Epicardial development in lamprey supports an evolutionary origin of the vertebrate epicardium from an ancestral pronephric external glomerulus
Abstract
SUMMARY The epicardium is the outer layer of the vertebrate heart. Both the embryonic epicardium and its derived mesenchyme are critical to heart development, contributing to the coronary vasculature and modulating the proliferation of the ventricular myocardium. The embryonic epicardium arises from an extracardiac, originally paired progenitor tissue called the proepicardium, a proliferation of coelomic cells found at the limit between the liver and the sinus venosus. Proepicardial cells attach to and spread over the cardiac surface giving rise to the epicardium. Invertebrate hearts always lack of epicardium, and no hypothesis has been proposed about the origin of this tissue and its proepicardial progenitor in vertebrates. We herein describe the epicardial development in a representative of the most basal living lineage of vertebrates, the agnathan Petromyzon marinus (lamprey). The epicardium in lampreys develops by migration of coelomic cells clustered in a paired structure at the roof of the coelomic cavity, between the pronephros and the gut. Later on, these outgrowths differentiate into the pronephric external glomerulus (PEG), a structure composed of capillary networks, mesangial cells, and podocytes. This observation is consistent with the conclusion that the primordia of the most anterior pair of PEG in agnathans have been retained and transformed into the proepicardium in gnathostomes. Glomerular progenitor cells are highly vasculogenic and probably allowed for the vascularization of a cardiac tube primarily devoid of coronary vessels. This new hypothesis accounts for the striking epicardial expression of Wt1 and Pod1, two transcription factors essential for development of the excretory system.
INTRODUCTION
The heart in vertebrates is constituted of three cell layers, epicardium, myocardium, and endocardium, with a contribution of neural crest cells. Both myocardium and endocardium derive from the precardiac mesoderm. The epicardium is the outer layer of the vertebrate heart, and it gives rise to a mesenchymal cell population which is critical to heart development, as these epicardially derived cells (EPDC) contribute to the formation of the coronary vasculature and the cardiac interstitium (Vrancken-Peeters et al. 1999; Pérez-Pomares et al. 2002; Guadix et al. 2006). Furthermore, epicardium and EPDC induce the proliferation of the myocardium and it has been reported that their activity is essential for proper development of the ventricular compact layer (Stuckmann et al. 2003; Lavine et al. 2005; Merki et al. 2005). For this reason, anomalous epicardial development may cause severe heart malformations (Kwee et al. 1995; Yang et al. 1995).
The embryonic epicardium arises from an extracardiac and originally paired progenitor tissue called the proepicardium, a proliferation of coelomic cells at the limit beetween the liver and the cardiac sinus venosus in all the vertebrates so far studied (Manner et al. 2001). Both proepicardial primordia develop in fishes (Muñoz-Chápuli et al. 1994) whereas only the right one fully forms in birds (Schulte et al. 2007). In mammals both proepicardial primordia fuse in the midline forming a crescent that covers all the septum transversum area (Schulte et al. 2007). In all cases, proepicardial coelomic cells attach to the cardiac surface and spread on the myocardium, giving rise to the epicardium.
In contrast to the vertebrate cardiac bauplan, the hearts in non-vertebrate metazoans are usually formed by one or several myoepithelial cell layers but they always lack a epicardium. Despite this main difference, no hypothesis has hitherto been proposed about the evolutionary origin of this tissue and its proepicardial progenitor whose formation is dependent on molecular and cellular mechanisms that are poorly known (Schlueter et al. 2006).
We have studied the epicardial development in a representative of the most basal living lineage of vertebrates, the agnathan P. marinus (lamprey). Our results have revealed an unsuspected scenario on the evolutionary origin of the proepicardium, relating this tissue with the primitive excretory system of vertebrates. Surprisingly, a relationship between the epicardium and the kidneys had been previously suspected due to some common features in gene expression, but an evolutionary and developmental basis accounting for this relationship was lacking in the literature. The aim of this paper is to show our findings on the epicardial development in lampreys and to provide a novel model on the origin of the epicardium in vertebrates.
MATERIAL AND METHODS
This study was carried out in early prolarvae of the sea lamprey (P. marinus L.; n=22) reared from artificially fertilized eggs. Gametes were obtained from sexually mature adult lampreys caught during their upstream migration (from late May to early July) in the Ulla and Miño Rivers (northwest of Spain). The methods for maintaining the embryos and prolarvae were essentially the same as those described by Piavis (1971). The fertilized eggs were transferred to the hatchery incubators and placed into plastic trays. They were kept with circulating water and under appropriate conditions of darkness and temperature (18°C). Under these conditions, in our broods hatching occurred at 12–13 days postfertilization (dpf). Stages of prolarvae are indicated according to their age in days after fertilization. All experiments were conducted in accordance with European Community guidelines on animal care and experimentation to minimize pain and discomfort.
Prolarvae were fixed in Bouin's fixative, washed in alcohol, dehydrated in a graded series of ethanol, and embedded in paraffin. Transverse and sagittal sections (5–8 μm thick) were cut on a Leica RM 2145 microtome (Leica Microsystems, Barcelona, Spain) and subsequently stained with hematoxylin–eosin.
Dogfish embryos (Scyliorhinus canicula L.) were used for comparative purposes. Fertilized eggs were obtained from adult females collected in the Bay of Malaga (Western Mediterranean) by commercial trawl vessels. The eggs were kept in indoor tanks of well-aerated seawater. Egg capsules were open at intervals and the embryos were anesthetized in 0.04% tricaine methanesulfonate (MS-222, Sigma-Aldrich Co., St. Louis, MO, USA) in seawater and measured. Some embryos were fixed in 1.25% glutaraldehyde and 1% paraformaldehyde and embedded in Araldite 502 as described previously (Muñoz-Chápuli et al. 1996). Semithin sections (0.5 μm) were obtained with an ultramicrotome and stained with toluidine blue. Other embryos were fixed in methanol–acetone–water (2:2:1), paraffin embedded, sectioned, and stained as described above.
Immunolocalization of the Wilms' tumor suppressor transcription factor (Wt1) in avian embryos was performed as described previously (Carmona et al. 2001). Briefly, the embryos were excised and cryoprotected in 10%, 20%, and 30% sucrose solutions, embedded in OCT and snap frozen in liquid nitrogen-cooled isopentane. Cryostat sections were collected on poly-l-lysine-coated slides and fixed for 10 min in 1:1 methanol–acetone at −20°C. Then, the sections were rehydrated, the endogenous peroxidase activity was quenched by incubation for 30 min with 3% hydrogen peroxide in Tris-phosphate buffered saline (TPBS). Non-specific binding sites were saturated for 30 min with 16% sheep serum, 1% bovine serum albumin, and 0.5% Triton X-100 in TPBS (SBT). Endogenous biotin was blocked with the avidin–biotin blocking kit (Vector, Burlingame, CA, USA). Sections were subsequently incubated overnight at 4°C in polyclonal anti-human Wt1 (Sc-192, Santa Cruz, Heidelberg, Germany), diluted 1:500 in SBT, washed, incubated for 1 h at room temperature in biotin-conjugated anti-rabbit goat IgG (Sigma-Aldrich Co.) diluted 1:100 in SBT, washed again, and incubated for 1 h in avidin–peroxidase complex (Sigma-Aldrich Co.) diluted 1:150 in TPBS. After washing, peroxidase activity was developed with Sigma Fast® 3,3′-diaminobenzidine (D4168; Sigma-Aldrich Co.) tablets according to the indications of the supplier.
RESULTS
The epicardium in lampreys develops between 17 and 26 dpf, from a paired outgrowth of coelomic cells in the dorsal part of the coelomic cavity, between the pronephros and the gut (Fig. 1). Ciliated nephrostomes were always located cephalic and caudal to these cell clusters. The coelomic outgrowths are not yet developed in embryos of 12 dpf (Fig. 1A) while in prolarvae of 14 dpf, the earliest evidences of cell outgrowth in this area are already present (Fig. 1B). In prolarvae of 17 dpf, the right cell cluster attaches to the dorsal side of the ventricle, close to the atrioventricular junction (Fig. 1C). From this area, epicardial cells can be seen apparently spreading over the ventricular surface. However, the atrium and the ventral side of the atrioventricular groove are devoid of epicardium. In prolarvae of 20 and 23 dpf, the attachment between the right outgrowth of coelomic cells has spread over the dorsal and right surfaces of the ventricle forming a flattened, squamous-monostratified epithelium (Fig. 1, D–F). As a consequence, most of the ventricular and atrial surfaces are lined by epicardial cells. The contact between the right cell outgrowth and the cardiac surface is disappearing by 26 dpf (Fig. 1, G and H). In later stages, there is no contact between the coelomic cell clusters, which become the right and left pronephric external glomerulus (PEG) (Fig. 1, I–K), and the heart surface. Blood-containing vessels are abundant in these glomeruli from these stages on.
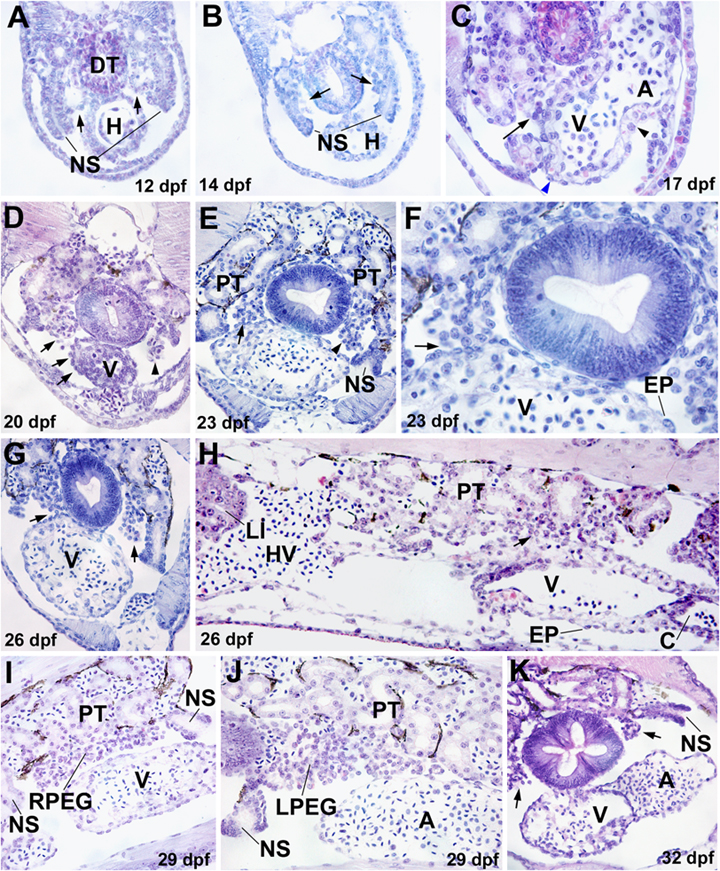
Epicardial development in the lamprey Petromyzon marinus. (A) Embryo of 12 days post-fertilization (dpf). The coelomic epithelium covering the developing pronephros shows no signs of outgrowth towards the coelom (arrows). DT, digestive tube; H, heart; NS, nephrostome. (B) Newly hatched prolarva of 14 dpf. Clusters of coelomic cells (arrows) can be seen between the nephrostomes (NS) and the digestive tube (DT). These clusters represent the primordia of the pronephric external glomerulus (PEG). (C) Prolarva of 17 dpf. The right cell outgrowth contacts the ventricle surface (arrow) close to the limit between the ventricle (V) and the atrium (A). Note the lack of epicardial lining in the ventral surface of the heart (arrowheads). (D–F) Prolarvae of 20 (D) and 23 (E, F) dpf. Pronephric tubules (PT) are already well developed in the pronephros. The right PEG primordium is widely attached to the ventricle (V) (arrows, section shown in D is located slightly ahead of the attachment site). Epicardial cells (EP) apparently spread over the cardiac wall. The left PEG primordium does not attach to the heart (arrowheads). (G–H) Transverse and sagittal sections of a 26 dpf P. marinus prolarvae, respectively. Large PEG primordia are indicated by arrows. The attachment of the right PEG primordium persists on the dorsal and posterior part of the ventricle (V). Note the long distance between the liver (LI) and the ventricle. C, Conus arteriosus. HV, hepatic vein. (I–J) The attachment of the right PEG (RPEG) to the ventricle has disappeared by 29 dpf. The right and the left PEG (LPEG) are shown in I and J, respectively. A, Atrium. (K) Transverse section of a 32 dpf P. marinus prolarvae. Both PEG (arrows) are already well separated from the heart.
For comparison purposes, Fig. 2 shows the localization and structure of the proepicardium in embryos of a basal gnathostome, the dogfish (S. canicula). In this species the proepicardium is paired, and it releases free-floating cells into the pericardial cavity that adhere to the myocardium (Muñoz-Chápuli et al. 1994). However, in earlier stages there is an attachment between the right proepicardium and the ventricular surface (Fig. 2, A and C). Later in development, the proepicardium is located on the developing septum transversum, between the liver and the sinus venosus, and it does not have any contact with the heart surface. When the epicardial development is completed, the proepicardial cells showed signs of apoptosis (not shown).

Features of proepicardial development in fish and avian embryos. (A–D). Proepicardial development in early dogfish embryos (Scyliorhinus canicula). Transverse sections showing the proepicardium as a paired outgrowth of the coelomic epithelium of the developing septum transversum (ST). Note how the right proepicardium (arrow and RPE) contacts the surface of the ventricle (V) where epicardial cells (EP) can already be seen over the myocardium (M). The left proepicardium (arrowhead and LPE) releases cells in the coelom. These floating cells also adhere to the myocardium (Muñoz-Chápuli et al. 1994). OE, oesophagous. (E). Sagittal section of a late dogfish embryo. The proepicardium (PE) is located on the developing septum transversum, between the liver (LI) and the sinus venosus (SV), just at the level of the hepatic vein (HV). An epicardium (EP) containing epicardial-derived mesenchymal cells is already developed on the ventricle. Note the distance between the proepicardium and the pronephric/mesonephric tubules (P/MN). (F–H). Expression of the transcription factor Wt1 in quail embryos. Wt1 is expressed by epithelial (arrows in H) and mesenchymal cells of the proepicardium (PE), epicardium (E) and mesonephros (MN), but not by the nephric ducts (ND). It is shown in F the attachment of the right proepicardium to the ventricle (V), close to the atrioventricular groove (AV). Note the proximity of the proepicardium to the liver (LI).
Figure 2 also shows the similarities in the expression pattern of the transcription factor Wt1 in the proepicardium and in the mesonephros of quail embryos. In both cases Wt1 is strongly expressed in the coelomic lining and also, with different intensity, in the adjacent mesenchyme. Wt1 expression apparently decreases as these mesenchymal cells differentiate, as suggested by the decrease of staining at the inner areas and also by the lack of expression in the epithelial structures of the mesonephros.
DISCUSSION
The proepicardium is an originally paired structure present in all the vertebrate models so far studied. It is originally extracardiac (it does not apparently form from the early primary or secondary heart fields), but it attaches to the heart surface and spreads lining the myocardium and giving rise to the epicardium. No hypothesis had been hitherto made about its origin and relationships to other embryonic systems. We herein propose that the proepicardium is the reminiscence of the PEG progenitor that was conserved through the evolution essentially due to its role in providing vasculogenic cells to the heart. The paired PEG primordia gave rise to the bilateral proepicardia of fishes (Muñoz-Chápuli et al. 1994), but the right PEG primordium/proepicardium seems to have been favored by the evolution, because it is the only one that attaches to the heart in lamprey, dogfish, and birds. Thus, the attachment of the right PEG primordium/proepicardium to the heart surface is highly conserved during the evolution of vertebrates. In mammals, both primordia fuse in the midline giving rise to a single proepicardium (Schulte et al. 2007), but attachment to the myocardium also occurs (Nesbitt et al. 2006). The unequal contribution of the PEG primordia to the epicardium is probably related with the asymmetrical development of the heart, because the rightward looping of the early cardiac tube faces the ventricular surface to the right primordium. Thus, proepicardial asymmetry seems to be a consequence of the cardiac asymmetry.
A pair of PEG is present in lamprey and amphibian larvae (Kluge and Fischer 1990), and they are composed of capillary networks, mesangial cells, and coelomic-derived podocytes. Rudimentary PEGs have also been described in the chick embryo, although their functionality is doubtful (Hiruma and Nakamura 2003). However, PEGs are functional in both lamprey and amphibian larvae, thus retaining the original pattern of the vertebrate excretory system, i.e., a blood-filtering glomerulus which releases the filtrate into the coelomic cavity, where it is aspirated by the ciliated funnels of the pronephric ducts. The glomerular filtrate was, in amniote vertebrates, released into the nephrocele. Consequently, the PEG disappeared from adult vertebrates.
Proepicardium had never been related with the excretory system, and in fact it is anatomically associated with the liver and/or the septum transversum in all the vertebrate models hitherto studied. Therefore, an open question is how did the PEG primordium become associated with the liver/septum domain? We think that the disappearance of the most anterior part of the pronephros, the progressive enlargement of the liver, and especially the expansion and looping of the cardiac inflow tract account for this new localization of the proepicardium (Fig. 3). The enlargement of the cardiac inflow tract was probably concomitant with a looping of the whole area, thus accounting for the dorsal location of the atrium and the new position of the proepicardium caudal to the ventricle. In fact, in lamprey embryos and prolarvae the liver is far away from the heart, the atrium is located laterally to the ventricle and a defined sinus venosus is not present at the time of epicardial development (1, 3). On the other hand, the evolutionary expansion of the cardiac inflow tract is probably recapitulated during the development of amniote vertebrates. A mesenchymal population which does not express Nkx2.5 (a marker of the myocardial progenitors) and expresses instead podoplanin (a podocytic marker), the T-box gene Tbx18 and Wt1 incorporates to the venous pole of the embryonic heart (Christoffels et al. 2006; Gittenberger-de Groot et al. 2007; unpublished observations). It is important to remark that Tbx18 is strongly expressed in the proepicardium and genital ridges (Haenig and Kispert 2004). In addition, the liver growth towards the dorsal and right side, intermingling with the septum transversum mesenchyme, probably uncoupled the PEG progenitor from the primitive nephrogenic area. Thus, the anatomical association of the proepicardium with the liver and the septum transversum seems to be purely contingent.
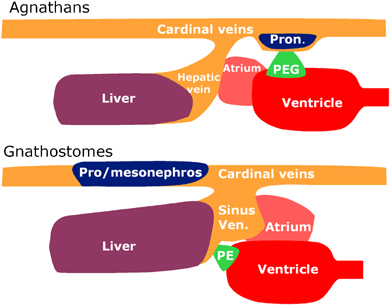
Comparison between the anatomical arrangement of the pronephric external glomerulus (PEG) in agnathans and the proepicardium (PE) of gnathostomes. The different localization of the proepicardium in gnathostomes is probably due to the disappearance of the most anterior part of the pronephros, the enlargement of the liver, the incorporation of venous areas to the cardiac inflow tract giving rise to the sinus venosus and a further cardiac rotation leaving the atrium dorsal and the proepicardium caudal to the ventricle. In this way the primitive PEG primordium became associated to the liver and septum transversum.
A main support of our hypothesis is provided by the strong epicardial expression of Wt1 and Pod1, two transcription factors essential for genitourinary development. (Moore et al. 1999; Cui et al. 2003). The Wilms' tumor suppressor transcription factor Wt1 is essential for development of kidneys and gonads, two organs which do not develop in Wt1-deficient mouse embryos (Kreidberg et al. 1993). In these embryos, the epicardial development is defective, showing premature and anomalous differentiation (endothelial differentiation is particularly impaired) as well as reduced proliferation (Wagner et al. 2005; J. M. Pérez-Pomares et al. unpublished observations). On the other hand, the bHLH transcription factor epicardin/Pod1 is strongly expressed in podocytes, epicardial, and epicardial-derived cells. Although Pod1-null embryos show glomerular defects, spleen agenesis and hypoplasic lungs and gonads (Quaggin et al. 1999; Lu et al. 2000; Cui et al. 2003), no epicardial defects have been reported.
Two signaling systems, mediated by retinoic acid (RA) and the activin receptor ALK2, respectively, are also essential for both epicardial/proepicardial development (Lavine et al. 2005; Merki et al. 2005; Olivey et al. 2006) and PEG differentiation (Osafune et al. 2002). The myocardial signals that promote myocardial proliferation are RA-dependent (Lavine et al. 2005), and proepicardial apoptosis has been described in RXRα-null embryos (Jenkins et al. 2005). On the other hand, the proepicardial epithelial–mesenchymal transition is induced by TGFβ1 and TGFβ2 through ALK2 activation (Olivey et al. 2006). These molecular mechanisms parallel the role of RA and activin for the induction of the pronephric glomus and tubules in Xenopus (Osafune et al. 2002).
Finally, a striking feature of the proepicardium can also be explained by its evolutionary relationship with the pronephros. The presence of blood island-like structures in the epicardium of the mammalian embryos is known since long time ago (Komiyama et al. 1987; Hirakow 1992). However, the heart is not considered a true hematopoietic organ. Recently, some evidence has been provided about hematopoietic cells in the heart (basically erythropoietic) derived from proepicardial-derived progenitors (Kattan et al. 2004; Tomanek et al. 2006; Wilting et al. 2007). This fact can be now explained by the pronephric origin of the proepicardium, since the pronephros is known to be a main hematopoietic site in fish and amphibians (Carpenter and Turpen 1979; Willett et al. 1999).
The evolutionary conservation of the cell transfer from the PEG primordium to the heart illustrates the importance of these cells for heart evolution. We think that the primary contribution made by glomerular cells to the cardiac development probably was to supply the heart with vascular cells. The embryonic heart is primarily devoid of blood vessels, which limits the thickness of the myocardial wall and, consequently, its performance. Glomerular progenitor cells transferred to the heart and bearing a high vasculogenic potential might represent the first evolutionary step to accomplish a complete myocardial vascularization through an epicardial-derived coronary vascular bed. In this way, the vertebrate heart could increase its thickness and its performance. Secondarily the epicardial and epicardial-derived cells probably acquired a signaling role, producing factors actively inducing the myocardial proliferation.
We can finally speculate about the relationships between the proepicardium/heart connection and a striking feature of the hemichordates, the heart–kidney complex. This structure is located in the proboscis, above the stomochord—a mouth diverticle-, and it is constituted by a pulsatile blood sinus associated to an excretory glomerulus. The apparently dorsal location of the heart in hemichordates historically ruled out an evolutionary correspondence with the vertebrate heart. However, recent studies on gene expression in the hemichordate Saccoglossus kowalevskii have shown that the dorsoventral axis of this species is reversed respect to that of vertebrates (Lowe et al. 2006). This surprising finding opens the possibility that the heart–kidney complex of hemichordates can be related with the heart–pronephros connection observed in lamprey embryos and prolarvae. In this case, the proepicardium could be the last vestige of an ancestral link between the primitive heart and the primitive excretory system of deuterostomes.
We believe that the recognition of the proepicardium as an evolutionary derivative of the PEG will provide new avenues on the molecular mechanisms involved in its development and the impact of the epicardium on cardiac morphogenesis.
Acknowledgments
We thank David Macías for supplying dogfish sections. This work has been supported by the European Community's Sixth Framework Programme contract (“HeartRepair”) LSHM-CT-2005-018630 and by grants BFU05-00483, SAF2006-26666E, 07V1A12, and BFU2006-14127.




