Nodal proteins are target antigens in Guillain-Barré syndrome
Abstract
Neurofascin-186 (NF186), neuronal cell adhesion molecule (NrCAM), and gliomedin are adhesion molecules playing a central role in the formation of nodes of Ranvier. In Guillain-Barré syndrome (GBS), immune attack toward the nodes may participate in the disabilities. Autoantibodies to NF186 and gliomedin have been detected in a rat model of GBS. Here, we investigated the prevalence of antibodies against nodal adhesion molecules in patients with GBS or chronic inflammatory demyelinating polyneuropathy (CIDP). Sera from 100 GBS patients, 50 CIDP patients, 80 disease controls, and 50 healthy controls were tested for their ability to bind the nodes of Ranvier. To characterize the antigens, we performed cell binding assays against NF186, gliomedin, contactin, and NrCAM. We found that 43% of patients with GBS and 30% of patients with CIDP showed IgG fixation at nodes or paranodes. In eight patients with GBS or CIDP, we identified that IgG antibodies recognized the native extracellular domain of NF186, gliomedin, or contactin. Also, 29 patients showed IgM against nodal adhesion molecules. However, we did not detect IgM fixation at nodes or paranodes. Antibodies to gliomedin or NF186 were mostly detected in demyelinating and axonal GBS, respectively. The adsorption of the antibodies to their soluble antigens abolished IgG deposition at nodes and paranodes in nerves, indicating these were specific to NF186, gliomedin, and contactin. In conclusion, gliomedin, NF186, and contactin are novel target antigens in GBS. At nodes, additional epitopes are also the targets of IgG. These results suggest that antibody attack against nodal antigens participates in the etiology of GBS.
Introduction
The node of Ranvier is a key site of injury in several immune neuropathies: Guillain-Barré syndrome (GBS), chronic inflammatory demyelinating polyneuropathy (CIDP), and multifocal motor neuropathy (MMN) (Santoro et al., 1990; Griffin et al., 1996; Hafer-Macko et al., 1996a; 1996b). In acute motor axonal neuropathy (AMAN), an axonal variant of GBS, immune attack directed against gangliosides located at the nodal and paranodal membranes may lead to axonal degeneration and paralysis (Hafer-Macko et al., 1996a; Kusunoki et al., 1997; Sheikh et al., 1999; Gong et al., 2002; Susuki et al., 2007). Autoantibodies to gangliosides are associated with AMAN (Willison and Yuki, 2002). Also, rabbits immunized with gangliosides developed anti-GM1 antibodies and an axonal neuropathy (Susuki et al., 2003). Acute inflammatory demyelinating polyneuropathy (AIDP) is the most common form of GBS in Europe and North America (Hadden et al., 1998). In AIDP, the immune attack seems to target the Schwann cell membrane and to result in vesicular demyelination (Hafer-Macko et al., 1996b), however, the immune targets remain unknown.
The molecular complexes governing node formation and reciprocally nodal dysfunction in peripheral neuropathies have recently been uncovered (Devaux and Scherer, 2005). At nodes, the interaction between gliomedin, expressed on the Schwann cell microvilli, and neurofascin-186 (NF186) on nodal axolemma is crucial for voltage-gated sodium (Nav) channel aggregation (Eshed et al., 2005; Thaxton et al., 2011). In addition, the paranodal axoglial junctions composed of contactin-associated protein (Caspr)/contactin/neurofascin-155 (NF155) form a barrier to the lateral diffusion of nodal channels (Bhat et al., 2001; Boyle et al., 2001; Charles et al., 2002; Zonta et al., 2008; Feinberg et al., 2010; Thaxton et al., 2010). In a rat model of AIDP, we showed that paranodal demyelination together with the disruption of NF186 and gliomedin at nodes resulted in the dispersion of Nav channel clusters and severe conduction loss (Lonigro and Devaux, 2009). These alterations were associated with autoantibodies against neurofascin and gliomedin. This suggested that antibodies to nodal adhesion molecules may participate in the pathogenesis of AIDP. Here, we examined whether nodal and paranodal proteins are the targets of the immune response in patients with GBS and CIDP.
Materials and Methods
Sera
Patients with GBS (n = 100) and CIDP (n = 50) were referred to the neuroimmunological laboratory at Dokkyo Medical University (Tochigi, Japan) from hospitals throughout Japan for serum anti-ganglioside antibody testing. The Ethical Committee of Dokkyo Medical University approved the study. Written informed consent was obtained from all patients. Sera from GBS patients were collected within 3 weeks of the onset of neurologic symptoms and before the administration of any treatments. Information on patients regarding the age, antecedent illnesses, initial symptoms, and neurologic signs during the course of the illness was obtained. GBS patients were then classified as having AMAN (n = 50) or AIDP (n = 50) according to the electrodiagnostic criteria (Ho et al., 1995). CIDP patients were classified in accordance with the diagnostic criteria published by the American Academy of Neurology AIDS Task Force (1991). Patients' disabilities at nadir were evaluated using the Hughes functional grading scale (Hughes et al., 1978). Serum IgG and IgM antibodies to GM1, GM1b, GD1a, GalNAc-GD1a, GD1b, GT1a, and GQ1b were measured by ELISA as described previously (Odaka et al., 1998). Normal control (NC) sera were obtained from 50 healthy volunteers. Patients with other neurological disorders (OND; n = 80) included patients with acute disseminated encephalomyelitis (n = 20), multiple sclerosis (n = 20), myasthenia gravis (n = 20), and amyotrophic lateral sclerosis (n = 20).
Constructs
Rat gliomedin (NM_181382.2) was amplified by PCR from a rat sciatic nerve cDNA library and the PCR product was sub-cloned into the mammalian expression vector pcDNA3.1 (Invitrogen, Paisley, UK) at KpnI and EcoRV sites. Myc epitope was then inserted at the C-terminus of the protein by site-directed mutagenesis. Full length rat NF186 (NM_001160314.1; HA-tagged), rat contactin (NM_057118), rat neuronal cell adhesion molecule (NrCAM) (NM_013150; HA-tagged) were the kind gift of Drs. Vann Bennett (Duke University Medical Center, Durham, NC), Elior Peles (The Weizmann Institute of Science, Rehovot, Israel), and Catherine Faivre-Sarrailh (CNRS-UMR6231, Marseille, France).
Cell binding assay
Human embryonic kidney (HEK) cells were transiently transfected with NF186, gliomedin, contactin, or NrCAM using JetPEI (Polyplus-transfection, Illkirch, France). Using our protocol, about 60% of the cells expressed NF186, gliomedin, contactin, or NrCAM. One day after transfection, cells were trypsinated, suspended in serum free Opti-MEM medium (Invitrogen), and plated onto poly-l-lysine coated glass coverslips in 24-well plates at a density of 100,000 cells/well. After 1 day, living cells were incubated for 20 min with 50 µl of serum diluted at 1/20 to 1/5,000 in blocking solution with FITC conjugated anti-human IgM (1/50) or TRITC conjugated anti-human IgG (1/50). Cells were washed thrice in PBS, fixed, and blocked as described above. Cells were then incubated for 1 h with primary antibodies - rat monoclonal antibodies against hemagglutinin (HA; 1/200; Roche, Basel, Switzerland), mouse monoclonal antibodies against Myc (1/500; Roche), or a goat antiserum against contactin (1/2,000; R&D Systems, Minneapolis, MN, USA). The cells were then washed and revealed with the appropriate Alexa conjugated secondary antibodies (1/500; Invitrogen). Cells were stained with DAPI, and mounted with Mowiol plus 2% DABCO (Sigma-Aldrich, St. Louis, MO, USA). Patients with serum IgG or IgM that specifically co-localized with NF186, gliomedin, contactin, or NrCAM at cell surface were considered positive. Patients with serum IgG or IgM that bound to non-transfected cells were considered negative. For competition experiments, the extracellular domain of NF186 (NF186-Fc), gliomedin (gliomedin-Fc), and contactin (contactin-Fc) fused to human Fc were obtained as described previously (Charles et al., 2002; Eshed et al., 2005). Sera were pre-incubated for 30 min at room temperature with the Fc fusion proteins (0.5 µg) before incubation with living HEK cells.
Nerve staining
Teased fibers from sciatic nerves of adult C57BL/6J mice and adult Wistar rats were prepared as previously described (Lonigro and Devaux, 2009). Teased fibers were permeabilized by immersion in −20°C acetone for 10 min, blocked for 1 h in blocking solution containing 0.1% Triton X-100 and incubated overnight at 4°C with sera diluted at 1/200 to 1/5,000. In some experiments, tissues were double-stained with mouse antibodies against Nav channels (1/500; Sigma-Aldrich) or a rabbit antiserum against Caspr (1/3,000) (Menegoz et al., 1997). The slides were washed and incubated with the appropriate Alexa conjugated secondary antibodies (1/500; Invitrogen). Slides were mounted and examined using an ApoTome fluorescence microscope (ApoTome, AxioObserver and AxioCam MRm, Carl Zeiss MicroImaging GmbH, Jena, Germany). Digital images were manipulated into figures with Adobe Photoshop. For competition experiments, sera were pre-incubated for 30 min at room temperature with the Fc fusion proteins (0.5 µg) before immunolabeling.
Statistical analysis
Differences in group frequencies were compared using GraphPad Prism by chi-square test with Yate's correction or by Fisher's exact test with Bonferroni's adjustment (GraphPad Software, La Jolla, CA, USA). p Values inferior to 0.05 were considered significant. Correlation between the presence of antibodies to gangliosides and the presence of antibodies to NF186/gliomedin/contactin/NrCAM was examined by Spearman's rank correlation test.
Results
GBS and CIDP sera target the nodes of Ranvier
First, we investigated whether IgG from GBS and CIDP patients may bind the nodes of Ranvier or the flanking paranodes. Because of the high background observed when using sera on rat nerves or at a 1/50 dilution on mouse nerves, we tested the GBS, CIDP, and control sera at a 1/200 dilution on teased sciatic nerves from mice. We found that many sera from GBS and CIDP patients labeled the nodal and paranodal regions (Table 1), and particularly co-localized with Nav channels at nodes or with Caspr, a paranodal adhesion molecule (1, 4). Nodes were more frequently stained than the paranodes, and 22% of GBS sera stained both structures. IgG that bound to the nodes were detected at a similar frequency in AIDP and AMAN (34%). Eight AIDP patients showed IgG binding restricted to the nodal region, compared to four AMAN patients (Fig. 1A and 1B). Many patients' sera still labeled the nodal or paranodal regions at a 1/500 and a 1/2,000 dilution. In non-permeabilized tissue, these sera stained the nodes and microvilli (data not shown), suggesting they target extracellular epitopes. By comparison, no sera from healthy controls stained nodes or paranodes at a 1/200 dilution (Table 1). Also, only 3 patients with OND (n = 80), which included immune mediated neurological disorders (acute disseminated encephalomyelitis, multiple sclerosis, and myasthenia gravis), showed IgG binding to the nodal region at a 1/200 dilution. This suggested that nodal staining was specific and not simply due to a rise in immunoglobulin levels. Our results thus emphasized that a high percentage of AIDP (44%), AMAN (42%), and CIDP (30%) patients showed antibodies to nodal antigens. Worth noting, the profiles of IgG deposition were reminiscent of the localization of cell adhesion molecules at nodes and paranodes, suggesting that autoantibodies target the axoglial junctions.
| Percentage of sera that stained | NC | GBS | AIDP | AMAN | CIDP | OND |
|---|---|---|---|---|---|---|
| Node | 0 | 34 (12)* | 34 (16)* | 34 (8)* | 20 (12)† | 3.8 (3) |
| Paranode | 0 | 31 (9)* | 28 (10)* | 34 (8)* | 18 (8)† | 0 |
| Any compartments | 0 | 43* | 44* | 42* | 30 | 3.8 |
- Sera were diluted 1/200, tested on mouse sciatic nerves, and revealed for IgG deposition. The percentage of sera that stained only the indicated axonal compartment is shown in parentheses.
- AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; CIDP, chronic inflammatory demyelinating polyneuropathy; GBS, Guillain-Barré syndrome; NC, normal control; OND, other neurological disorders.
- *p < 0.001.
- †p < 0.05 compared with NC and OND using a χ2 test with Yate's correction or Fisher's exact test with Bonferroni's correction.
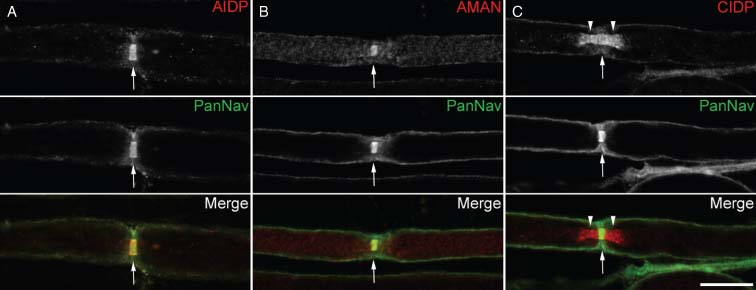
Serum IgG from Guillain-Barré syndrome (GBS) and chronic inflammatory demyelinating polyneuropathy (CIDP) patients bind the nodes of Ranvier and paranodes. These are distinct sera from (A) acute inflammatory demyelinating polyneuropathy (AIDP), (B) acute motor axonal neuropathy (AMAN), and (C) CIDP patients tested at a 1/500 dilution on mouse sciatic nerve fibers. Teased fibers were double-stained for Nav channels (PanNav; green) and human IgG (red). IgG from several AIDP (A) and AMAN patients (B) bound specifically to the nodal region (arrows) and co-localized with Nav channels. In other patients, here CIDP patients, IgG specifically labeled the paranodal (arrowheads) and nodal domains (arrows; C). Scale bar: 10 µm.
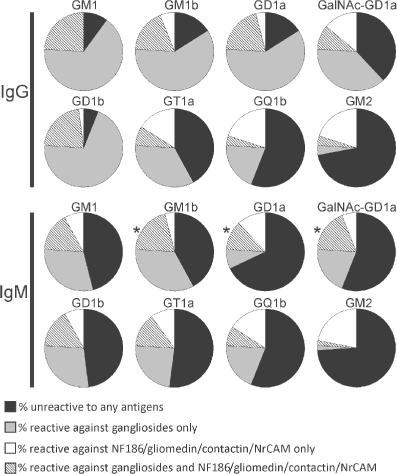
Serum reactivity of acute motor axonal neuropathy (AMAN) patients against gangliosides. These graphs represent the proportion of AMAN patients with IgG (upper panel) and IgM (lower panel) against gangliosides that are reactive (shaded) or non-reactive (gray) against neurofascin-186 (NF186)/gliomedin/contactin/NrCAM. The proportion of patients displaying autoantibodies to NF186/gliomedin/contactin/NrCAM, but not to the indicated gangliosides is shown in white. Those who did not react against any of these antigens are represented in black. The presence of antibodies to NF186/gliomedin/contactin/NrCAM was significantly correlated with the presence of IgM anti-GM1b, -GD1a, or -GalNAc-GD1a antibodies (*p < 0.05 using a χ2 test, Fisher's exact test, and Spearman correlation test).
NF186, gliomedin, and contactin are target antigens in GBS patients
Recent genetic investigations identified the constituents of the axoglial junctions at nodes and paranodes (Bhat et al., 2001; Boyle et al., 2001; Charles et al., 2002; Eshed et al., 2005; Zonta et al., 2008; Feinberg et al., 2010; Thaxton et al., 2011). Because these proteins play a central role in saltatory conduction, we examined whether GBS and CIDP patients show antibodies targeting NF186, gliomedin, contactin, or NrCAM. In order to quantify exactly the serum titers, we tested serial dilutions of the sera by immunofluorescence on living cells transfected with these adhesion molecules. This procedure enables staining of cell surface antigens as in flow cytometry analyses, and has the advantage to allow convenient screening of large cohorts of patients. In addition, we found that this technique was more reliable than ELISA to discriminate true antibody reaction against cell adhesion molecules. We found that many sera presented IgG or IgM that bound selectively to NF186, contactin, or gliomedin (Table 2) and co-localized with these at cell surface but did not stain untransfected cells (Fig. 2). The data in Table 2 represent the proportion of patients that complied with these criteria. Sera that stained untransfected cells were considered negative. AIDP patients presented a significantly higher prevalence of antibodies directed against gliomedin (Table 2; p < 0.05; χ2 test with Yate's correction or Fisher's exact test with Bonferroni's adjustment) compared to healthy controls and OND. By contrast, antibodies against NF186 were more frequently detected in AMAN patients. The prevalence of antibodies against NrCAM was not significantly increased in any neurological disorders. These results indicated that gliomedin and NF186 are autoantigens in GBS patients. Of interest, many patients showed autoantibodies recognizing a single antigen (Table 2). When examined regardless of the targeted antigen or immunoglobulin class, it appeared that 26% of GBS and 24% of CIDP patients showed autoantibodies to nodal adhesion molecules with titers ranging from 1/50 to 1/1,000. In particular, the titers of anti-NF186 antibodies were higher in AMAN patients (mean titer around 1/200) compared to anti-gliomedin or anti-contactin antibodies (mean titer around 1/50). Only 2 of 50 control sera reacted against NF186, contactin, or NrCAM. Patients with OND or healthy controls did not present an increased humoral response against the tested proteins. The cell binding assay thus suggested that the humoral reactivity toward nodal adhesion molecules was more prevalent in GBS and CIDP.
| Reactivity against | |||||||
|---|---|---|---|---|---|---|---|
| NF186 | Gliomedin | NrCAM | Contactin | Any antigens | Only one antigen | Multiple antigens | |
| GBS | 15 (3) | 12 (1) | 4 | 12 (2) | 26† | 16 | 10 |
| AIDP | 12 (1) | 14 (1)† | 6 | 16 (2) | 28* | 16 | 12 |
| AMAN | 18 (2)† | 10 | 2 | 8 | 24† | 16 | 8 |
| CIDP | 12 (2) | 6 | 2 | 16 (2) | 24† | 16 | 8 |
| OND | 0 | 1.3 | 0 | 3.8 | 3.8 | 2.5 | 1.3 |
| NC | 2 | 0 | 2 | 2 | 4 | 2 | 2 |
- Sera were tested at dilutions ranging from 1/20 to 1/5,000 on living human embryonic kidney (HEK) cells transfected with NF186, gliomedin, NrCAM, or contactin and were then revealed for IgM and IgG deposition. Table values represent the percentage of sera with IgM or IgG against nodal proteins. The respective numbers of IgG-positive sera are indicated in parentheses.
- AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; CIDP, chronic inflammatory demyelinating polyneuropathy; GBS, Guillain-Barré syndrome; NC, normal control; NF186, neurofascin-186; OND, other neurological disorders.
- *p < 0.01.
- †p < 0.05 compared with NC and with OND using a χ2 test with Yate's correction or Fisher's exact test with Bonferroni's correction.
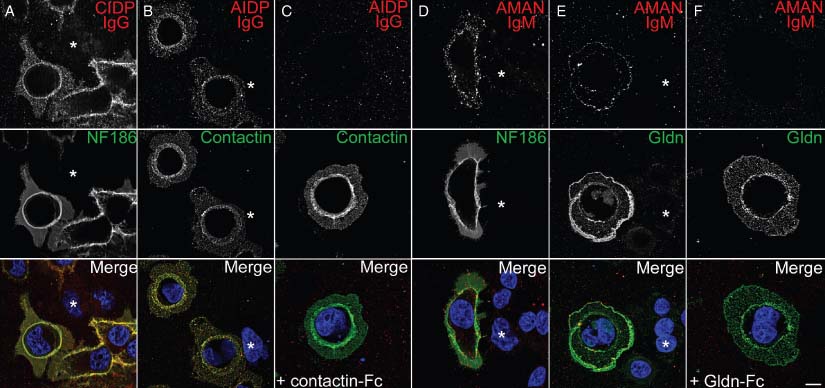
Guillain-Barré syndrome (GBS) and chronic inflammatory demyelinating polyneuropathy (CIDP) sera bind onto transfected human embryonic kidney (HEK) cells. HEK cells were transiently transfected with NF186 (A, D), contactin (B, C), or gliomedin (E, F), and living cells were incubated with representative sera from (A) acute inflammatory demyelinating polyneuropathy (AIDP), (C, D) acute motor axonal neuropathy (AMAN), or (B) CIDP patients. HEK cells were then stained for neurofascin-186 (NF186), contactin, or gliomedin (Gldn) as indicated. IgG or IgM from these patients specifically bound to the surface of transfected cells and co-localized with the target antigens, but did not bind untransfected HEK cells (asterisks). Pre-incubation of sera with soluble contactin-Fc (C) or gliomedin-Fc (Gldn-Fc; F) abolished the binding of IgG and IgM to the surface of transfected cells. Scale bar: 10 µm.
To confirm the specificity of IgG and IgM binding, positive sera were pre-incubated with the soluble extracellular domains of contactin, gliomedin, or NF186. The binding of IgG and IgM to the surface of transfected cells was completely antagonized by the soluble antigens (Fig. 2), corroborating that nodal adhesion molecules are the targets of antibodies in GBS and CIDP patients.
Autoantibodies from GBS patients target adhesion molecules in peripheral nerves
As indicated in Fig. 1 and Table 1, we found that 58 patients with GBS or CIDP showed IgG deposition at nodes of Ranvier and paranodes. Using cell binding assay, we found that eight of these patients exhibited IgG to NF186, gliomedin, or contactin (Table 2). In addition, 30 patients showed IgM to NF186, gliomedin, contactin, or NrCAM (Table 2). We thus reinvestigated more closely whether autoantibodies against NF186, gliomedin, or contactin may be responsible for IgG deposition. Also, we examined whether serum IgM from positive GBS and CIDP patients bound to the nodal/paranodal regions. We did not detect IgM deposition at nodes or paranodes with any of the tested sera, even at a 1/50 dilution. By contrast, the sera from the eight patients with IgG to NF186, gliomedin, or contactin stained the nodal and/or paranodal regions in permeabilized sciatic nerve fibers (Fig. 3A–D). Both sensory and motor axons were labeled with the sera, but also Remak bundles. In particular, two sera with anti-NF186 IgG stained specifically the nodes and co-localized with Nav channels. Four sera stained the nodes and paranodal junctions, these showed anti-NF186 IgG (Fig. 3) or IgG to multiple antigens. Finally, two sera with anti-contactin IgG labeled specifically the paranodal junctions (Fig. 3A). In order to test the specificity of these autoantibodies, we incubated the sera with the soluble extracellular domains of contactin or NF186. The staining of the nodal or paranodal regions was abolished after incubation of the sera with the soluble antigens (Fig. 3B and 3D), indicating they specifically target these nodal and paranodal adhesion molecules. Nerve fibers were colabeled for Caspr, a marker of paranodal junctions. Caspr staining was not affected by the soluble antigens, confirming the specificity of patients' autoantibodies. Altogether, this indicated that NF186, gliomedin, and contactin are target autoantigens in nerve tissues. Importantly, among the 58 patients with GBS or CIDP that showed IgG fixation at nodes and paranodes, 50 patients did not exhibit IgG to NF186, gliomedin, or contactin. Figure 1 shows labeling obtained with these patients' sera. These results emphasized that autoantibodies may target a variety of nodal antigens. These may be adhesion molecules or other antigens.
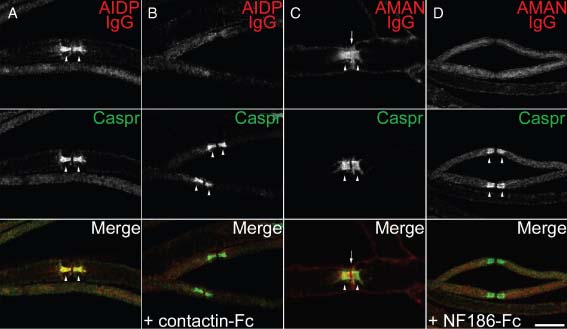
The binding of Guillain-Barré syndrome (GBS) and chronic inflammatory demyelinating polyneuropathy (CIDP) patients' IgG to nodes of Ranvier is blocked by soluble antigens. Mouse sciatic nerve fibers were incubated with sera (red) from (A, B) acute inflammatory demyelinating polyneuropathy (AIDP) or (C, D) acute motor axonal neuropathy (AMAN) patients and were stained for Caspr (green) to label paranodes. Pre-incubation of AIDP serum with soluble contactin-Fc (B) abolished the binding of IgG to contactin at paranodes (arrowheads). Also, pre-incubation of AMAN serum with soluble NF186-Fc (D) abolished the binding of IgG to neurofascin at nodes (arrows) and paranodes (arrowheads). Scale bar: 10 µm.
Clinical features of GBS and CIDP patients reactive to nodal adhesion molecules
The clinical features of the GBS patients with antibodies to gliomedin, contactin, NF186, and NrCAM are shown in Table 3. A higher proportion of AMAN patients with antibodies to nodal adhesion molecules were older than 60 years (58% vs. 18% in patients lacking antibodies to nodal adhesion molecules). Also, a higher percentage of these patients presented respiratory disturbances. These disturbances were not related to the age of the patients and were observed in both young and elderly patients. No correlation was found between antecedent illness and the presence of antibodies to nodal adhesion molecules. However, we observed that antibodies to NF186/gliomedin/contactin/NrCAM were significantly correlated with the presence of IgM against GM1b, GD1a, and GalNAc-GD1a in AMAN patients (Fig. 4; p < 0.05 using a Spearman correlation test). In AIDP patients, the clinical features were not significantly different between patient groups (Table 3).
| AIDP | AMAN | |||
|---|---|---|---|---|
| Unreactive to nodal antigens | Reactive to nodal antigens | Unreactive to nodal antigens | Reactive to nodal antigens | |
| Patient characteristics | n = 36 | n = 14 | n = 38 | n = 12 |
| Median age (range) | 48 (1–76) | 53 (1–84) | 45 (16–79) | 63 (14–83) |
| Patients older than 60 (%) | 46 | 51 | 18 | 58* |
| Antecedent illnesses | ||||
| Patients with antecedent infectious symptoms (%) | 72 | 50 | 82 | 83 |
| Patients with fever (%) | 53 | 29 | 37 | 50 |
| Patients with respiratory infection (%) | 50 | 57 | 29 | 42 |
| Patients with gastroenteritis (%) | 17 | 14 | 58 | 58 |
| Patients with headache (%) | 11 | 7 | 8 | 8 |
| Initial symptoms | ||||
| Patients with upper limb weakness (%) | 22 | 7 | 50 | 0* |
| Patients with lower limb weakness (%) | 22 | 43 | 29 | 50 |
| Patients with both weaknesses (%) | 8 | 7 | 10 | 25 |
| Neurological signs during the course of the illness | ||||
| Patients with respiratory disturbance (%) | 17 | 29 | 8 | 42* |
| Patients with neck weakness (%) | 39 | 36 | 68 | 67 |
| Patients with upper limb weakness (%) | 92 | 100 | 97 | 92 |
| Patients with lower limb weakness (%) | 89 | 93 | 97 | 92 |
| Patients with ataxia (%) | 11 | 21 | 3 | 8 |
| Grade at nadir | ||||
| Median (range) | 4 (1–5) | 4 (1–6) | 3 (1–5) | 3.5 (1–6) |
| Patients with grade ≥5 (%) | 17 | 29 | 8 | 33 |
- AIDP, acute inflammatory demyelinating polyneuropathy; AMAN, acute motor axonal neuropathy; GBS, Guillain-Barré syndrome; NF186, neurofascin-186.
- *p < 0.05 compared with patients unreactive to nodal antigens using a χ2 test with Yate's correction or Fisher's exact test with Bonferroni's correction.
Discussion
In summary, we discovered that 43% of GBS and 30% of CIDP patients present IgG targeting nodal and paranodal antigens in rodent nerves. Using combined cell binding and nerve binding assays, we identified NF186, gliomedin, and contactin as the immune targets of autoantibodies in many of these patients. Particularly, we showed that patients' IgG colocalized with Nav channels at nodes and with Caspr at the paranodal septate-like junctions. We did not test whether GBS sera bind human nerve fibers. First, because motor nerve biopsies cannot be obtained from healthy donors; then, because the detection of nodal and paranodal proteins is highly sensitive to fixation procedures, which precludes the use of autopsies. Nonetheless, the amino acid sequence of nodal and paranodal components are highly homologous (87%–99%) in humans and rodents, and the organization of nodes of Ranvier is similar in both species (Li et al., 2005; Saporta et al., 2009). These data thus corroborate that the node is the locus of the immune attack in these neuropathies and suggest that several antigens located at nodes and paranodes in human nerves are targets of IgG antibodies.
A recent investigation using ELISA has also revealed that NF155 is a target of autoantibodies in GBS (Pruss et al., 2011). Here, we examined the prevalence of autoantibodies against four nodal proteins in a wider group of patients with definite cases of AMAN, AIDP, or CIDP. In a first attempt, we used cell-ELISA assays to detect serum reactivity against nodal adhesion molecules. However, many sera positive by ELISA displayed unspecific aggregation by cell-binding assay and were false positive. In cell-binding assays, we showed that IgG or IgM fixation to the cell surface was specific and was antagonized by soluble antigens. It thus appears that cell-binding assay or flow cytometry are more reliable techniques to discriminate a positive antibody reaction against cell adhesion molecules. We did not examine the prevalence of antibodies against NF155 which differ modestly from NF186 (Davis et al., 1996). Nonetheless, some sera reactive against NF186 stained the nodes and paranodes, suggesting these may recognize both isoforms. Staining was abolished after pre-incubation with soluble NF186, demonstrating that these sera recognized the extracellular domain of nodal and paranodal neurofascin isoforms.
Our study also indicates that many sera bound nodal and paranodal antigens other than NF186, gliomedin, contactin, or NrCAM. Antibodies to NF155 and Caspr may account for some of the staining. We did not examine whether Caspr is an immune target, because Caspr is poorly expressed at the cell surface and requires contactin for surface expression (Bonnon et al., 2003). However, proteomic approaches may help to identify novel antigens. Considering gangliosides are found concentrated at nodal and paranodal regions (Kusunoki et al., 1997; Sheikh et al., 1999; Gong et al., 2002), IgG anti-ganglioside antibodies may also account for some of the nodal/paranodal staining. IgG antibodies against GM1, GM1b, GD1a, and GalNAc-GD1a are strongly associated with AMAN, but are detected in <20% of AIDP patients (Yuki, 2007). In many patients, we found that IgG fixation co-localized with Nav channels expressed at nodal membrane or with Caspr at paranodal septate-like junctions. Albeit, anti-GM1 or anti-GD1b antibodies, or cholera toxin stain nodal and paranodal structures, they do not stain specifically the nodal axolemma or paranodal septate-like junctions (Kusunoki et al., 1997; Molander et al., 1997; Sheikh et al., 1999; Susuki et al., 2007). Hence, it seems implausible that all the positive sera were targeting gangliosides, and more particularly in AIDP. Owing to the broad clinical and epidemiological pictures of GBS, this is not surprising that a wide range of antigens are involved in the pathogenesis of GBS. Further works are needed to identify the variety of nodal and paranodal antigens, notably whether these antigens are axonal or glial.
Here, we revealed that NF186, gliomedin, and contactin are novel target antigens in patients with GBS. Clinical analyses showed that anti-NF186 antibodies were more prevalent in AMAN, whereas anti-gliomedin antibodies were more prevalent in AIDP. This is in accordance with the axonal or glial localization of these molecules. We did not observe a significant antibody reaction toward these molecules in patients with central demyelinating disorders (multiple sclerosis and acute disseminated encephalomyelitis) or motor neuron disease (amyotrophic lateral sclerosis). This indicates that autoantibodies to nodal adhesion molecules are more prevalent in GBS forms and are not related to secondary immune reactions against demyelinated or damaged myelinated fibers. Antibodies to neurofascin were previously described in some patients with multiple sclerosis in a group of 26 individuals (Mathey et al., 2007). Here, we did not detect any reaction against neurofascin in a group of 20 patients with multiple sclerosis. This indicated that the prevalence of these autoantibodies in multiple sclerosis may vary considerably. The causes generating these autoantibodies in GBS remain, however, unknown. The presence of autoantibodies did not correlate with any antecedent illnesses in particular. Worth noting, antibodies to NF186/gliomedin/contactin/NrCAM were closely associated with the presence of IgM to gangliosides in AMAN patients. Also, all patients with antibodies against NF186/gliomedin/contactin showed IgG to GM1. Molecular mimicry between Campylobacter jejuni lipo-oligosaccharides and human gangliosides has been implicated in triggering IgG/IgM to GM1 and GD1a (Yuki et al., 2004). Similarly, we can suspect that infectious agents showing molecular mimicry with nodal proteins may trigger the development of autoantibodies against NF186/gliomedin/contactin/NrCAM and simultaneously the development of IgM against gangliosides. Examination of broader populations showing autoantibodies against a single antigen should help to define the causes of autoimmunity.
Surprisingly, we did not detect IgM deposition at nodes or paranodes, albeit many patients showed IgM antibodies against gliomedin, NF186, contactin, or NrCAM. The importance of IgM against nodal adhesion molecules in GBS pathology is therefore uncertain. IgM typically bind with low affinity to antigens, and the mean titer of IgM to nodal adhesion molecules was around 1/100. At a 1/100 or lower dilution, we found that patients' sera generated an important background staining. In addition, our data indicate that most AMAN patients with IgM to nodal adhesion molecules also showed IgM to gangliosides. One explanation could thus be that GBS and CIDP patients have a large repertoire of IgM antibodies reactive against numerous glycoproteins or glycolipids that may preclude the detection of IgM deposition at nodes. The significance of IgM to nodal adhesion molecules remains to be clarified.
Are IgG autoantibodies to NF186, gliomedin, and contactin pathogenic? Because the genetic ablation of neurofascin or contactin leads to important conduction deficits (Boyle et al., 2001; Zonta et al., 2008), we can suspect that antibodies against these molecules may have deleterious effects on conduction either by blocking the association of neurofascin with contactin and gliomedin or through complement-mediated injury. In a model of AIDP, we found that alterations of nodal and paranodal adherent junctions were associated to IgG autoantibodies to gliomedin and NF186, and resulted in important conduction deficits (Novakovic et al., 1998; Lonigro and Devaux, 2009). Similarly, the administration of antibodies to neurofascin was shown to exacerbate axonal injury in an animal model of multiple sclerosis (Mathey et al., 2007). Taken together, these results suggest that these autoantibodies may actively participate in the pathogenesis of GBS. Surprisingly, GBS patients with autoantibodies to nodal adhesion molecules did not display more severe clinical symptoms. A plausible explanation for this could be that we examined sera from patients with definite cases of AMAN and AIDP that are likely to present similar clinical features. In addition, our data indicate that nearly 50% of the patients have antibodies recognizing nodal and paranodal antigens. The remaining patients may exhibit humoral or cellular responses against myelin or axonal antigens (proteins or glycolipids). Hence, all patients may show distinct immunological responses that lead to identical neurologic signs. Our observations do not, therefore, argue against a pathogenic function of antibodies to nodal antigens. In future prospects, the ability of patients' IgG to bind nodes of Ranvier should be taken into account, as this may influence the diagnosis and treatment of these particular GBS patients showing a humoral response against nodal antigens.
In conclusion, these results highlight the importance of the immune response against nodal glycoproteins in the etiology of GBS. In order to understand the pathogenesis of GBS, it appears clearly that the target antigens of IgG need to be identified, as well as the pathogenic function of these autoantibodies. Considering conduction block occurs proximally at nerve roots or distally at nerve endings, antibodies to nodal antigens may favor conduction failure at proximal or intermediate sites along the nerves. This should be taken into consideration in future investigations.
Acknowledgements
We thank Drs. E. Peles, V. Bennett, and C. Faivre-Sarrailh for generously providing constructs or antibodies and for thoughtful comments. This study was supported by the Association Française contre les Myopathies (MNM1 2010-14580), the GBS/CIDP Foundation International, and the CNRS.




