A BATCH CULTURE METHOD FOR MICROALGAE AND CYANOBACTERIA WITH CO2 SUPPLY THROUGH POLYETHYLENE MEMBRANES1
Received 27 July 2009. Accepted 28 January 2010.
Abstract
A new method for CO2 supply to photoautotrophic organisms was developed, and its applicability for measuring specific growth rates in shaken batch cultures of cyanobacteria and unicellular algae was shown. Small bags containing a concentrated carbonate buffer with a CO2 partial pressure of 32 mbar were prepared from a thin foil of low density polyethylene (LDPE). These bags were inserted as CO2 reservoirs (CRs) into polystyrene culture flasks with gas-permeable screw caps, which were suitable to photometric growth measurement. CO2 was released directly into the medium with membrane-controlled kinetics. The CRs were not depleted within 1 week, although the atmosphere in the culture vessel exchanged rapidly with the ambient air. Rates of initial growth and final densities of the cultures of six different unicellular algal species and one cyanobacterium were markedly increased by diffusive CO2 supply from the CR. In the presence of a CR, growth was exponential during the first 2 d in all cultures studied. The method described allowed a high number of measurements of specific growth rates with relatively simple experimental setup.
Abbreviations:
-
- CR
-
- CO2 reservoir
-
- LDPE
-
- low density polyethylene
-
- OD
-
- optical density
-
- PE
-
- polyethylene
-
- PPFD
-
- photosynthetic photon flux density
Carbon limitation is a problem for culturing algae and cyanobacteria in large-scale biomass production and as well in small-scale batch cultures. Continuous supply of inorganic carbon is essential to maintain optimum conditions for photosynthesis, at least above a critical cell concentration (Eriksen 2008). Carbon limitation is undesirable for studying factors that might have an impact on growth since it can mask the effects to be analyzed. In chemostate or turbidostate cultures, carbon limitation can be prevented by maintaining the pH and bicarbonate concentration at an optimum. Yet if a large number of variants are to be analyzed in parallel, small-scale batch cultures are much more suitable. Batch supply of bicarbonate to a culture assimilating in normal air rapidly leads to an increase in pH. This effect, in turn, can reduce the availability of inorganic carbon and growth (Talling 1976, Kohl and Nicklisch 1988). An optimum CO2 concentration in the medium is usually maintained by shaking the culture vessels in a chamber with enhanced CO2 partial pressure or by passing air with an enhanced CO2 partial pressure through the culture fluid. Another option is the use of a concentrated carbonate buffer as a CO2 source within the culture vessel. Special vessels with a separate chamber for the carbonate buffer have been applied as Warburg manometers to measure photosynthesis (Warburg and Krippahl 1960) and, occasionally, also for culturing photoautotrophs (Hüsemann and Barz 1977, Tripathi et al. 2001). Here we describe a new type of CR that can be applied in any culture vessel. These CRs are tubes or bags that are prepared from a thin gas-permeable polyethylene (PE) foil and contain a concentrated carbonate buffer. The CO2 release from the CR into the growing culture is controlled by the PE membrane. Advantages of such CRs for studying exponential growth in small-scale batch cultures will be demonstrated.
Materials and Methods
Cultures and mineral media. The following axenic stock cultures were used: Synechocystis sp. PCC 6803, Ankistrodesmus fusiformis Corda ex Korshikov (SAG 2005), Monoraphidium braunii (Nägeli) Komárk.-Legn. (SAG 2006), Monoraphidium minutum (Nägeli) Komárk.-Legn. (SAG 243-1), Desmodesmus communis E. Hegewald (SAG 276-4b, syn. Scenedesmus communis), Pseudokirchneriella subcapitata (Korshikov) Hindák (SAG 61.81), and Chlamydomonas reinhardtii P. A. Dang. (SAG 83.81). Cultures used for inoculation were grown under axenic conditions on 100 mL of autoclaved medium in shaken 500 mL glass flasks under the light and temperature conditions applied for the growth studies (see below). The following media were applied: a nitrate-containing BGx11 medium (Rippka et al. 1979) for Synechocystis sp., Tris acetate phosphate (TAP) medium with 18 mM acetate (Gorman and Levine 1965) for C. reinhardtii, and Kuhl medium (Kuhl and Lorenzen 1964) for the other green algae.
CR. Using a bag sealer, small bags with a diameter of 0.9 cm and a length of 10 cm, open at one side, were made from a 20 μm thick foil consisting of LDPE. The bags were filled with 2.5 mL of a buffer solution obtained by mixing four volumes of 3 M KHCO3 with one volume of 3 M K2CO3.. The CO2 partial pressure above the applied buffer solution was 32 mbar (Kleinzeller et al. 1965). The bags were closed by a knot and an additional heat seal (Fig. 1A). CRs could be stored before use in a closed vessel containing the carbonate buffer.
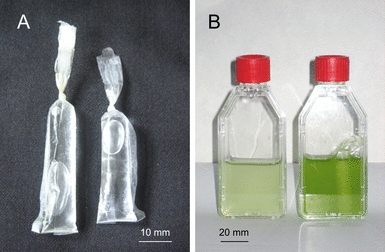
CO2 reservoirs (A) and culture flasks (B) with Pseudokirchneriella subcapitata after 4 d of growth. Left flask: culture grown without CO2 reservoir; right flask: culture grown with CO2 reservoir.
Culture vessels and culture conditions. Growth was studied in 50 mL Cellstar® culture flasks, supplied from Greiner Bio-One GmbH (Frickenhausen, Germany). The filter caps of these vessels are equipped with a porous hydrophobic membrane allowing rapid gas exchange with the atmosphere. Before inoculation, the culture vessels were filled with 25 mL of the autoclaved medium under a sterile atmosphere. Subsequently, for variants with CR, one PE bag with the carbonate buffer (Fig. 1A) was shortly bathed in 70% ethanol, washed twice with sterile water, and placed into the medium. Culture flasks with CR (Fig. 1B) and control variants without CR were shaken in a horizontal position. For experiments with green algae, a linear shaker (frequency 160 · min−1) was used in a phytochamber (HPL 400; Heraeus-Vötsch GmbH, Balingen-Frommern, Germany) at 22°C under continuous illumination with fluorescence lamps at a photosynthetic photon flux (PPFD) of 40 μmol photons · m−2 · s−1. For variants growing with convective aeration under the above-mentioned conditions, an air stream from a membrane pump was passed through a sterile filter and introduced into the medium of the culture flask by silicon rubber tubing.
The Synechocystis culture was grown on a rotary shaker (MTS 4, IKA Labortechnik; Janke & Kunkel GmbH & Co. KG, Staufen, Germany) with 12g at room temperature (20°C–23°C) and illuminated with a light:dark (L:D) cycle of 16:8 (23°C/20°C) by lamps Philips SON-T Agro 400 in combination with the lamps Philips LPI-T Plus (Philips Deutschland GmbH, Hamburg, Germany). The applied PPFD was 60 μmol photons · m−2 · s−1.
Growth measurement. The optical density (OD) at 750 nm of the cell suspension was determined with a photometer (Spekol, Carl Zeiss Jena, Germany) using a self-made adapter for the flasks. The linear path length through the suspension in the polystyrene flasks was 20 mm. Before the OD measurement, each vessel was shaken, and the PE bag with the buffer (CR) was allowed to settle to one side of the vessel outside of the light path (Fig. 1B). Biomass concentration [mg dry weight · mL−1] was obtained from OD using calibration curves. Specific growth rates obtained in the presence of a CR were determined from the linear increase of Ln (OD) during the first 2 d of the culture. Within this period, the OD remained <0.5, and calibration curves (dry biomass per flask vs. OD) were approximately linear.
CO2 release from CR to sodium hydroxide solution. CRs were rinsed with water and then placed in sealed 15 mL Falcon tubes (Technic Plastic Products AG, Trasadingen, Switzerland) containing 10 mL of 50 mN NaOH. The tubes were gently shaken at room temperature. After 24 h each, in the course of a week, CRs were removed and placed in sealed tubes with 10 mL fresh NaOH solution, as above. The carbonate ions formed in the NaOH solution were precipitated by addition of 1 mL of 0.5 M barium chloride solution and determined by titration of this solution with HCl. In control experiments, it was shown that the LDPE bags did not release sodium ions.
CO2 flux from ambient air into culture vessels. To measure the unidirectional CO2 diffusion across the gas-permeable screw cap, 25 mL of a 0.025 N NaOH solution was pipetted into the flasks and shaken subsequently in the growth chamber for 24 h as described above. Control vessels containing the same amount of NaOH were hermetically sealed with impermeable screw caps. The carbonate ions trapped within the NaOH solution were precipitated and determined by titration as described above.
Statistical analyses. Every culture experiment was carried out at least twice with at least three parallel flasks per experiment. Data represent means with standard deviations. To evaluate differences between mean values, the Student’s (t) test was applied after performing an F test.
Results
The CRs markedly stimulated the growth of all species investigated. In cultures with a CR, a linear phase in the semilogarithmic plot (Fig. 2A) and the sigmoid increase in OD (2, 3) indicate that growth was exponential during the first 2 d. Specific growth rates determined for this period are listed in Table 1. At day 3, when OD reached values >0.5, growth in the presence of a CR was not exponential and approached a maximum value. Without CR, specific growth rates decreased from the beginning of the experiment, and growth ceased at lower cell densities than growth in the presence of CR. Final OD values in the presence of a CR were several times higher than those without CR. Specific growth rates found in the presence of a CR were >1.0 with C. reinhardtii, Synechocystis sp., P. subcapitata, and M. braunii. If the medium contained an organic carbon source (18 mM acetate), C. reinhardtii also grew rapidly in the absence of a CR, but nevertheless the mixotrophic growth of this species was further stimulated by the CR (Fig. 3). The relative increase of both initial growth and final biomass due to additional CO2 supply by the CR was highest (∼4-fold) in the P. subcapitata and Synechocystis sp. cultures.
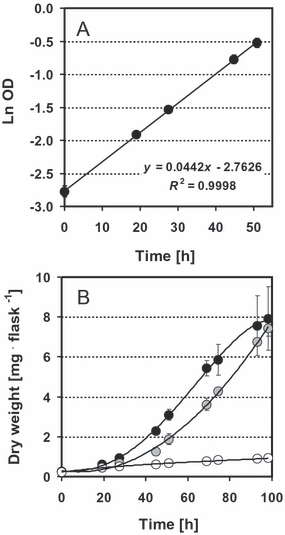
Growth of Pseudokirchneriella subcapitata. (A) Increase in OD in the logarithmic scale; (B) increase in dry biomass during the culture period. Closed symbols: culture shaken in the presence of a CO2 reservoir (CR). Gray symbols: aerated culture. Open symbols: culture shaken without CR. Mean values of three parallels with SD. Mean values of all three variants are significantly different with a probability level of 99% after the second culture day.
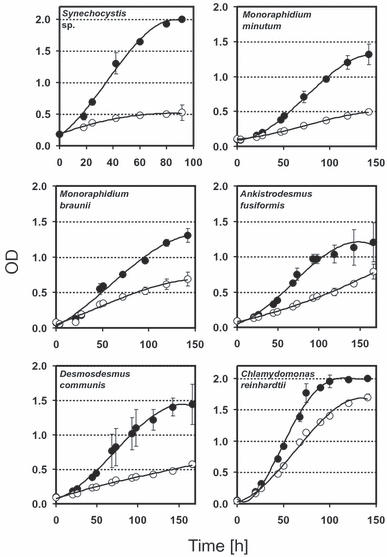
Growth of studied organisms in the presence (closed circles) and absence (open circles) of the CO2 reservoir (CR). Means values of three replicates with SD.
| Species | Specific growth ratea [d−1] | Increase in ODa,c [% of control] | Final ODb,c [% of control] |
|---|---|---|---|
| Synechocystis sp. | 1.126 ± 0.075 | 441 | 382 |
| Ankistrodesmus fusiformis | 0.690 ± 0.024 | 224 | 184 |
| Desmodesmus communis | 0.726 ± 0.023 | 229 | 294 |
| Monoraphidium braunii | 1.019 ± 0.072 | 193 | 190 |
| Monoraphidium minutum | 0.699 ± 0.020 | 295 | 268 |
| Pseudokirchneriella subcapitata | 1.040 ± 0.066 | 422 | 411 |
| Chlamydomonas reinhardtii (grown with 18 mM acetate) | 1.317 ± 0.093 | 163 | 118 |
- aDuring the first 2 d of cultivation, six to 16 replicates.
- bBased on OD measured after 4 d (Synechocystis) or 6 d (green algae).
- cMean values of variants with CR and those of corresponding controls without CR differed from each other with a probability of error <1%.
Shaken control cultures of P. subcapitata were compared with cultures that were aerated by bubbling a stream of air through the cell suspension. Growth of aerated cultures was significantly faster than the nonaerated controls. However, until day 4, the increase in OD obtained in convectively aerated cultures remained significantly lower than the increase measured in the presence of a CR (Fig. 2B). The whole experiment shown was repeated with the same result two times. We can exclude that the difference between the growth rate of the aerated suspension and the suspension supplied with the CR was due to different ratios between the OD and the dry weight (data not shown).
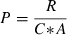 (1)
(1)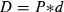 (2)
(2)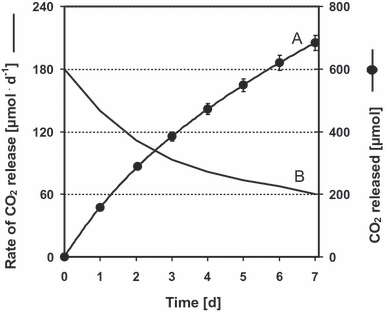
Release of CO2 from CO2 reservoirs (CRs) to sodium hydroxide solution. Mean values of 10 parallel experiments with SD. (A) Cumulative function of the daily measurements (scale on the right axis). The equation [y = −0.1096x4 + 2.4472x3 – 23.501x2 + 179.88x] fits to the measuring points (R2 = 1.000). (B) Time dependence of the CO2-releasing rate (scale on the left axis).
Measured values for CO2 diffusion and the derived parameters P and D are given in Table 2. During the first 2 d, the measured daily CO2 release from a CR was more than two times higher than the daily CO2 influx through the gas-permeable screw cap.
| CO2 diffusion from ambient air into the culture flask through the screw cap (zero-concentration inside) | 72 ± 3 μmol · d−1 |
| Initial rate of CO2 release from a CR (R), derived from curve B (Fig. 4) | 180 μmol · d−1 = 2.1 nmol · s−1 |
| Permeability coefficient of the membrane for CO2 (P) | 1.36 μm · s−1 |
| Diffusion coefficient of the membrane material (D) | 27.2 μm2 · s−1 |
- Calculation of P and D is based on the following dimensions of the CR: thickness of the LDPE membrane, 20 μm; mean surface area (A), 1,194 mm2; initial CO2 concentration difference across the membrane (ΔC), 1.28 mol · m−3 (32 mbar). CR, CO2 reservoir.
A comparison of CO2 supply by a CR with the carbon demand of net photosynthesis has been carried out using the data on dry weight increase of P. subcapitata (Table 3). During the first culture day, the amount of CO2 released from a CR would suffice for the formation of 4.7 mg of carbohydrate, whereas the dry weight increase in the culture of P. subcapitata during the first day was only 0.5 mg. To compare supplied with required molar amounts, the measured increase in dry weight was transformed to potential carbon consumption assuming a carbon content of 1 mol per 30 g dry biomass (the carbon content of carbohydrate). Although this calculation does not consider the different carbon content of carbohydrates, lipids, and proteins and neglects the minerals, an excess of carbon supply over carbon net assimilation during the first 2 d is indicated by the data shown in Table 3. However, at day 3, when the increase in dry weight reached its maximum, the estimated carbon requirement for assimilation and the CO2 supply from the CR became comparable in size.
| Culture day | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Increase in dry biomass in the culture flaska [mg · d−1] | 0.50 | 1.97 | 2.93 | 2.13 |
| Carbohydrate producible by CO2 supplied from a CR [mg CH2O · d−1] | 4.7 | 4.0 | 2.9 | 2.6 |
| CO2 supplied by a CRb [μmol CO2 · d−1] | 157.7 ± 3.8 | 131.8 ± 7.1 | 95.5 ± 3.8 | 86.8 ± 3.8 |
| Carbon required for the increase in biomass in the culture flaskc [μmol C · d−1] | 16.7 | 65.7 | 97.6 | 71.0 |
- aMeasurement with cultures of Pseudokirchneriella subcapitata (Fig. 2).
- bDaily measurements, compare Materials and Methods.
- cBased on an assumed ratio of 30 g dry biomass per mol of assimilated carbon.
Discussion
In all cultures studied, the presence of the CR in the culture flask allowed exponential growth and high specific growth rates during the first 2 d of culture. In the absence of a CR, growth was significantly lower than in its presence and seemed to be nearly linear. This proves a carbon limitation in control flasks without CR. Interestingly, CO2 supply by the CR stimulated growth of C. reinhardtii even in the presence of acetate in the growth medium. As mentioned in the introduction, the supply of inorganic carbon as CO2 can prevent a nondesirable increase in pH and alkalinity of the culture medium. Indeed, the pH of the cultures with CR showed only a moderate increase (from ∼6 to ∼8) during the first 3 d (data not shown).
The technique described for supply of CO2 has the advantage that it can be used with any culture vessel. In comparison to aeration with an optimum CO2 concentration, the use of the technique described here is especially efficient if numerous batch cultures have to be studied in parallel. A large set of axenic cultures can be grown without carbon limitation under identical conditions, and their growth can be conveniently analyzed. Since the flasks were shaken in horizontal position, the length of the light path through the shaken culture was shorter (∼10 mm) than the light path for the measurement of the OD (20 mm). Therefore, a sensitive growth measurement was possible before the light intensity within the culture was strongly reduced.
Previous applications of carbonate buffers for CO2 supply to cultures were based on special culture flasks with an inserted chamber for the buffer. Using such a technique, it is not possible to combine sustainable CO2 supply with rapid gas exchange between the vessel atmosphere and ambient air. Rapid gas diffusion through the porous membrane of the screw cap of the 50 mL Cellstar® vessel is an important feature of the method described here, since it prevents accumulation of oxygen in the culture medium.
Although complete depletion of the CR over a short time can be avoided by membrane control of CO2 release during the first days of culture, growth and carbon demand were increasing, whereas the rate of carbon supply was decreasing. With the chosen dimensions of the CR, carbon limitation could be prevented only during the first 2 d. During this period, growth was exponential, and the molar CO2 supplied by the CR was several times higher than required for assimilation. Subsequently, when growth became stationary, the carbon requirement was comparable to carbon release from a CR (Table 3).
CO2 release decreased to one-half of its initial rate within 4 to 5 d (Fig. 4, curve B). At the same time, the amount of total inorganic carbon in a CR decreased only slightly (from 7.5 to 6.95 mmol). The rapid decline in CO2 release reflects the change in CO2 partial pressure of the buffer enclosed in the LDPE bag, which in turn, is due to an increase in its alkalinity. The permeability coefficient of the LDPE membrane and the diffusion coefficient of the membrane material for CO2 (Table 2) are in the range known for thin LDPE foils (Liesl 2002). These parameters can be used for designing CRs with higher capacity and better sustainability.
Diffusion of CO2 from ambient air through the gas-permeable screw cap of the culture flasks could be limiting for assimilation in the absence of the CR, and this might explain the relatively slow growth of the control variants, and also the growth-stimulating effect of bubbling air through the medium (Fig. 2). However, a strong limitation of growth by the diffusion resistance of the screw cap is not in accord with our measurements. Unidirectional diffusion of CO2 from ambient air into the flasks amounted to 72 μmol · d−1, whereas the daily dry weight increase of photoautotrophic cultures in the absence of a CR was <0.5 mg · d−1, corresponding to a synthesis of <16 μmol CH2O. Obviously, the permeability of the screw cap for CO2 was high enough to prevent a severe reduction of the CO2 concentration within the gas space of the flask, at least during the first days. A possible explanation of the difference between the different variants of carbon supply from ambient air might be limitations of the carbon flux by the resistances of gas dissolution and diffusion. Indeed, we found a significant growth promotion for P. subcapitata in the control variant, when the shaking frequency was increased (A. Wüstenberg, unpublished data). Further experiments are needed to determine whether growth stimulation by the CR in the studied cultures was due to a relatively high value of the concentration of inorganic carbon, which is optimal for growth, or merely due to limitations of the carbon flow from the gas phase to the cells in the absence of the CR.
All the cultures studied here were freshwater organisms studied in a medium without bicarbonate. Since aerated seawater has a much higher equilibrium concentration of inorganic carbon than the studied freshwater media, it might be interesting in future to investigate whether growth of marine algae can be stimulated by the CR.
Finally, we would like to emphasize that the CRs described are suitable for preventing carbon limitation and maintaining exponential growth in shaken small-scale batch cultures of algae and cyanobacteria. The method allows the measurement of specific growth rates without special equipment and can be carried out in a short time with a large number of cultures in parallel. It was not the aim of this study to increase the biomass concentration or to improve the efficiency of energy utilization in photobioreactors. However, our results might encourage the application of diffusive carbon supply through thin PE membranes in biotechnological applications.
Acknowledgments
The authors thank Dr. Christian Steinberg for critical comments.