TWO SPECIFIC CAUSES OF CELL MORTALITY IN FREEZE-THAW CYCLE OF YOUNG THALLI OF PORPHYRA YEZOENSIS (BANGIALES, RHODOPHYTA)1
Received 2 August 2009. Accepted 28 January 2010.
Abstract
Porphyra yezoensis Ueda is an important marine aquaculture crop with single-layered gametophytic thalli. In this work, the influences of thallus dehydration level, cold-preservation (freezing) time, and thawing temperature on the photosynthetic recovery of young P. yezoensis thalli were investigated employing an imaging pulse-amplitude-modulation (PAM) fluorometer. The results showed that after 40 d of frozen storage when performing thallus thawing under 10°C, the water content of the thalli showed obvious effects on the photosynthetic recovery of the frozen thalli. The thalli with absolute water content (AWC) of 10%–40% manifested obvious superiority compared to the thalli with other AWCs, while the thalli thawed at 20°C showed very high survival rate (93.10%) and no obvious correlation between thallus AWCs and thallus viabilities. These results indicated that inappropriate thallus water content contributed to the cell damage during the freeze-thaw cycle and that proper thawing temperature is very crucial. Therefore, AWC between 10% and 40% is the suitable thallus water content range for frozen storage, and the thawing process should be as short as possible. However, it is also shown that for short-term cold storage the Porphyra thallus water content also showed no obvious effect on the photosynthetic recovery of the thalli, and the survival rate was extremely high (100%). These results indicated that freezing time is also a paramount contributor of the cell damage during the freeze-thaw cycle. Therefore, the frozen nets should be used as soon as time permits.
Abbreviations:
-
- AWC
-
- absolute water content
-
- F 0
-
- intrinsic fluorescence
-
- F m
-
- maximum fluorescence yield
-
- F m′
-
- maximum fluorescence yield in illumination
-
- F v
-
- variable fluorescence
-
- F v/Fm
-
- maximal PSII quantum yield
-
- SP
-
- saturation pulse
-
- WC
-
- water content
-
- Y(NO)
-
- quantum yield of nonregulated energy dissipation
-
- Y(NPQ)
-
- quantum yield of regulated energy dissipation
-
- YII
-
- effective PSII quantum yield
Porphyra (Bangiales, Rhodophyta) is a genus that is distributed worldwide (Conway 1964, Brown et al. 1990, Griffin et al. 1999, Coll and Oliveira 2001). In some countries, the cultivation of certain species of Porphyra is of paramount importance to their marine aquaculture industry (Tseng 2001, Park et al. 2003, Niwa et al. 2005, 2008). P. yezoensis, one of the most widely cultivated Porphyra species, is cultured in China, Japan, and Korea (Zemke-White and Ohno 1999). Currently, it is usually cultured using the “pole system” or “floating system.” Whether the pole system or floating system is used, however, P. yezoensis thalli are cultured on synthetic nets, which are hung between poles fitted in the sea bottom or attached to buoys floating on the surface of the sea (Oohusa 1984).
The cold storage of seeded nets permits an extended growing season of algae (Lobban and Harrison 1994). In Porphyra aquaculture, a technique known as “frozen nets,” which means storing the cultivation nets carrying the young thalli of Porphyra at a very low temperature, was developed in 1965. In Japan, this technique has long been widely used to avoid adverse marine climatic conditions (Oohusa 1984, Miura 1992). The young Porphyra thalli with a length of 1–3 cm are withdrawn from the culture field together with the nets, dried to a certain degree in the air, and then put into vinyl bags and stored in a freezer at −20°C. The nets can be brought back to the culture field whenever it is needed to continue the culture cycle (Oohusa 1984, 1993). As it has helped farmers to avoid heavy production damages, the development of this method is of great importance (Oohusa 1984). More recently, this technique has also been found to be very useful in China (Zhang 1988, Chen 1994, Ma et al. 1998). However, the suggested dehydration levels of Porphyra thalli have been different in the literature (Chen 1994, Ma et al. 1998). Consequently, many aquaculture farmers still rely purely on experience, which inevitably leads to variation in the effectiveness of the cold storage. Thus, it is very important to reinvestigate the influence of the thalli’s dehydration level.
Both qualitative and quantitative methods have been employed to assess viability following the freezing and thawing of algae (Taylor and Fletcher 1999). However, due to the lack of sensitive and nondestructive monitoring approaches for viability assaying following the thawing of the cold-preserved thalli, the cryopreservation studies of seaweeds have been hindered (Day et al. 1998, Taylor and Fletcher 1999). Indirect methods, such as the uptake of a particular stain, have been used extensively (Kuwano et al. 1996, Liu et al. 2004, Zhou et al. 2007). Cell motility and oxygen evolution measurements are also used. But these simple methods are prone to overestimate the recovery potential (Day et al. 1998, Taylor and Fletcher 1999). As a photosynthetic organism, the photosynthetic recovery of P. yezoensis thalli after cold storage is of paramount importance for the recovery of the thalli after being brought back into sea, and the recovery conditions of thalli are closely connected to the output of the reentered culturing nets. However, to date, research into this aspect is rare in the literature. Therefore, it would be very useful to investigate the viability of the Porphyra thalli after freezing and thawing via photosynthetic parameters.
Chl a fluorescence technology has become an important noninvasive tool to investigate the utilization and dissipation of absorbed light energy in photosynthesis (Krause and Jahns 2004). With PAM fluorometry and saturation pulse method, a wide range of photosynthetic parameters can be obtained, giving insight into the physiological state of most photosynthetic organisms (Schreiber 2004). Using chl fluorescence imaging technique, with imaging PAM fluorometry, the heterogeneity of algal photosynthesis within a thallus can be investigated conveniently, and the photosynthetic investigation can reach the cellular level (Endo and Omasa 2004). Therefore, using imaging PAM fluorometry, the photosynthetic parameters of the single-layered P. yezoensis thalli can be obtained in an effective and convenient way. The aim of this study was to employ imaging PAM fluorometry to investigate the influences of dehydration level, freezing time, and thawing temperature on the recovery of the cold-preserved young P. yezoensis thalli.
Materials and Methods
P. yezoensis thalli were collected from the second bathing beach of Qingdao (36°08′ N, 120°58′ E) during low tide. Experiments were performed immediately after sampling. Healthy P. yezoensis thalli with length of 1–3 cm were chosen as study objects. Before experiments, the chosen thalli were thoroughly cleaned in sterilized seawater using a soft brush.
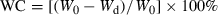 (1)
(1)Dehydration and WC determination of the dehydrated thalli. In total, 87 P. yezoensis thalli were chosen and evenly divided into three thallus groups. After removal of the thallus surface water, the chosen thalli were thoroughly spread on a piece of paper and dehydrated in the air. To achieve different degrees of dehydration of the thalli, random time intervals were applied to different Porphyra thalli within each group.
Immediately after dehydration, the thalli were weighed, sealed in self-sealing plastic bags, put into a freezer, and maintained at −20°C.
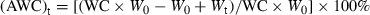 (2)
(2)Within each group, six P. yezoensis thalli (three of them with and three without surface water) were preserved in the same way and used as control, as their AWCs were the same (100%).
Thawing and cultivation of the P. yezoensis thalli after cold storage.
After 10 h of cold storage, the first group of P. yezoensis thalli was revived by plunging the frozen thalli into 10°C seawater and maintained at 10°C under irradiance of 15 μmol · m−2 · s−1, 12:12 light:dark (L:D) cycle.
The other two groups of thalli were revived after 40 d of cold storage. One group of the Porphyra thalli was also revived by plunging the frozen samples into 10°C seawater and maintained at 10°C; the other group of thalli was revived and maintained in 20°C seawater. The other culture conditions of the two groups of thalli were the same: irradiance 15 μmol · m−2 · s−1, 12:12 L:D.
 (3)
(3)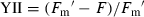 (4)
(4) (5)
(5) (6)
(6)Results
After thawing and short recovery, the photosynthetic capability of the Porphyra thalli was often unevenly distributed within the thalli. The basal parts were generally of higher photosynthetic capabilities compared with the upper parts of the frozen thalli (Fig. 1). Therefore, to one specific photosynthetic parameter, the average value of the whole thallus was used to represent the thallus’s value of the photosynthetic parameter.
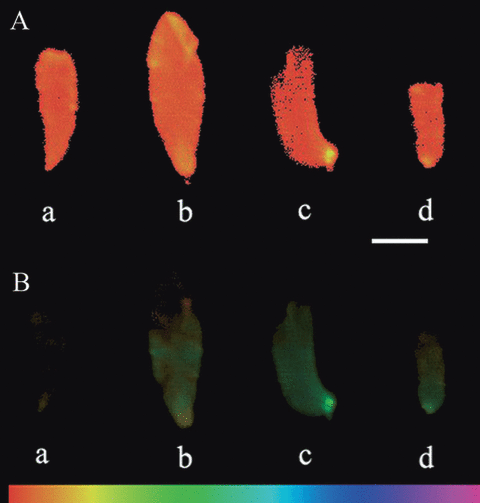
Pulse-amplitude-modulation (PAM) images of representative Porphyra yezoensis thalli thawed and recovered 12 h under 10°C after 40 d of cold storage. (A). The intrinsic fluorescence (F0) image of the thalli. (B) The maximal PSII quantum yield (Fv/Fm) image of the thalli. The absolute water contents (AWCs) of the thalli were 4% (a), 10% (b), 40% (c), and 43% (d), respectively, before being frozen. The color of the figures, as noted on the bottom of (B), indicates the value of the parameters. The average Fv/Fm values of thallus (a), (b), (c), and (d) were 0, 0.178, 0.387, and 0.247, respectively. Scale bar, 1 cm.
It can be seen in Figure 2A that in terms of maximal PSII quantum yield, Fv/Fm, after 10 h of frozen storage, no obvious difference can be found between the P. yezoensis thalli with different AWCs. The overall viability of the recovered thalli was extremely high; the survival rate was 100%, and the mortality rate was zero. As can be seen in Figure 2A, in terms of Fv/Fm, the six thalli with AWC of 100% were almost evenly distributed vertically, and no obvious differences could be seen among the Porphyra thalli that were frozen with or without surface water. Thus, for the short-term cold-preserved thalli, the photosynthetic performances of the differently dehydrated thalli showed no obvious differences with each other.
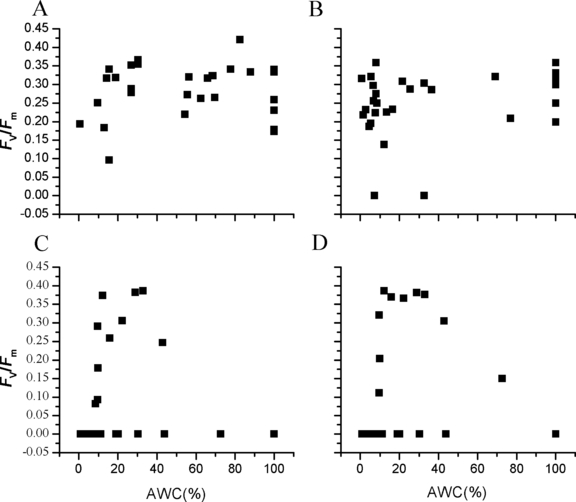
The maximal PSII quantum yield (Fv/Fm) of thawed and recovered Porphyra yezoensis thalli. (A) The Fv/Fm of P. yezoensis thalli thawed and recovered 0.5 h under 10°C after 10 h of cold storage. (B) The Fv/Fm of P. yezoensis thalli thawed and recovered 12 h under 20°C after 40 d of cold storage. (C) The Fv/Fm of P. yezoensis thalli thawed and recovered 12 h under 10°C after 40 d of cold storage. (D) The Fv/Fm of P. yezoensis thalli thawed and recovered 80 h under 10°C after 40 d of cold storage. AWC, absolute water content.
It is shown that after 40 d of cold storage, for the P. yezoensis thalli thawed and recovered at 20°C, no obvious difference of Fv/Fm value could be seen between the P. yezoensis thalli with different AWCs. The overall viability of the recovered thalli was also extremely high, and the survival rate was very high (93.10%), as only two of the 29 thalli showed zero Fv/Fm value. The six thalli with AWC of 100% were also almost evenly distributed, and no obvious differences could be seen among the Porphyra thalli that were frozen with or without surface water (Fig. 2B).
However, within the group of Porphyra thalli that were thawed and recovered at 10°C, there was an obvious correlation between thallus photosynthetic capability and the AWCs of the thalli. After 10 h of recovery, the P. yezoensis thalli with AWCs between 10% and 45% had the highest maximal PSII quantum yield. In comparison with recovery status of the thalli that were frozen for 10 h and recovered at 10°C, the overall viability of the recovered thalli was relatively low, and the survival rate was also low (37.93%). Eighteen of the 29 thalli showed zero Fv/Fm value. The six thalli with AWC of 100% all showed zero Fv/Fm value, and no difference can be seen among the Porphyra thalli that were frozen with or without surface water (Fig. 2C). Therefore, the low survival rate and the low viability of the thalli were caused by the relatively longer term of preservation.
It is shown that after 80 h of recovery, compared to the thalli with other AWCs, the P. yezoensis thalli with AWCs between 10% and 40% had the highest maximal PSII quantum yield. And Fv/Fm of the thalli with AWCs between 10% and 40% were of almost the same level. The six thalli with AWC of 100% all showed zero Fv/Fm value, and no difference could be seen among the Porphyra thalli that were frozen with or without surface water (Fig. 2D).
The distribution of the effective PSII quantum yield, YII, of the 40 d preserved P. yezoensis thalli, which were thawed and recovered for 12 h at 10°C, were very similar with the maximal PSII quantum yield (Fv/Fm) of P. yezoensis thalli. The P. yezoensis thalli with AWCs between 10% and 40% also showed comparably higher YII value. This result indicated that the photosynthetic parameter, Fv/Fm, can effectively represent the real photosynthetic capabilities of the recovered Porphyra thalli (Fig. 3).
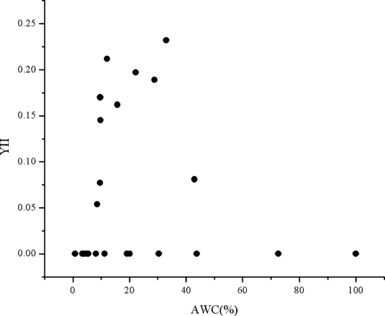
The effective PSII quantum yield (YII) of Porphyra yezoensis thalli thawed and recovered 12 h under 10°C after 40 d of cold storage. AWC, absolute water content.
A high Y(NPQ), quantum yield of regulated energy dissipation, value shows that the sample has retained the physiological means to protect itself by regulation. After being thawed and recovered 12 h at 10°C, the P. yezoensis thalli manifested relatively higher Y(NPQ) values when the thallus AWCs were within 10%–40%. And the Y(NPQ) values of the thalli were the highest when the thallus AWCs were ∼30%. The six thalli with AWC of 100% all showed zero Y(NPQ) value, and no difference could be seen among the Porphyra thalli that were frozen with or without surface water (Fig. 4A).
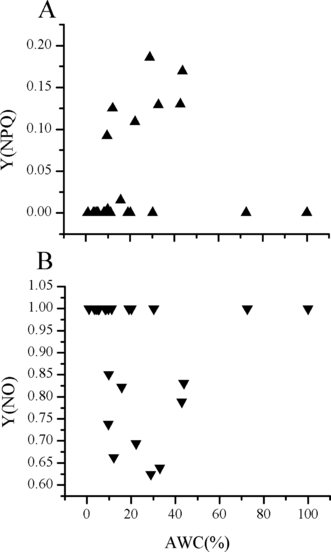
The quantum yield of regulated and nonregulated energy dissipation of thawed and recovered Porphyra yezoensis thalli. (A) The quantum yield of regulated energy dissipation, Y(NPQ), of P. yezoensis thalli thawed and recovered 12 h under 10°C after 40 d of cold storage. (B) The quantum yield of nonregulated energy dissipation, Y(NO), of P. yezoensis thalli thawed in 10°C seawater and 12 h of recovery under 10°C after 40 d of cold storage.
After 12 h of recovery, the quantum yield of nonregulated energy dissipation, Y(NO), values of P. yezoensis thalli were lower when the thallus AWCs were within 10%–40%, and the Y(NO)s were the lowest when the thallus AWCs were ∼30%. A high Y(NO) value indicates that both photochemical energy conversion and protective regulatory mechanisms are inefficient. Thus, it is indicative of the plant having serious problems coping with the incident radiation. Either it is already damaged or it will be photodamaged upon further irradiation. Therefore, the thalli with AWCs ∼30% were with the least-damaged photosynthetic apparatus. The Y(NO) value of the six thalli with AWC of 100% were all one, and no difference could be seen among the Porphyra thalli that were frozen with or without surface water (Fig. 4B).
Discussion
Desiccation has been reported to improve thermotolerance capability of the intertidal algae (Hunt and Denny 2008). When performing thallus thawing under 10°C, the water content of the preserved thalli, which were kept at −20°C, showed obvious effects on the recovery of the P. yezoensis thalli. In terms of photosynthetic performances, after 40 d of cold storage, at −20°C, the thalli with AWCs of 10%–40% manifested obvious superiority in comparison with the others. This result showed that AWC of 10%–40% is the suitable thallus water content range for the frozen storage of P. yezoensis thalli; and thallus AWC <10% or >40% has negative effects on the photosynthetic recovery of the Porphyra thalli. Our results showed that the photosynthetic capabilities of the Porphyra thalli were the highest when the thalli were desiccated to AWC ∼30%. Therefore, when using frozen nets, it is better to dehydrate the thallus AWCs to ∼30%. And it is very important that the Porphyra thallus AWCs should not be >40% or <10%.
Pearson and Davison (1994) showed that the physiological responses of this brown alga Fucus distichus to osmotic dehydration and freezing stress were similar, and these responses resemble the responses of desiccation in air. It is well known that during desiccation, osmotic concentration increases. The hypothesis presented by Muldrew and McGann (1994) is that the osmotically driven water efflux that occurs in cells during freezing is responsible for some of the cell damage, and the pressure that develops during freezing due to water flux is found to be sufficient to cause a rupture of the plasma membrane; Dumont et al. (2006) noted that the crystallization of the solutes within the cells was one of the important reasons that caused the cell mortality during freeze-thaw cycles. Thus, after proper desiccation, with the upgraded osmotic concentration in the thallus cells, the water efflux was inhibited partially or completely. Consequently, cell damage caused by such water efflux was prevented completely or partially. On the other hand, a higher osmotic concentration can also lower crystallization temperature of the solution. Dumont et al. (2006) showed that at −20°C relatively lower cell water content can effectively prevent cell mortality of the cold-preserved cell. Furthermore, compatible solutes, such as K+, glutamate, and glutamine, accumulate away from proteins, forcing water nearby and thus stabilizing them and possibly stabilizing dry membranes (Rothschild and Mancinelli 2001). Therefore, it is possible that when the AWCs of the Porphyra thalli were ∼10%–40%, the osmotic concentrations within the thallus cells were better balanced and the cell membranes were in a better shape.
Shen and Dai (2000) pointed out that temperature was very important for the reviving of the cells of P. yezoensis after cold storage and that the suitable temperature range for the thallus reviving was 10°C–20°C. However, our results showed that in comparison with the thalli that were thawed at 10°C, when performing thawing under 20°C, the correlations between thallus photosynthetic capability and the AWCs of the thalli disappeared almost completely. The relatively higher seawater temperature had positive effects on the recovery of Porphyra thalli: 10 h after thawing, under relatively higher seawater temperature (20°C), no obvious influence of thallus water content was observed. The P. yezoensis thalli thawed at 20°C showed obvious photosynthetic superiority compared to the thalli thawed at 10°C. The results of Cañavate and Lubian (1997) showed that rapidly warmed cryopreserved microalgae cells have an obvious advantage in cell viability in comparison with the cells that were warmed slowly. Dumont et al. (2006) also showed that cell viability could be considerably improved with an increased warming rate. It is proposed that when thawing is fast enough, the ice crystals in the cells melt before growing into injurious sizes (Sakai and Otsuka 1967). Therefore, it is possible that the evaluated thawing rate under the relatively higher temperature better preserved the Porphyra cells’ viability. This result indicated that proper thawing temperature is crucial and that the thawing process should be as short as possible. And it is suggested that thawing and short recovery in seawater of proper controlled temperature before the use of the frozen nets may improve the photosynthetic performance of the revived P. yezoensis thalli.
Our results also showed that, compared to relatively long-term cold storage, water content showed no obvious effect on the recovery of the P. yezoensis thalli, after relatively short-term (10 h) cold storage; after recovery for half an hour at 10°C, the viability of the thalli was very high, and the mortality rate was very low. These results indicated that although not preserved with appropriate water content, the P. yezoensis thallus cells are prone to be damaged during the freeze-thaw cycle; however, the cells are not necessarily damaged after thawing. On the other hand, another important function of frozen nets is to get rid of attached unwanted algae, such as Ulva sp., the most commonly seen competitive algae in Porphyra cultivation farms. They often can be removed after the cold preservation because these algae cannot survive the frozen preservation as well as P. yezoensis thalli. Previous results have shown that the mortality rates of these competitive alga thalli are closely related to the freezing time, and the longer the time, the higher the mortality rates of the thalli of these algae (Chen 1994, Ma et al. 1998).
It is reported that the rates of most chemical reactions during freezing decrease exponentially with decreasing temperature (Taylor and Fletcher 1999). Warren et al. (1997) suggested that to prevent cell deterioration, the temperature of at least −135°C is necessary. Below this temperature, the degradative enzymatic reactions continue to occur in all frozen cells, resulting in a continual loss of viability with time (Taylor and Fletcher 1999). Thus, preserved at −20°C, the Porphyra thallus cells were undergoing continuous deterioration during the whole cold-storage process. Therefore, when using the frozen nets technique in P. yezoensis cultivation, the cell damage during the freeze-thaw cycle and the cell deterioration during the freezing period are all responsible for cell damage after reviving. Taking this into consideration, we suggest that the frozen nets should be used as soon as time permits.
Acknowledgments
The work was supported by the National Natural Science Foundation of China (30830015), Project for Supporting the National Development (No. 2006BAD09A04), and the 863 Project of China (Nos. 2006AA10A413, 2006AA10A402, 2007AA09Z406).