COORDINATED CAROTENOID AND LIPID SYNTHESES INDUCED IN PARIETOCHLORIS INCISA (CHLOROPHYTA, TREBOUXIOPHYCEAE) MUTANT DEFICIENT IN Δ5 DESATURASE BY NITROGEN STARVATION AND HIGH LIGHT1
Received 1 August 2009. Accepted 28 January 2010.
Abstract
The responses to PAR intensity and nitrogen deficiency have been investigated in the Δ5-desaturase-deficient mutant (P127) of the microalga Parietochloris incisa (Reisigl) Shin Watan. (Chlorophyta, Trebouxiophyceae). The mutant accumulates dihomo-γ-linolenic acid (DGLA, C20:3 ω6) instead of arachidonic acid (C20:4 ω6) characteristic of the wildtype. The growth, fatty acid and pigment composition, and light absorption by P127 cell suspensions were studied for the first time during cultivation on complete and N-free BG-11 medium at 35, 130, and 270 μE · m−2 · s−1. On complete medium under high irradiance, an increase in biomass was observed, and total fatty acid (TFA) and DGLA contents were higher than in N-starving cultures. A distinct irradiance-dependent rise in carotenoid-to-chl ratio was recorded in P127 due to an increase in carotenoids (on complete medium) or by a decline in chl (on N-free medium). Cultivation under high and medium irradiances caused a decline in light-harvesting xanthophylls and an increase in β-carotene, localized predominantly in cytoplasmic oil bodies (OB). The P127 mutant, similar to wildtype, responded to the stresses by coordinated induction of fatty acid and carotenoid syntheses, but displayed the same magnitude of the response as was observed in wildtype under 30% lower irradiance. The changes in optical properties of the P127 cultures tightly correlated with their pigment composition, and hence with fatty acid content, making it possible to develop a nondestructive technique for the assay of TFA and DGLA. The peculiarities of the stress responses in the wildtype and the mutant are discussed.
Abbreviations:
-
- AA
-
- arachidonic acid
-
- Antn
-
- antheraxanthin
-
- car
-
- carotenoid(s)
-
- chl
-
- chlorophyll(s)
-
- D(λ)
-
- spectrum of optical density
-
- DGLA
-
- dihomo-γ-linolenic acid
-
- DW
-
- dry weight
-
- D λ
-
- optical density at wavelength λ
-
- OB
-
- oil bodies
-
- PUFA
-
- polyunsaturated fatty acids
-
- RMSE
-
- root mean square error
-
- STD
-
- standard deviation
-
- TAG
-
- triacylglycerols
-
- TFA
-
- total fatty acids
-
- Vio
-
- violaxanthin
-
- WT
-
- wildtype
-
- Zea
-
- zeaxanthin
Stress-induced coordinated synthesis of nonmembrane lipids, such as triacylglycerols (TAG), and carotenoids (car) (Whitelam and Codd 1986), was documented in several algal species including chlorophytes dwelling in harsh natural environments, such as halotolerant Dunaliella salina (Ben-Amotz et al. 1982, Pick 1998, Rabbani et al. 1998, Mendoza et al. 1999, Borowitzka and Siva 2007), Haematococcus pluvialis under high light and nitrogen stress (Boussiba 2000, Zhekisheva et al. 2002, Wang et al. 2003), and Parietochloris incisa isolated from a high altitude (Merzlyak et al. 2007, Solovchenko et al. 2008a,b). Some lines of evidence suggest that accumulation of the neutral lipids in cytoplasmic OB facilitates adaptation to the unfavorable conditions by serving as the sink for the excessive photosynthates and as a source of energy (Thompson 1996) and polyunsaturated fatty acid (PUFA) moieties during growth restoration (Khozin-Goldberg et al. 2005). In P. incisa, OB serve also as the depot for the extraplastidic secondary car, which are supposed to provide photoprotection via screening chloroplasts from the excessive light (Merzlyak et al. 2007, Solovchenko et al. 2008a).
Previously, we determined that high light and nitrogen deprivation trigger coordinated lipid and pigment syntheses in the green oleaginous freshwater microalga P. incisa (Trebouxiophyceae, Chlorophyta) modulated by the illumination intensity during cultivation of the alga. A rapid increase in car/chl ratio observed under high-light conditions was accompanied by a buildup of high amounts of TAG enriched in the ω-6 arachidonic acid (AA; see Khozin-Goldberg et al. 2002), along with a deposition of β-carotene in the OB (Solovchenko et al. 2008a). Remarkably, the changes in spectral absorption of the alga accompanying the alteration of pigment composition appeared to be tightly related with the dynamics of both TFA and AA, making it possible to estimate the lipid content nondestructively in cell suspensions (Solovchenko et al. 2008a, 2009).
Recently, a Δ5-desaturase mutant of P. incisa (P127) was obtained in the Laboratory of Microalgal Biotechnology (Ben-Gurion University, Israel), which was shown to be almost incapable of desaturating (DGLA) to AA (I. Khozin-Goldberg and Z. Cohen, personal observation). The mutant accumulates high contents of DGLA (mainly incorporated in TAG), up to 39% of TFA and 14% of dry weight (DW) and only traces of AA. In fungi, algae, and invertebrates, DGLA normally occurs only as an intermediate in AA biosynthesis and does not accumulate in any appreciable amounts. DGLA has pharmacological significance related to its anti-inflammatory activity and for treating atopic eczema, psoriasis, asthma, and arthritis (Fan and Chapkin 1998). Therefore, the mutant could serve as a potential source of the nutraceutically important PUFA. In this context, it was interesting to study the effects of light and nitrogen deficiency on the pigment and lipid metabolism of P. incisa and elucidate to what extent Δ5-desaturase deficiency, and the decrease in TAG unsaturation caused by it, modifies the stress responses in the mutant. At the same time, this information is important for estimating the impact of the altered lipid metabolism on high-light tolerance and optimizing the conditions for the production of DGLA. In this study, we report for the first time the interrelationships between fatty acid accumulation, changes in pigment content and composition, and optical properties of the P127 mutant cells.
Materials and Methods
Cultivation conditions. The Δ5-desaturase mutant of P. incisa, P127, was obtained in the Microalgal Biotechnology Laboratory, J. Blaustein Institutes for Desert Research by nitrosoguanidine mutagenesis (NNG). The mutant was cultivated on complete, [+N], and nitrogen-free, [−N], BG-11 medium (Stanier et al. 1971) in 1 L glass columns (6 cm ID) under constant illumination (by daylight fluorescent lamps) of three different intensities (35, 130, and 270 μE · m−2 · s−1 PAR as measured in the center of the empty column) and with constant bubbling of CO2: air mixture (1: 99, v/v) at 25°C. Prior to the experiment, cultures were daily diluted to maintain logarithmic growth. In all cases, initial chl content and DW were maintained at 30 mg · L−1 and 1 g · L−1, respectively, to prevent photodamage of the cultures at high irradiance (Solovchenko et al. 2008a). The nitrogen content in the medium was checked during the experiment using the nitrate assay kit (Merckoquant 1.10020.001, Merck, Darmstadt, Germany). For nitrogen-starvation, cells were washed three times with sterilized distillated water and resuspended in [−N] BG-11. The DW, TFA, and pigment contents and composition (see below) were determined at d 0 and following 3, 7, 10, and 14 d of growth.
Fatty acid analysis. Capillary gas-chromatography was used for fatty acid quantification; the analysis was performed according to Cohen et al. (1993). The data shown represent mean values with a range of <5% for major peaks (>10% of fatty acids) and 10% for minor peaks, of at least two independent samples, each analyzed in duplicate.
Isolation thylakoids and OB. Thylakoid- and OB-enriched fractions were isolated from the cells of 14 d cultures following the rupture of the biomass in liquid nitrogen as described in (Solovchenko et al. 2008a). The OB fractions were immediately extracted with 4 mL of diethyl ether and the thylakoids—with 5 mL of chloroform-methanol mixture (2:1, v/v) per 2 mL of the thylakoid fraction. After centrifugation (Beckman L5-75B, Beckman Instruments Inc., Palo Alto, CA, USA) of the extracts for 10 min at 1,500g for phase separation, the chloroform phase was collected, evaporated in a stream of nitrogen to dryness, and redissolved in acetone for further analysis. All manipulations were carried out under dim illumination at 4°C.
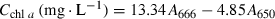 (1)
(1)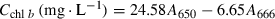 (2)
(2) (3)
(3) (4)
(4)Spectral measurements. The spectra of P127 cell, thylakoid, and OB extracts were recorded with the Cary 50 Bio spectrophotometer. Due to rapid cell sedimentation, the optical properties of the P. incisa P127 suspensions were determined using glass-fiber filter technique (Mitchell 1990). The spectra were taken upon cell deposition on 25 mm GF/F glass-fiber filters (Schleicher & Schuell Inc., Keene, NH, USA) against an empty filter soaked in BG-11 medium. The wet filters were mounted as overheads (deposited cells facing the detector) on the output windows of cuvette compartment of the spectrophotometer. Optical density (D) of the filters was expressed as −log10T, where T is transmission.
Statistical treatment. The experiments were carried out in two biological replications with three analytical replications for each of them. In the figures, average values together with standard deviations are presented. The significance of differences was tested using analysis of variance (ANOVA) via Microsoft Excel (Microsoft Corp., Redmond, WA, USA).
Results
Accumulation of biomass. The time-course of the changes in biomass of the P. incisa P127 cultures under different conditions is plotted in Figure 1. Under low PAR (LL, 35 μE · m−2 · s−1) intensity, the cultures showed relatively slow increase in biomass (Fig. 1, curves numbered 1) regardless of the medium composition (average increase rates of ca. 0.16 mg DW · d−1 for LL–N and LL+N) and attained DW of ca. 2.2 g · L−1 by the end of cultivation period (14 d). Under irradiances of 130 μE · m−2 · s−1 (medium light, ML), the [ML+N] cultures possessed higher final biomass (ca. 6.1 g · L−1 by 14 d at the average rate of 0.47 mg DW · d−1) than [ML−N] cultures; the latter reached DW of ca. 4.1 g · L−1 at the average rate of 0.29 mg DW · d−1 (Fig. 1, curves numbered 2). Under high PAR irradiance (HL, 270 μE · m−2 · s−1), the [HL+N] cultures reached the highest biomass (ca. 7 g · L−1; curve 3 in Fig. 1a) at the average rate of 0.47 mg DW · d−1, whereas the biomass and its accumulation rate in the [HL−N] culture did not differ significantly from [ML−N] (cf. curves 2 and 3 in Fig. 1b).

Biomass accumulation by P127 mutant on complete (a) and N-free (b) medium under low (1), medium (2), and high (3) irradiance. DW, dry weight.
Fatty acid accumulation. In all cultures except for [LL+N], the volumetric content of TFA built up with time along with an increase in DW (Fig. 2). In cultures grown on complete medium, the extent of TFA accumulation depended on the irradiance. Specifically, in the [LL+N] culture, TFA did not show significant changes (Fig. 2, a and c, curves numbered 1). In [ML+N], the TFA percentage increased, after a 5% dip for the first 3 d, reaching 20% by day 14 (Fig. 2a, curve 2); volumetric content in this case also increased (Fig. 2c, curve 2). The highest volumetric contents of TFA were detected in the [HL+N] cultures (Fig. 2c, curve 3).
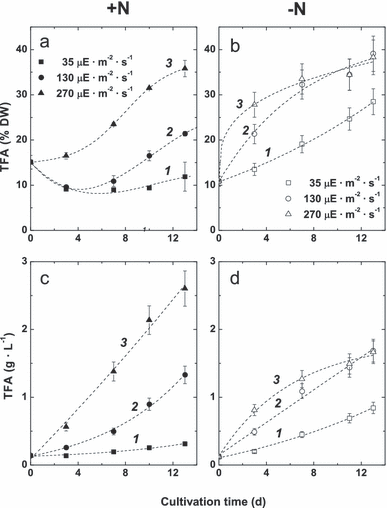
The dynamics of total fatty acid (TFA) percentage (a, b) and volumetric content (c, d) in the cells of the P127 mutant grown on complete (a, c) and N-free (b, d) medium under low (1), medium (2), and high (3) illumination. DW, dry weight.
In all cultures grown on N-free medium, TFA content continuously increased (Fig. 2, b and d). The [LL−N] cultures accumulated more TFA than the corresponding [+N] cultures (cf. Fig. 2, b and d, and Fig. 2, a and c, curve 1). The [ML−N] and [HL−N] cultures demonstrated a similar increase in TFA percentage (Fig. 2b, curves 2, 3) close to that of the [HL+N] cultures (Fig. 2a, curve 3). Due to slow down of an increase in biomass (Fig. 1), cultivation under [HL−N] and [ML−N] conditions resulted in a lower volumetric contents of TFA (ca. 1.5 g · L−1; Fig. 2d, curves 2, 3), which was only a little higher than in the case of [ML+N] (Fig. 2c, curve 2). Notably, the [HL+N] cultures accumulated ca. 40% more TFA per unit volume than the corresponding nitrogen-starved cultures (cf. curves numbered 3 in Fig. 2, c and d). However, under ML and LL, the TFA volumetric contents were higher in the [−N] cultures.
The DGLA percentage on a DW basis demonstrated a linear relationship (r2∼ 0.80) with the percentage of TFA (Fig. 3). Volumetric accumulation of DGLA followed approximately the same trends as TFA (not shown). Accordingly, the highest percentages of DGLA were seen in the [HL+N] and [HL−N] cultures, whereas the highest volumetric DGLA content was observed in the case of [HL+N] (Fig. 3).
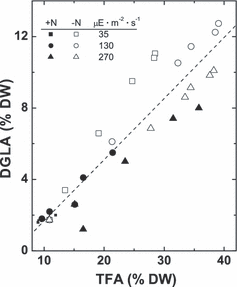
Relationships between total fatty acid (TFA) and dihomo-γ-linolenic acid (DGLA) content in P127 mutant biomass grown on complete (closed symbols) and N-free medium (open symbols) at different irradiance. DW, dry weight.
Changes in the contents of chl and car and their ratio. The dynamics of chl and car contents as well as their ratios are shown in 4, 5. Relatively slow and nearly linear increase in chl was observed in the [LL+N] cultures (Fig. 4a, curve 1). Chl content in the [HL+N] cultures peaked on day 7 of cultivation, reaching 75 mg · L−1, and underwent a gradual decrease thereafter, apparently manifesting the depletion of the nitrogen from the medium (Fig. 4a, curve 3). The [ML+N] cultures possessed the highest chl content, reaching ca. 130 mg · L−1 by day 10 (Fig. 4a, curve 2). The chl content in cultures grown on N-free medium (Fig. 4b) declined, after a moderate increase during the first 3 d of cultivation, as in the case of [LL−N] and [ML−N] (Fig. 4b, curves 1 and 2, respectively), or immediately after the start of the experiment ([HL−N]; Fig. 4b, curve 3). Regardless of the experimental conditions, the chl a/b ratio did not change significantly and remained in the range of 2.3–2.5 (not shown).
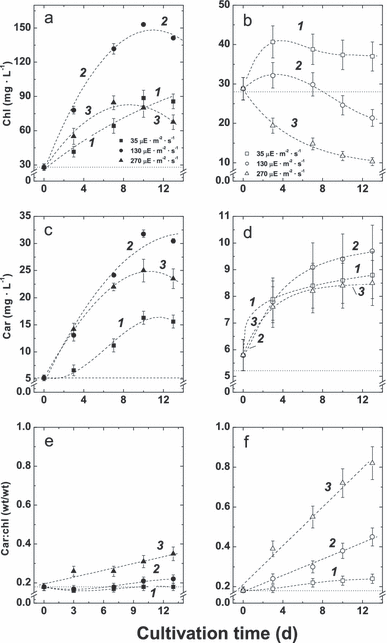
The dynamics of total chl (a, b) and carotenoid (c, d) contents and their ratio (e, f) in the cells of the P127 cells grown on complete (a, c, e) and N-free medium (b, d, f) at low (1), moderate (2), and high (3) irradiance. Car, carotenoids.
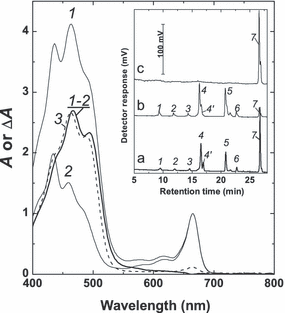
Normalized to the red chl maximum absorbance spectra of chloroform extracts of the P127 cells grown for 14 d on complete medium at high (270 μE · m−2 · s−1; curve 1) and low (35 μE · m−2 · s−1; curve 2) irradiances, and their difference spectrum (1–2). Curve 3 is the spectrum of the hexane extract of oil bodies isolated from the cells used for recording the spectrum 1. Inset: HPLC chromatograms of total pigment extract from the P127 cells used for recording the spectrum 1 (a) as well as thylakoids (b) and oil bodies (c) isolated from the same cells. Detection at 455 nm. 1, neoxanthin; 2, violaxanthin; 3, antheraxanthin; 4, lutein; 4′, zeaxanthin; 5, chl b; 6, chl a; 7, β-carotene.
On complete medium, a distinct irradiance-dependent increase in car content took place (Fig. 4c). A moderate increase in car following an S-shaped trend was recorded in the [LL+N] culture (Fig. 4c, curve 1); cultures grown at medium and high irradiances showed steeper increase in car content, which was the highest in the [ML+N] culture (Fig. 4c, curve 2) and less pronounced, tending to decline after day 10 in the [HL+N] culture (Fig. 4c, curve 3). Under N-deprivation, car content was considerably lower relative to that observed during cultivation on complete medium and did not exceed 11 mg · L−1 in all these cases (Fig. 4d).
According to the dynamics of the changes in chl and car contents described above, the ratio of those pigments depended on cultivation conditions (Fig. 4, e and f). Thus, in the [LL+N] and [ML+N] cultures, the car/chl ratio did not change significantly (Fig. 4e, curves 2, 1) and remained almost the same on the background of a considerable increase in chl. The [HL+N] culture displayed ca. 50% increase in the car/chl ratio (Fig. 4e, curve 3), which rose more rapidly with an increase in chl. The most pronounced (up to 4-fold in [HL−N]) irradiance-dependent rise in the car/chl ratio was recorded in the absence of N: the higher the PAR irradiance, the higher the extent of a linear increase in car/chl with cultivation time (Fig. 4f). As could be seen from Figure 5, the car/chl ratio rapidly increased along with the decline in chl following similar nonlinear trend regardless of the irradiance level.
Car composition of the P127 cells, thylakoids, and OB. The proportions of the individual carotenes and xanthophylls were monitored at the beginning and at the end of the experiment (day 14). Regardless of cultivation conditions, car of the P127 was composed of neoxanthin, violaxanthin, anteraxanthin, lutein, zeaxanthin, and β-carotene (Fig. 5). The car profile of the P127 underwent similar irradiance-dependent changes in the [+N] and [−N] cultures. Among these changes, the most conspicuous was a decrease in lutein and an increase in β-carotene at higher irradiances (see, e.g., Fig. 5, inset); these trends were more pronounced in the [−N] cultures.
To reveal the subcellular localization of pigments, their composition was determined in isolated thylakoid- and OB-enriched fractions (see inset in Fig. 5). On the whole, the car composition of the thylakoid fraction resembled that of whole cells with significantly lower proportion of β-carotene (cf. curves a and b, the inset of Fig. 5). On the contrary, β-carotene was the dominant car in OB (inset in Fig. 5, curve c) regardless of cultivation conditions. Only traces of xanthophylls and chl (probably due to contamination of the OB preparations with chloroplast membranes in the course of isolation) were detected in OB. The changes in absolute β-carotene content in OB and thylakoids followed the same trends as total car content determined for the whole cells (Fig. 4, c and d). The highest and lowest β-carotene contents were found in the OB isolated from [HL+N] and [LL+N] cells, respectively. By 14 d of cultivation under high-light conditions, ca. 70% of the total β-carotene was localized in the OB, whereas that of total β-carotene and that of thylakoid-localized β-carotene amounted to ca. 30% (inset in Fig. 5).
Relationships between the changes in fatty acid and pigment contents and ratio. The car/chl ratio exhibited a tight, nonlinear relationship with TFA contents per DW or suspension volume unit (Fig. 6; r2 = 0.86–0.99) regardless of the cultivation conditions, whereas a weak (r2 < 0.3) correlation was found between individual car or chl content and volumetric content of TFA (not shown). The relationships “TFA%DW versus [car]/[chl]” (Fig. 6a) and “TFAvol versus [car]/[chl]” for all cultures were successfully fitted with exponentials (Fig. 6b).
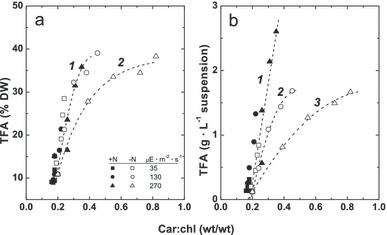
Relationships between carotenoid-to-chl ratio and total fatty acid (TFA) percentage (a) or volumetric content (b) in the P127 cells grown on complete (closed symbols) and N-free medium (open symbols) at different irradiation levels (shown on the figure). Car, carotenoids; DW, dry weight.
Relationships between lipid and pigment content and algal cell spectral absorption. Figure 7 displays the spectra of P127 cells recorded on the glass-fiber filters, normalized at 678 nm, D(λ) · D678−1. The difference, ΔD(λ) · D678−1, spectra (see insets in Fig. 7) contained features in the 600–720 nm range evident of progressive narrowing of the red chl absorption peak and a shift of its maximum toward shorter wavelengths. As could be seen, an increase in car/chl ratio taking place in P127 under higher irradiances (Fig. 4) was accompanied by a considerable increase in absorption in the blue region of the spectrum. This effect was weaker in the [+N] cultures (Fig. 7a), whereas in the [−N] cultures, it was two to three times higher (Fig. 7b). The difference spectra (insets in Fig. 7) had maxima near 480, 460, and 420 nm, attributable to car.
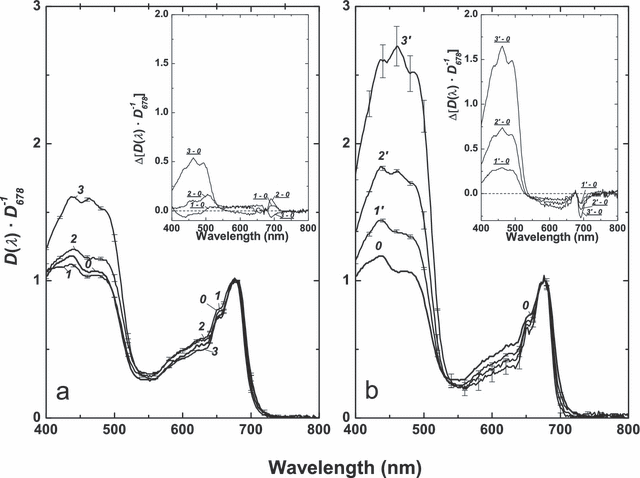
Spectra of optical density of GF/F glass-fiber filters with P127 cells deposited on them for the inoculum (0) and cultures grown for 14 d on complete (a) and N-free (b) media at low (1, 1′), medium (2, 2′), and high (3, 3′) irradiances. Average spectra (n = 6) normalized to the red chl maximum are presented. Bars correspond to STD. Insets: corresponding difference spectra.
The relationship of D(λ) with absolute car or chl content was weak (r2 < 0.5; not shown); by contrast, that of “car/chl versus D(λ) · D678−1” was characterized by a tight correlation, which varied from strong negative (–0.98) in the 600–700 nm band governed by chl absorption to strong positive (>0.99) in the region of combined absorption by car and chl (Fig. 8). As a result of a tight interrelationship between the car/chl ratio, TFA and DGLA contents (3, 6), a strong correlation was found between the volumetric content as well as percentages of the FA contents and D(λ) · D678−1 (Fig. 8). For both [+N] and [−N] cultures, the highest correlation (r2 > 0.95) was found in the blue-green range of the spectrum, whereas negative peaks were observed in the red region of the spectrum dominated by chl absorption. In the NIR, the region where pigments do not absorb, a weak correlation (r < 0.5) was observed.
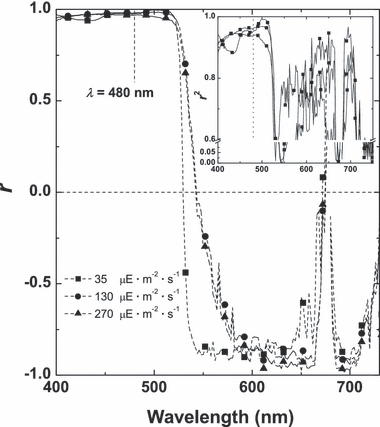
Spectra of correlation coefficient between total fatty acid (TFA) content and normalized to the red chl maximum spectral absorption of the cells of P127 mutant grown on N-free medium deposited on glass-fiber filters calculated for all sampling times.
The correlation spectra, r{D(λ) · D678−1 vs. [DGLA]}, were essentially the same as those, r{D(λ) · D678−1 vs. [TFA]}, presented in Figure 8. However, the parameters of approximation of these relationships for the [+N] and [−N] were different. In the 400–500 nm band containing high values of correlation coefficient (see, e.g., 8, 9), exponential models provided the best fit of the “D480 · D678−1 versus [TFA%DW]” (Fig. 9a). Essentially, the same models related the percentage and volumetric content of TFA with D480 · D678−1 (Fig. 9, a and b, curves numbered 1); at the same time, the relationship “D480 · D678−1 versus [TFAvol]” was linear (Fig. 9b, curve 2). Based on these findings, different algorithms for estimation of the contents of TFA and DGLA in the P127 cell suspensions grown on complete and N-free media were suggested (Table 1). These algorithms allowed the determination of the TFA percentage in the full range studied (8%–40% DW) with an RMSE of 2.20%. A similar algorithm provided the accuracy of the volumetric TFA assay of 0.13 g · L−1 in the TFA range of 0.1–2.8 g · L−1 (Table 1).
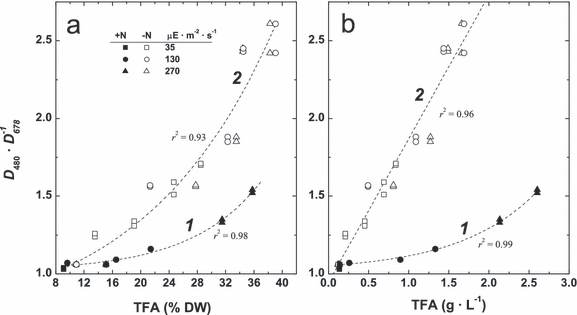
Relationship between total fatty acid (TFA) percentage (a) and volumetric content (b) and the index D480 · D678−1 in the cultures of the P127 mutant grown on complete (1) and N-free (2) media under different irradiances (shown on the figure). DW, dry weight.
| Growth conditions | Range | Estimation algorithm | RMSE | r 2 | n |
|---|---|---|---|---|---|
| TFA, % DW | |||||
| Complete BG11 | 8.0–38 | [TFA] = 44.01 + 15.31 ln (D480/D678–0.93) | 1.58 | 0.95 | 30 |
| N-free BG-11 | 8.0–40 | [TFA] = 27.06 + 17.48 ln (D480/D678–0.68) | 2.20 | 0.95 | 30 |
| TFA, g · L−1 | |||||
| Complete BG11 | 0.1–2.8 | [TFA] = 27.06 + 17.48 ln (D480/D678–0.68) | 0.13 | 0.97 | 30 |
| N-free BG-11 | 0.1–1.7 | [TFA] = 1.04 (D480/D678–0.95) | 0.12 | 0.96 | 30 |
| DGLA, % DW (via TFA%DW) | |||||
| All | 1.2–13 | [DGLA] = 0.35 [TFA]–1.84 | 1.44 | 0.90 | 60 |
- DGLA, dihomo-γ-linolenic acid; TFA, total fatty acid; DW, dry weight.
Discussion
The patterns of biomass accumulation recorded in P. incisa (Fig. 1) are compatible with our previous observations of WT cultures of P. incisa grown under similar conditions (Solovchenko et al. 2008a,b). The data on biomass increase and FA accumulation (Fig. 2) agree with the hypothesis that lipid accumulation by green algae is determined by a balance of carbon fixation and absorption of N from the medium (Mayzaud et al. 1989). Thus, during the stage of a rapid increase in biomass (first 3 d of cultivation) under low and moderate irradiances, FA content could even decrease (Fig. 2a, curves 1, 2). Under high fluxes of PAR (Fig. 1a, curve 3, and Fig. 4), the cellular C/N balance apparently shifts toward lipid accumulation (Leman 1997) even on N-replete medium (Fig. 2a). Under N-starvation, moderate irradiances seem to saturate photosynthesis, which resulted in cessation of biomass (Fig. 1b) and FA accumulation after 3–4 d of cultivation (Fig. 2b).
The cultivation of P127 at different irradiances on complete and N-free media was accompanied by significant changes in its pigment content and composition (4, 5). [+N] cultures demonstrating moderate increase in car/chl ratio (Fig. 4e) contained more car (Fig. 4c) and less chl (Fig. 4a) under increasing irradiance. The data presented in Figure 4d suggest that on N-free medium the cultures were restricted in their ability to synthesize car and responded to elevated irradiance with a decline in chl (Fig. 4b), similar to the WT (Merzlyak et al. 2007, Solovchenko et al. 2009). One could think that an increase in chl under [LL−N] and [ML−N] conditions during the first 4 d of cultivation occurred at the expense of endogenous N reserves of the algal cells (Droop 1983); when they were exhausted, chl started to decrease. As a result, more rapid changes in car/chl ratio were observed (Fig. 4f) in comparison with the [+N] cultures. These trends of changes in the pigment content represent a typical response of WT P. incisa (Solovchenko et al. 2008a) and a number of other chlorophytes to high irradiance (Mendoza et al. 1999, Young and Beardall 2003, Masojídek et al. 2004).
P127 had the same car composition of the WT, and changes in the car profile of P127 under high irradiances were also similar to those registered in WT (Solovchenko et al. 2008a). The main trends of the changes in the car composition of P127 included (i) a considerable decrease in lutein responsible for light harvesting (Niyogi 1999, Demmig-Adams and Adams 2006) and (ii) a dramatic increase in the proportion of β-carotene (data not shown). Within thylakoids, β-carotene is localized predominantly in the reaction centers preventing their photooxidation (Green and Durnford 1996). Since the stoichiometry of pigments in these structures is highly conserved, it is unlikely that high amounts of β-carotene could be incorporated in the reaction centers of the photosynthetic apparatus. These considerations drove us to the investigation of the subcellular localization of β-carotene accumulated by P127 under stress.
According to our estimates, based on the comparative analysis of car profiles of whole cells, isolated OB and thylakoids (Fig. 5), the bulk (up to 70%) of β-carotene accumulated by P127 under high light and nitrogen deficiency was localized in cytoplasmic OB. Taking into account the data on absolute car content (Fig. 4, c and d), one may argue that during cultivation under high irradiance, β-carotene is mainly synthesized de novo. However, we cannot rule out a partial translocation of β-carotene from decomposed thylakoid membranes to OB. This suggestion is compatible with previously established deep rearrangement of the ultrastructure of the P. incisa plastids accompanied by degeneration of the grana and lamellae. There are also grounds to believe that the car stored within OB serve in their protection: β-carotene is a powerful antioxidant (Edge et al. 1997) that is able to prevent peroxidation of unsaturated lipids within OB.
The changes in algal metabolism, both developmental and stress-induced, are often accompanied by dramatic and specific changes in pigment content and composition, which subsequently affect optical properties of algal cells and cell suspensions (Fig. 7, see also Berner et al. 1989, Sosik and Mitchell 1991, Merzlyak et al. 2007, Solovchenko et al. 2009). The measurements of the D(λ) spectra of P127 were complicated by strong clotting of the cells and rapid sedimentation of the cell aggregates, which was much more expressed than in WT. Therefore, we chose the approach presuming deposition of the algal cells on glass-fiber filters, which is extensively used in the plankton research (Mitchell 1990). The spectra of P127 measured with this technique did not show influence of scattering in the NIR (Fig. 7). Although no correction for light pathlength amplification effect (Mitchell 1990) was done, the spectra of P127 deposited on GF/F filters contained distinct peaks attributable to car and chl (Fig. 7, curves 1–3) and were compatible with scattering-compensated absorption spectra of the WT cell suspensions (Merzlyak et al. 2007).
The cultivation of P127 under high irradiances was accompanied by a considerable increase in the absorption of algal cells in the blue region of the spectrum (Fig. 7). Taking into account the shape of the difference spectra as well as positions of their maxima in this range (insets in Fig. 7), one could ascribe the increase of the absorption in the blue to the buildup of the contribution of car (Britton 1995, Solovchenko et al. 2009) to the overall absorption. This suggestion is in accord with the existence of a strong (r2 > 0.96) positive correlation in the blue-green region of the spectrum between car/chl ratio and normalized to the red chl maximum D(λ) values (not shown). Analysis of the correlation spectra, r{D(λ) · D678−1 vs. [car/chl]} and r{D(λ) · D678−1 vs. [DGLA]}, showed that the spectral region that is most sensitive to variation in the car/chl ratio and to the FA volumetric content (regardless of illumination intensity and nitrogen availability) is situated in the broad band of 400–540 nm (Fig. 8). The existence of this intercorrelation could be explained by the fact that the increase in the car/chl ratio under high-light and/or N-starvation conditions in P127 mutant (Fig. 6), as in the WT of P. incisa, is closely related to accumulation of FA (6, 8, 9; Solovchenko et al. 2009). We tried to exploit this relationship for the development of an algorithm for nondestructive assay of TFA and AA contents. As a result, the ratio D480 · D678−1 correlated closely with TFA and DGLA percentages and volumetric contents (3, 8; Table 1) was suggested as a proxy for the assay of FA. This finding enabled the development of several algorithms for nondestructive estimation of TFA and DGLA (Table 1).
Interestingly, the normalized D(λ) · D678−1 spectrum exhibited a strong negative correlation with car/chl ratio (not shown), TFA and DGLA contents in the region governed solely by chl absorption (Fig. 8). A similar effect was found in Dunaliella tertiolecta (Sosik and Mitchell 1991) and P. incisa WT grown under low light (Merzlyak et al. 2007) and under conditions close to those used in this work (Solovchenko et al. 2009). This region contained spectral features readily apparent in the difference-normalized spectra (insets in Fig. 7) evident of narrowing the red chl absorption maximum. This seems to stem from the changes in so called “packaging” effect, which strongly depends on chl content in the algal cells (Berner et al. 1989). Taking these observations into account, one could speculate that high correlation “car/chl versus D(λ) · D678−1” in the red stems from tight relationship of the spectral changes attributable to the package effect and variation of contribution of car into light absorption in the blue.
Conclusion
The comparison of the results obtained in this work with our previous findings with the WT (Merzlyak et al. 2007, Solovchenko et al. 2008a, 2009) indicated that the stress responses of the Δ5-desaturase mutant of P. incisa (producing gross amounts of less unsaturated long-chain PUFA DGLA instead of AA) to high-light irradiation and N-deprivation were, in general, similar to that observed in the WT cells, but the mutant displayed the same magnitude of the response under 30% lower irradiance. The mutation did not alter the patterns of coordinated irradiance-dependent responses of lipid and pigment syntheses obviously aimed to diminish the potentially high photodynamic effect of chl and to reestablish the balance of C and N fluxes in the cell without the risk of photooxidative damage. Similar to WT, a considerable part of extraplastidic car (mainly β-carotene) was deposited in OB, where it could efficiently trap the excessive light and protect the PUFA contained within OB against photooxidation. Finally, the revealed intercorrelation of car/chl, FA, and optical properties existing under wide range of cultivation conditions was exploited for direct nondestructive assay of the nutraceutically valuable compounds, car and DGLA, in the biomass.
Acknowledgments
The authors would like to thank Shoshana Didi-Cohen for the dedicated technical assistance. This work was supported in part by fellowships from the Blaustein Center for Scientific Cooperation (BCSC) to A. E. S. and M. N. M. The financial support from the Russian Foundation for Basic Research (Grant # 09-04-00419) and the Russian President’s Grant Council (Ministry of Science of the Russian Federation, Grant # MK-3433.2008.4) is also acknowledged. Dedicated to the memory of Professor Mark N. Merzlyak.