Delineation of Two Sibling Red Algal Species, Gracilaria Gracilis and Gracilaria Dura (Gracilariales, Rhodophyta), Using Multiple DNA Markers: Resurrection of the Species G. Dura Previously Described in the Northern Atlantic 200 Years Ago1
Received 18 July 2009. Accepted 7 January 2010.
Abstract
Molecular markers belonging to the three different genomes, mitochondrial (cox2-cox3 spacer), plastid (rbcL), and nuclear (internal transcribed spacer [ITS] 2 region), were used to compare samples of the two morphologically related species Gracilaria gracilis (Stackh.) Steentoft, L. M. Irvine et Farnham and G. dura (C. Agardh) J. Agardh collected along Atlantic coasts. In northern Europe, the distinction between these two species is ambiguous, and they are currently recognized under the single name of G. gracilis. The low but congruent patterns of genetic divergence observed for markers of the three genomic compartments highly suggest that these two taxa correspond effectively to two different genetic entities as previously described 200 years ago, based on morphological traits. However, thanks to the combination of different DNA markers, occurrence of “incongruent” cytotypes (i.e., mitotypes of G. dura associated with chlorotypes of G. gracilis) in individuals collected from Brittany, suggests interspecific hybridization between the two sibling species studied.
Abbreviations:
-
- ITS
-
- internal transcribed spacer
-
- K2P
-
- Kimura 2 parameter
Species delineation is an old methodological problem that has recently received an increased level of attention in the context of the barcoding approach (Chase et al. 2005, DeSalle et al. 2005, Petit and Excoffier 2009). We address this question for the cosmopolitan Gracilaria Grev. genus of red seaweeds, in which many species are poorly delimited (Gurgel and Fredericq 2004). The taxonomy of this genus is particularly difficult due to the relatively small number of diagnostic characters available compared to the high number of species described and their wide distribution (Gurgel et al. 2004). In this genus, the precise taxonomic identification of cylindrical specimens is often problematic, or nearly impossible, when only small and nonreproductive individuals are available in the field. Short sequences of nuclear or organelle DNA were thus developed in the 1990s, such as the plastid-encoded intergenic RUBISCO spacer (Destombe and Douglas 1991) and the nuclear ITS spacer regions (Goff et al. 1994), which allowed cohesive taxonomic entities to be defined and to delineate the species in this genus. More recently, different genetic markers have been used for red algal taxonomy (Provan et al. 2004, Saunders 2005, Robba et al. 2006). For a more accurate identification of sibling species, and because detection of hybridization/introgression cannot be reliably accomplished by examination of a single DNA region, Chase et al. (2005) suggested the use of a multilocus approach including mitochondrial, plastid, and nuclear ribosomal DNA markers.
In this paper, we investigate one of these taxonomic problems using two species: G. gracilis and G. dura. Both species are very similar morphologically and occur in the lower intertidal and upper subtidal. G. gracilis was originally described as Fucus verrucosus by Hudson (1762) based on material collected in the United Kingdom. This species was for a long time confused with Gracilariopsis longissima under the name Gracilaria verrucosa (Huds.) Papenf. (Steentoft et al. 1995). The wide amphi-Atlantic distribution of G. gracilis (Bird and Kain 1995) was confirmed by the use of cytoplasmic and nuclear DNA markers (Destombe and Douglas 1991, Wattier et al. 1997). This species is commonly found on northern Atlantic coasts from Morocco to Norway (Guillemin et al. 2008) and on southern Atlantic coasts of Namibia (Wattier et al. 1997, Iyer et al. 2005) and Argentina (Destombe and Douglas 1991). G. dura was described for the first time in 1822 by C. Agardh from samples collected on the Atlantic coast of southern Spain (Cadiz) by Cabrera (C. Agardh 1822); later this species was also reported on the northern coast of Brittany by Crouan and Crouan (1830) (cited in Chalon 1905 and Piquenard 1912) and also on the Atlantic coast of Morocco (Raphaélis 1929, Dangeard 1949). The plants collected in the Mediterranean Sea by J. Agardh (1842) in the Gulf of Naples were referred to as G. dura var. lyra.
In Morocco, G. dura and G. gracilis can easily be distinguished based on their gross morphology. G. dura has a bright red color and small lateral branches often located on the same side of the main axis (see Gayral 1958, p. 359), whereas G. gracilis usually has dark reddish-brown, irregularly branching thalli (Steentoft et al. 1995). In northern Europe, distinguishing between these two terete species is much more difficult. Thus, they are probably regularly misidentified and generally recorded under the name of G. gracilis. Recently, a barcoding approach based on a sequence of the RUBISCO spacer indicated that these two morphological species, occurring together in the same site in Morocco, corresponded to two sister species (Guillemin et al. 2008). In this context, the observed weak morphological difference between G. dura and G. gracilis in the northern Atlantic compared to the coast of Morocco raises the question as to whether this difference is due to environmental factors or to hybridization events.
By using a combination of markers from the three different genome compartments—mitochondria, plastid, and nuclear—the aim of this study was to delineate more precisely these two morphological species and to detect the occurrence of putative hybridization.
Materials and methods
Sampling and DNA extraction. Forty-two samples from 22 locations were selected to be representative of the G. gracilis and G. dura species distribution (Fig. 1 and Table S1 in the supplementary material). Samples were stored in silica gel for later molecular analysis. DNA was extracted from the algae using a modified Chelex method (Cohen et al. 2004). Individuals from Morocco previously identified as G. gracilis (MGR3) and G. dura (MGR177a) and deposited as dried samples preserved in silica gel in the collection at Roscoff-UMR7144 were used as references (Guillemin et al. 2008).
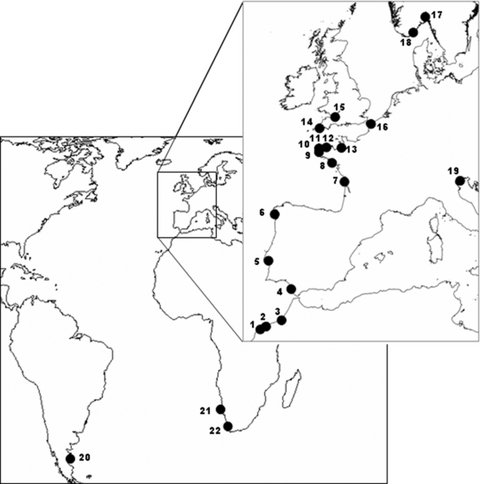
Map showing the sample sites given in Table S1 (see supplementary material).
PCR reactions and sequencing. The three different genome compartment markers used in this study are as follows: for the nucleus, ITS of rDNA (Hamby and Zimmer 1992); for the choloroplast, rbcL (Gurgel and Fredericq 2004); and for the mitochondrion, cox2-cox3 spacer (Zuccarello et al. 1999a). A set of primers published by Guillemin et al. (2008) for ITSs (ITS2-F and ITS2-R) and RUBISCO (rbcL-RFLP-F and rbcL-RFLP-R), and by Zuccarello et al. (1999b) for cox2-cox3 spacer (cox2F and cox3R) were chosen for the amplification reactions. These reactions were performed in a total volume of 30 μL using the following final concentrations: 1X reaction buffer IV, 0.2 μM dNTP, 2.5 mM MgCl2, 0.3 μM of each primer, 1 U Taq polymerase (ABgene, Surrey, UK), and 5 μL of Chelex-extracted template DNA. ITS amplification conditions were carried out as described in Guillemin et al. (2008). RUBISCO and cox2-cox3 spacer amplifications were carried out following the procedures described by Zuccarello et al. (1999a,b). PCR products were checked in an agarose gel stained with ethidium bromide. PCR products were purified (Millipore purification kit; Bedford, MA, USA) prior to cycle sequencing. Automated sequencing was performed using the amplification primers (except for the ITS2, for which we used the primers ITS2-R and TW81 published, respectively, by Guillemin et al. 2008 and Goff et al. 1994) on an ABI PRISM® 3100 Automated DNA Sequencer (Applied Biosystems, Foster City, CA, USA) after cycle sequencing of the purified PCR products with dye-labeled dideoxynucleotides (BigDye version 3.0, Millipore), according to the protocol provided by the manufacturer. All unique sequences have been deposited in GenBank (cox2-cox3 spacer, rbcL region, ITS2 region).
Data analysis. The alignment was performed using the program Clustal (under default conditions) integrated with MEGA 3.1 (Kumar et al. 2004). The alignment of the rbcL sequences included 39 newly determined sequences as well as sequences previously published for G. gracilis from Brittany (AY049399) and from Wales (AY049400) (Gurgel and Fredericq 2004) (Tables S1 and S2 in the supplementary material). The alignment of the cox2-cox3 spacer sequences included 38 newly determined sequences as well as sequences previously published for G. gracilis from Norway (AY423846) (Cohen et al. 2004) (Tables S1 and S3 in the supplementary material). The alignment of partial ITS2 regions included 37 newly determined and one sequence previously published for G. gracilis from Norway (U 21342) (Goff et al. 1994) (Tables S1 and S4 in the supplementary material). For each marker, the combination of primers provided unambiguous sequence data on both forward and reverse strands. Forward and reverse sequences were compared and corrected manually and aligned with other G. gracilis sequences previously published in GenBank (AY049399 and AY0494000 sequenced by Gurgel and Fredericq 2004, AY423846 sequenced by Cohen et al. 2004, and U21342 sequenced by Goff et al. 1994).
For each marker, sequence polymorphism and nucleotide diversity were computed using DnaSP software (Rozas et al. 2003). Within species, nucleotide variability was measured using θ, the proportion of polymorphic sites in a sample (Watterson 1975), and π, the average number of nucleotide differences between sequences in a sample (Nei and Li 1979). Sequence divergences were calculated using the Kimura 2 parameter (K2P) distance bootstrapped using MEGA3 (Kumar et al. 2004) with 1,000 replications to calculate the standard deviation. The genealogical relationships among the haplotypes were assessed with a haplotype network constructed using a median-joining algorithm as implemented in the software Network 4.201 (Bandelt et al. 1999).
Results
Alignment of the rbcL revealed 17 polymorphic sites over a total length of 1,309 bp (1.29%) and gave rise to eight chlorotypes (C1–C8; GenBank accession numbers GQ229494–GQ229500, Table 1, Fig. 2). The cox2-cox3 spacer sequences yielded an alignment of 308 bp of which 11 sites were polymorphic (3.6%) (Table 1). Ten mutations and one insertion–deletion mutation were observed, resulting in a total of six mitotypes designated M1 to M6 (Table 1, Fig. 2, GenBank accession numbers GQ229501–GQ229506). The ITS2 was 336 bp long and showed 27 polymorphic sites (18 mutations and nine insertion–deletion mutations, Table 1), resulting in a total of 11 ribotypes (designated R1 to R11; GenBank accession numbers GQ229507–GQ229517, Fig. 2).
| cox2-cox3 spacerAll sequences | rbcLAll sequences | ITS2All sequences | |
|---|---|---|---|
| Total data | |||
| Number of sequences | 39 | 41 | 38 |
| Sequence length | 308 | 1,309 | 336 |
| Number of haplotypes | 6 | 8 | 8 |
| Number of polymorphic sites | 11 | 17 | 27 |
| Number of parsimony informative sites | 9 | 11 | 13 |
| Number of gaps | 1 | 0 | 9 |
| π | 0.01313 (SD = 0.00106) | 0.00364 (SD = 0.00041) | 0.01584 (SD = 0.00247) |
| θ | 0.00847 | 0.00304 | 0.01306 |
| G. dura | |||
| Number of sequences | 12 | 13 | 11 |
| Number of haplotypes | 2 | 2 | 5 |
| Number of polymorphic sites | 2 | 1 | 17 |
| π | 0.00197 (SD = 0.00096) | 0.00029 (SD = 0.00010) | 0.00729 (SD = 0.00297) |
| θ | 0.00216 | 0.00025 | 0.00833 |
| G. gracilis | |||
| Number of sequences | 24 | 25 | 24 |
| Number of haplotypes | 4 | 6 | 6 |
| Number of polymorphic sites | 3 | 7 | 6 |
| π | 0.00106 (SD = 0.00043) | 0.00048 (SD = 0.00019) | 0.00025 (SD = 0.00023) |
| θ | 0.00261 | 0.00142 | 0.00081 |
| Individuals with incongruent cytoplasm | |||
| Number of sequences | 3 | 3 | 3 |
| Number of haplotypes | 1 | 1 | 3 |
| Number of polymorphic sites | 0 | 0 | 7 |
| π | 0 | 0 | 0.00402 (SD = 0.00134) |
| θ | 0 | 0 | 0.00402 |
| Between taxa G. gracilis/G. dura | |||
| Number of fixed differences | 6 | 9 | 8 |
| Average number of nucleotide differences between taxa | 7.833 | 4.977 | 11.587 |
| Average number of nucleotide substitution per site between taxa | 0.02552 (SD = 0.00757) | 0.00771 (SD = 0.00199) | 0.03533 (SD = 0.01135) |
- π: average number of nucleotide differences per site between two sequences (Nei and Li 1979) calculated on the total number of polymorphic sites; θ: the Watterson estimator of theta per base pair (Watterson 1975) calculated on the total number of polymorphic sites.
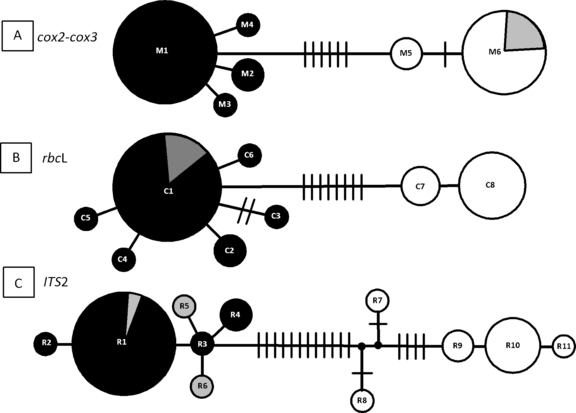
Median-joining networks of mitotypes (A: the cox2-cox3 spacer), chlorotypes (B: rbcL), and ribotypes (C: ITS2 region). Each circle represents a haplotype. The size of the circle is proportional to the number of sampled individuals with a given haplotype. White circles correspond to G. dura, and black circles to G. gracilis. For each DNA marker, the frequency of individuals showing an “incongruent cytotype” (i.e., possible hybrids: BATZ5, QUIH11, QUIB14; Table S1, see supplementary material) is represented as a shaded fraction of the circle. Each line between haplotypes, bars, and/or branch points represents one mutation step. Missing haplotypes, indicated by small black circles, were either not sampled or extinct.
Linkage disequilibria between species and among the three DNA genomic compartments. Irrespective of the genetic markers used, the results were congruent and showed a clear differentiation between G. gracilis and G. dura. The number of fixed differences between G. gracilis and G. dura varied from six for the cox2-cox3 spacer to nine for the rbcL (Table 1, Fig. 2). A high linkage was observed between cytoplasmic markers (Table 2). In G. gracilis, the cytotype M1C1 was the most frequent and found in all the sampled locations from Morocco to Norway, in the Adriatic sea, in Argentina, and in southern Africa. In G. dura, the most frequent cytotype, M6C8, was found only in Spain and Brittany. In addition to the mitochondrial and chloroplastic disequilibrium, a strong nucleo-cytoplasmic linkage desquilibrium was obvious (Table 3). In contrast, three samples collected in Brittany (QUIB14, QUIH11, and BATZ5) were recognized as “incongruent” cytotypes because they combined the most frequent G. dura mitotype (M6) with the most frequent G. gracilis chlorotype C1 (Table 3). These three “incongruent” cytotypes were all associated with the G. gracilis ribotypes R1, R5, and R6 (Table 3). If we exclude these last three individuals, the combinations of the three markers allowed us to assign without any ambiguity 12 of the study individuals to G. dura and 23 to G. gracilis (Table S1).
| rbcL (plastid) | cox2-cox3 spacer (mitochondrion) | ||||||
|---|---|---|---|---|---|---|---|
| G. gracilis | G. dura | ||||||
| M1 | M2 | M3 | M4 | M5 | M6 | ||
| G. gracilis | C1 | 16 | 2 | 1 | 3a | ||
| C2 | 2 | ||||||
| C4 | 1 | ||||||
| C5 | 1 | ||||||
| C6 | 1 | ||||||
| G. dura | C7 | 2 | 1 | ||||
| C8 | 9 | ||||||
- The number given for each genetic combination corresponds to the number of individuals bearing this genotype. The name of the samples analyzed for each combination is indicated in Table S1 (see supplementary material).
- aIndividual with incongruent cytoplasm.
| Cytoplasmic | Partial ITS2 (nuclear) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G. gracilis | G. dura | |||||||||||
| R5 | R6 | R1 | R2 | R3 | R4 | R7 | R8 | R9 | R10 | R11 | ||
| G. gracilis | C1-M1 | 14 | 1 | 1 | ||||||||
| C1-M2 | 2 | |||||||||||
| C1-M3 | 1 | |||||||||||
| C2-M1 | 2 | |||||||||||
| C4-M4 | 1 | |||||||||||
| C5-M1 | 1 | |||||||||||
| C6-M1 | 1 | |||||||||||
| G. dura | C7-M5 | 1 | 1 | |||||||||
| C8-M6 | 2 | 6 | 1 | |||||||||
| C1-M6 | 1a | 1a | 1a | |||||||||
- The number given for each genetic combination corresponds to the number of individuals bearing this genotype. The name of the samples analyzed for each combination is indicated in Table S1 (see supplementary material).
- aIndividual with incongruent cytoplasm.
rbcL. In G. gracilis, the six chlorotypes (C1–C6) differed by 1–4 bp changes between sequences, whereas in G. dura, the two chlorotypes (C7, C8) differed by only 1 bp. The genetic distances between the two “gracilis” and “dura” clusters were >16 times greater than those within clusters (Table 1). When the published sequence (AY049399, Gurgel and Fredericq 2004, corresponding to the chlorotype C3 in this study, Table S1) was removed from the analysis, the results gave rise to equal values for both clusters (G. gracilis without C3 within species K2P distance = 0.000313). Therefore, genetic distances between clusters were at least 24 times greater than those within clusters. The frequency distribution of chlorotypes resulted in one dominant chlorotype (C1) that accounted for 19 of the 25 individuals analyzed of G. gracilis (76%). The chlorotype C2 was found in two individuals (8%) in Quiberon, and the four remaining chlorotypes (C3 to C6) were only observed once. In G. dura, the Moroccan region was characterized by the chlorotype C7, while the Brittany and Welsh regions retained the chlorotype C8. The rbcL sequence of the Moroccan individual MGR177a (haplotype C7), previously identified as G. dura (Guillemin et al. 2008), differed by 10 bp from the G. gracilis individual MGR3 (haplotype C6) collected at the same site, confirming the co-occurrence of the two sibling species on the northwest African coast.
cox2-3. For the cox2-cox3 spacer sequences, the genetic distances between species were >13 times greater than the distances retrieved within species (Table 1). Four distinct mitotypes (M1 to M4) differing by 1–2 bp were recognized in G. gracilis, and two (M5, M6) differing by 2 bp in G. dura (Fig. 2). The mitotype M1 was the most common, detected in 20 of the 24 individuals of G. gracilis sequenced (83.3%), and corresponded to the sequence previously published for G. gracilis in Norway (GenBank AY423856, i.e., LIL1) by Cohen et al. (2004). M1 was found from Norway to South Africa, Italy, and Argentina. The mitotype M2 (5.1%) was only retrieved from two individuals from northern Brittany. Two mitotypes, M3 and M4, were observed in only one individual, in Charente and Portugal, respectively. In G. dura, the mitotype M6 was the most frequent (83.3%) and was observed in 10 individuals in Morocco, Spain, and southern and northern Brittany, whereas M5 was retrieved from only two individuals from Morocco.
ITS2. The ITS2 sequences distinguished six ribotypes (R1–R6) in G. gracilis and five ribotypes in G. dura (R7–R11). In G. gracilis, the six distinct ribotypes differed by 1–3 bp from each other, whereas in G. dura the five distinct ribotypes differed by 1–10 bp (Fig. 2). The genetic diversity in the G. gracilis cluster was 10 times lower than in the G. dura cluster (θ, the average number of nucleotide differences = 0.00081 and 0.00833, respectively, Table 1). In G. gracilis, the ribotype R1 was the more frequent (20 of the 24 individuals sequenced, 83%) and fit with the sequence of the individual from Norway (GR-NOR, GenBank number U21342) previously studied by Goff et al. (1994). The ribotype R1 was present across the entire range of G. gracilis distribution. The ribotype R2 was found only once in Norway, and R3 and R4 were found in Portugal. In the G. dura cluster, the ribotypes R7 and R8 were encountered in Morocco, whereas R9 was detected in two individuals in northern Brittany, and the two ribotypes R10 and R11 were found in Spain. The ribotype R10 corresponded to six of the seven sequences obtained from G. dura from Spain (86%).
Discussion
The standard DNA-barcoding approach based on a single gene (such as cytochrome oxydase subunit 1 gene) sequence divergence as a tool for species delimitation has been strongly criticized because it will not necessarily provide sufficient resolution needed to recognize the large range of species targeted by the barcoding initiative (DeSalle et al. 2005). As proposed by Chase et al. (2005), in our study, we used multilocus barcodes including three independent markers (plastid, mitochondria, and nuclear) to explore species limits in the G. gracilis species complex. This method not only improves the detection of species boundaries, but it also allows the incidence of hybridization and introgression to be tested, a phenomenon that occurs at a high frequency in plants and probably also in algae (Chase et al. 2005). Whatever the genetic marker, the intergroup divergence between G. gracilis and G. dura is five to 10 times higher than the intragroup divergence. These results are congruent with the boundaries used as guidelines for decisions concerning species validity (i.e., intergroup should be 10 times greater than intragroup differences: Hebert et al. 2004) and with previous taxonomic studies of the Gracilariaceae (Bellorin et al. 2002, Cohen et al. 2004, Yang et al. 2008). Indeed, the intergroup sequence pair-wise divergences observed in the present study for the ITS2, the rbcL gene, and the cox2-cox3 spacer fit with the values obtained previously between closest species of Gracilariaceae. The congruent divergences highly suggest that these two taxa correspond to two different genetic entities that have evolved separately and, therefore, correspond two distinct species according to the unified species concept developed by de Queiroz (2007), for which species are separately evolving metapopulation lineages. The fact that genetic diversity is higher between two individuals of G. gracilis and G. dura occurring together at the same geographic site than between two individuals of G. gracilis from Norway and South Africa or of G. dura from Morocco and Brittany indicates true discontinuities between groups (i.e., lineage separation) rather than genetic differentiation due to isolation by distance.
G. dura was first described, based on its morphological characteristics, from the northern Atlantic, in Morocco (Raphaélis 1929, Dangeard 1949), in southern Spain (Agardh 1822), and in Brittany (Chalon 1905, Piquenard 1912). However, because of the lack of clear-cut morphological character differences between G. dura and G. gracilis, the species G. dura disappeared totally from the inventory of the northern Atlantic flora after 1950 (Feldmann 1954, Steentoft and Farnham 1997). This species has also been reported from the Mediterranean Sea, in the Gulf of Naples by Agardh (1842) as G. dura var. lyra. However, the comparison of the rbcL sequences for G. dura from the Atlantic (GenBank numbers GQ229498 and GQ229499, this study) and from the Mediterranean (GenBank number AY651058, Gargiulo et al. 2006) clearly demonstrates that these two species are highly divergent and correspond to two different cryptic species (K2P pair-wise divergence >10%). Recently, Gargiulo et al. (2006) suggested a reexamination of these Mediterranean samples of G. “dura” from southern Italy that grouped, in all molecular analyses, with the clade containing Hydropuntia species.
The multilocus approach used in this study suggests that the two samples collected in Brittany and Wales and previously assigned to G. gracilis according to their rbcL gene plastid sequences by Gurgel and Fredericq (2004), while showing a genetic divergence of 0.95% (i.e., AY049399 and AY049400), correspond respectively to G. gracilis and G. dura. This finding suggests that the distribution range of G. dura (from Morocco to Wales) is much more restricted than that of G. gracilis (Wattier et al. 1997, Iyer et al. 2005, Gargiulo et al. 2006).
In the present study, the observed linkage disequilibrium between chloroplast and mitochondrion confirms uniparental inheritance of organelles. Organelle inheritances were studied only in a few Rhodophyta species (Zuccarello et al. 1999a,b, 2002, Choi et al. 2008). Organelle genomes were shown to be preferentially transmitted by the mother. However, in Porphyra yezoensis, low frequencies of paternal leakage and biparental inheritance were also reported (Choi et al. 2008).
Our data report high but incomplete linkage disequilibrium since “incongruent” cytotypes (i.e., a combination of G. dura mitotypes with G. gracilis chlorotypes) were observed in three individuals only and in sites where both species are co-occuring. The sample size does not allow us to definitively conclude between the two explanations for such incongruent associations: either shared ancestral polymorphism or interspecific hybridization. However, since incongruence was exclusively observed in sympatric populations, we speculate that hybridization is the most likely explanation. While the ITS regions have proved particularly helpful for inferring relationship at lower taxonomic levels, cases of recombination and gene conversion in these ribosomal repeat regions were reported following hybridization in higher plants and fungi (Hugall et al. 1999, Hughes and Petersen 2001). In our study, the clear relationship between cytoplasmic and ribosomal markers in G. gracilis and in G. dura suggests either that hybridization between the two species is uncommon or that ribosomal gene conversion occurred differently according to the parental species. The three putative hybrids (i.e., individuals with incongruent cytotypes) observed in our study advocate that hybrids between G. dura and G. gracilis may have been formed at some time, the result of either a mitochondrion or a chloroplast introgression.
Contrary to Phaeophyceae (such as Fucales: Coyer et al. 2007; and Laminariales: Druehl et al. 2005), only a few reports of hybridization have been published for red seaweeds. Using both nuclear and plastid DNA, Niwa et al. (2009) demonstrated the occurrence of plastid introgression from P. yezoensis to P. tenera. A possible case of differential organelle inheritance was also reported in Mastocarpus stellatus lineages by Zuccarello et al. (2005) in which some plants in natural populations exhibited a mixed cytoplasm composed of a mitochondrial haplotype of one breeding group with the plastid haplotype of the other breeding group.
Our genetic analysis of divergence between two sibling species based on a multilocus approach confirms the existence of two sibling species under the name of G. gracilis in the northern Atlantic. It illustrates clearly that sequences from different DNA compartments are needed to correctly diagnose species and retrace their phylogenetic history.
Acknowledgments
This study was partially supported by grants from the European Commission INCO-DEV Programme (INCO-EPIFIGHT ICA4-CT-2001-10021) to M.-L. G., the Fondation BETTENCOURT SCHUELLER, “COUP d’ELAN à la Recherche 2001,” the French Embassy in Chile, the Region Bretagne, and the LIA DIAMS (Associated International Laboratory between France and Chile: “Dispersal and Adaptation of Marine Species”), and the UACH-DID S-200801 project. We thank C. Engel, A. Mouradi, J. R. Andria, V. Peña Freire, N. Simon, J. Cabioch, M. Hommersand, C. Bird, J. Jueness, A. L. Boraso de Zaixso, P. I. Leonardi, C. Bird (K. Laufer), and R. Anderson for providing samples, and Cécile Godé and Stéphane Mauger for help in sequencing.