summer shift toward the remarkable dominance of large G. uberrimum (cell diameter ∼35 μm; note also its largest MCV in this lake, Table 2) was also observed during previous studies and resulted from its resistance to the grazing of Daphnia longispina (Vrba et al. 2003b). Because of the permanent CSPAB detected in G. uberrimum, ELFA-labeled species formed almost 100% of the total phytoplankton biomass in this lake. In Čertovo Lake, the majority of phytoplankton biomass was formed by G. uberrimum and P. umbonatum, with the exception of two samples, when Dinobryon was dominant, likely taking advantage of its phagotrophy and motility (Vrba et al. 2003b). As a fast-growing colonial mixotrophic flagellate (Bird and Kalff 1987), Dinobryon frequently formed summer peaks in both Čertovo and Prášilské lakes (Vrba et al. 2003b, Nedbalová et al. 2006).
Plešné Lake showed a distinct phytoplankton structure (Nedbalová et al. 2006), with several-fold higher total biomass caused by higher P input from the watershed (cf. Table 3), which likely favored the nonmotile green alga Monoraphidium dybowskii and filamentous cyanobacteria Limnothrix sp. and Pseudanabaena sp. (Vrba et al. 2006). The dominance of M. dybowskii in this lake also can be owing to its tolerance to high Al concentrations (Hörnström et al. 1995). Surprisingly, the production of extracellular phosphatases was detected only rarely in M. dybowskii during this study, although this species was ELFA labeled in all earlier studies (Štrojsová and Vrba 2006, 2009). We assume this fact likely reflects high P regeneration due to recent zooplankton grazing (see above). The same effect (i.e., higher ambient Pi supply) perhaps could partly explain lower CSPAB of all the dinoflagellate species in Plešné Lake. Their low biomass was certainly influenced by the relatively rare occurrence of large G. uberrimum; this species was never found before in Plešné Lake despite extensive observations (Nedbalová and Vrtiška 2000, Vrba et al. 2003a, Nedbalová et al. 2006). Its low abundance and smaller size in this lake could be caused by a specific predation of Heterocope saliens, dwelling solely in the epilimnion of Plešné Lake.
The effect of light availability. Although we are aware of dinoflagellate motility (e.g., Reynolds 1997), different light conditions could provide another explanation of the controversial CSPAB results. If we consider the Zeu:Zmix ratio as a proxy of light availability for algae, its median <1 clearly indicates common light insufficiency in the epilimnion of mesotrophic Plešné Lake compared to the oligotrophic lakes (Table 3) (cf. Kalff 2002). Consequently, the dinoflagellates living in this mesotrophic lake could be limited by light rather than P. On the other hand, sufficient light conditions in the both oligotrophic lakes likely allowed for close to maximal CSPAB of all three species (Fig. 3, Table 3). Similarly, the highest CSPAB of all the dinoflagellates in Plešné Lake occurred in June (Fig. 2) and coincided with the highest Zeu:Zmix = 1.6 (Table 3).
Seasonal coincidence of high bulk alkaline PA with good light conditions (high Zeu:Zmix ratio) was observed in a dimictic eutrophic reservoir (Nedoma et al. 2006). Our study indicates the possible importance of light for the production of extracellular phosphatases in natural dinoflagellate populations. Light availability may change cell stoichiometry from P to C limitation (Sterner and Elser 2002). Ecological stoichiometry (growth rate hypothesis) also predicts a high need in P during intensive cell growth (Sterner and Elser 2002). Both the high P need and optimal light conditions likely caused parallel peaks of each species-specific PA in all the lakes in June–July, whereas the persistence of ice cover till late April, and thus lower intensity of phytoplankton growth and its lowered P demand, could cause relatively low values of PA in early May (Fig. 2). This new view should be considered in further studies on P-limited phytoplankton.
At the same time, insufficient light conditions favor mixotrophic species in the phytoplankton competition, and dinoflagellates are potential mixotrophs; in particular, bacterivory of G. uberrimum has been reported (Callieri et al. 2006, Niesel et al. 2007). Its grazing on the abundant bacterioplankton might be a rich P source in Plešné Lake. Such a parallel uptake of both P and C could be an efficient alternative strategy to phosphatase expression under light deficiency. If this was the case, the mixotrophy of G. uberrimum also would explain the weaker interlake correlations of its cell-specific PA and other parameters compared to the other two dinoflagellates under study.
Conclusions
In this study, we observed rather large interlake and interspecies variations in cell-specific PA of the dinoflagellate populations. Rengefors et al. (2003) and Štrojsová et al. (2008) explained similar results by different P demand of particular species, various internal P supply, or distinct physiological conditions of species (cf. Wynne 1981). In the acidified lakes under study, the P cycling is yet impaired due to the complex Al effects (Bittl et al. 2001, Vrba et al. 2006). The large variation in the contribution of ELFA-labeled dinoflagellates to the total phytoplankton biomass among the lakes is likely a result of the interplay between their acidification status, P input, zooplankton grazing, light penetration, as well as species’ ability of mixotrophy. On the other hand, species-specific PA showed rather similar seasonal courses in all of the lakes. Species-specific variations in CSPAB in a single lake indicate that the same species may experience different P stress not only in response to both the surrounding microenvironment and internal P status of individual cells, but also due to changing deficit of light energy.
The present protocol of the FLEA assay combined with image analysis provides a standard method for PA measurements at the single-species level. This first autecological insight into cell-specific PA of the dinoflagellates suggests a possible context of sufficient light demand to employ extracellular phosphatases (e.g., in contrast to bacterioplankton). Regarding P depletion in aquatic ecosystems, this context is critical for bulk PA interpretation and needs to be further explored within natural plankton.
Acknowledgments
This project was supported by the Czech National Foundation (project no. 206/07/1200), Grant Agency AS CR (project no. A600170602), and partly by the projects MSM 0021620828, 6007665801, and AV0Z60050516. We acknowledge the laboratory and field assistance provided by our colleagues and, in particular, the instant methodological assistance (image analysis) by Jiří Nedoma as well as his critical comments and discussions on the earlier versions of the manuscript. We also thank to Alena Štrojsová and four anonymous reviewers for critical reading of the manuscript.