Cost-Effectiveness of Implantable Cardioverter-Defibrillators in Brazil: Primary Prevention Analysis in the Public Sector
ABSTRACT
Background: Several studies have demonstrated the effectiveness and cost-effectiveness of implantable cardioverter-defibrillators (ICDs) in chronic heart failure (CHF) patients. Despite its widespread use in developing countries, limited data exist on its cost-effectiveness in these settings.
Objective: To evaluate the cost-effectiveness of ICD in CHF patients under the perspective of the Brazilian Public Healthcare System (PHS).
Methods: We developed a Markov model to evaluate the incremental cost-effectiveness ratio (ICER) of ICD compared with conventional therapy in patients with CHF and New York Heart Association class II and III. Effectiveness was evaluated in quality-adjusted life years (QALYs) and time horizon was 20 years. We searched MEDLINE for clinical trials and cohort studies to estimate data from effectiveness, complications, mortality, and utilities. Costs from the PHS were retrieved from national administrative databases. The model's robustness was assessed through Monte Carlo simulation and one-way sensitivity analysis. Costs were expressed as international dollars, applying the purchasing power parity conversion rate (PPP US$).
Results: ICD therapy was more costly and more effective, with incremental cost-effectiveness estimates of PPP US$ 50,345/QALY. Results were more sensitive to costs related to the device, generator replacement frequency and ICD effectiveness. In a simulation resembling the MADIT-I population survival and ICD benefit, the ICER was PPP US$ 17,494/QALY and PPP US$ 15,394/life years.
Conclusions: In a Brazilian scenario, where ICD cost is proportionally more elevated than in developed countries, ICD therapy was associated with a high cost-effectiveness ratio. The results were more favorable for a patient subgroup at increased risk of sudden death.
Background
Chronic heart failure (CHF) is nowadays recognized as a major health problem, with increasing incidence and mortality in the past few years [1–4]. In 2006, it was responsible for almost 300,000 admissions and nearly 50,000 deaths in Brazil [5]. Nearly half of these patients die because of fatal arrhythmia. The implantable cardioverter-defibrillator (ICD) is a device targeted to terminate life-threatening arrhythmias, and has been studied in patients with left ventricular systolic dysfunction for more than two decades. After the MADIT-I trial [6] was published, nine other trials have evaluated the benefits of ICD in different CHF populations [7–15], and a meta-analysis of these studies has described a relative risk (RR) reduction of 25% for total mortality [16].
Although ICD therapy has been consistently associated with total death reduction in heart failure patients, its high cost prohibits large scale implantation, even in developed countries. It is estimated that more than 3 million North Americans meet eligibility criteria from the MADIT-II trial (that is, previous myocardial infarction and an ejection fraction of 30% or less) [17], and this huge population makes a broad implantation unaffordable. Several cost-effectiveness analyses have been reported aiming to offer data to physicians and policymakers on the incorporation of this technology [18–21]. However, most cost-effectiveness studies were based on individual data from the trials—mostly from MADIT-II [22–25]—or analyzed each trial separately [21]. Besides, none of them used cost data from developing country, which are different from those in the United States, Canada, and Europe.
In this report, we sought to evaluate the costs, effectiveness and cost-effectiveness of ICD implant in a hypothetical cohort of CHF patients with New York Heart Association (NYHA) functional class II and III and a left ventricular ejection fraction (LVEF) of 35% or less, applying cost and survival data from Brazil. As a secondary objective, we evaluated the effect of clinical and ICD related parameters in sensitivity analysis.
Methods
Target Population
The model assumed a baseline population of heart failure patients, 60 years old, NYHA class II and III, LVEF ≤ 35%, and independent of etiology. All patients were in primary prevention, that is, none of them had a history of life threatening arrhythmias. The choice of our target population characteristics was intended to reproduce the clinical features of the majority of patients included in the ten aforementioned trials. We incorporated in the model patients with NYHA functional class II or III, considering that the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) [15]—the largest ICD trial—had only patients with NYHA class II and III, and more than 80% of patients studied in the other trials were in these functional classes.
Decision Model Structure
We constructed a decision tree model with Markov transitional states using DATA Pro (TreeAge Software, Version 5.0, Inc. Williamstown, MA). The model tracked a hypothetical cohort of CHF patients over time who received an ICD plus conventional therapy—optimal pharmacological treatment—or conventional therapy alone (Fig. 1). We decided to evaluate only single chamber ICD, because of its equal efficacy, smaller cost, and lower rate of complications when compared with double chamber devices.
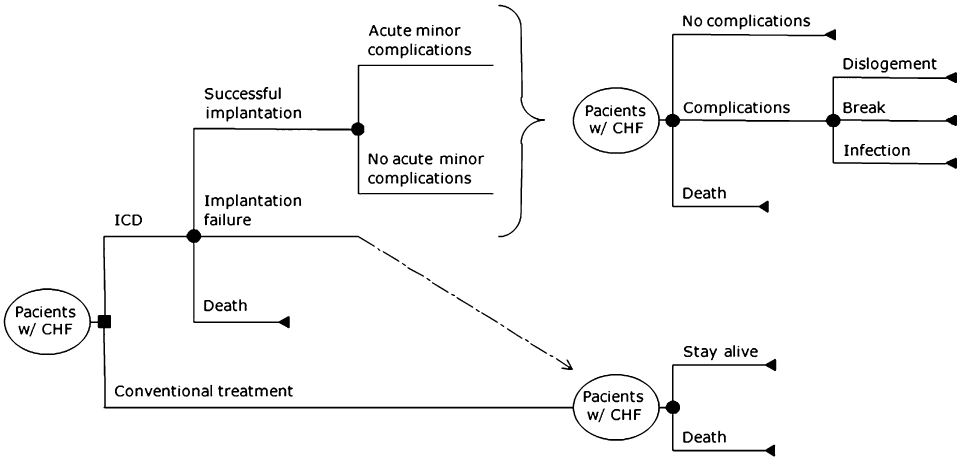
Schematic representation of the decision model. Patients with heart failure can either stay on conventional therapy or undergo implantable cardioverter-defibrillator (ICD) implantation. Patients assigned to ICD can suffer operative death or implant failure. Patients with successful implant can suffer minor procedure-related complications. After the acute phase, patients enter a Markov process that includes most common ICD chronic complications. Patients in the conventional arm also enter a Markov model of chronic heart failure (CHF).
The structure of our model is similar to the one constructed by Banz et al., who evaluated the cost-effectiveness of cardiac resynchronization therapy [26]. Patients in conventional treatment could remain stable or die in each yearly cycle. In the ICD arm, patients could suffer operative death or survive the procedure. Surviving patients could have implantation failure, when they entered a Markov process identical to conventional treatment, or could have a successful implantation, being susceptible to minor procedure-related complications, such as pneumothorax or deep vein thrombosis. Both patients with and without these complications entered a Markov process that simulated the natural history of patients treated with ICD. During each one year cycle, patients in this path could remain stable, experience any ICD complication or die. ICDs would have their generator replaced every 5 years in the base case, with a maximum replacement of three times.
Complications included in the model were infection—which demanded ICD replacement, in case of survival—lead dislodgement, break, or an insulation defect. We constructed the model using all cause mortality instead of stratifying death by cardiovascular or other causes, since most of the evidence about ICD efficacy reports total mortality reduction. A schematic representation of the decision tree is displayed in Figure 1.
The discount rate for both costs and effectiveness was 3% per year. We used the public third party payer perspective and a 20-year time horizon.
Survival Data
In order to build a model with reliable data regarding the natural history of heart failure in Brazil, we used data from a local cohort of heart failure patients followed by a heart failure team [27,28], whose characteristics are similar to the populations studied in the ICD clinical trials, specially SCD-HeFT and MADIT-II. This cohort was comprised of 386 subjects (63% male), with a median age of 59 years (interquartile range [IQR] 49–68) and had a median follow-up of 35 months (IQR 18–60). Forty-one percent of these patients had ischemic heart disease as the etiology of heart failure. There were 53% of patients with hypertension, 33% with diabetes, and 13% with current use of tobacco; 89% were on angiotensin-converting enzyme inhibitors and 73% were on beta blockers. None of the patients were either on ICD or cardiac resynchronization therapy. Considering the small number of patients with a follow-up longer than 7 years in this cohort, we needed to project survival beyond this time point. We undertook a comparison between the survival curve of this cohort and from the adult Brazilian population, in order to identify which mathematical function would best fit the data. Exponential function (compared to linear and quadratic) best fitted the curves, judged by its higher r2, and was therefore chosen for survival modeling. The final equation for survival prediction was Y = 0.000387 * (EXP[X * 7.922]), where X is the survival function for an individual from the general population at a given age, Y is the estimated survival for a heart failure patient at that given age, 0.000387 is the intercept of the function and 7.922 is the β coefficient. Survival function from general population was calculated with a Cox model, using data from National Demographic Census. Given the fact then the census has stratified mortality data only up to 79, the model's time horizon was set to 20 years.
Effectiveness Data
Clinical outcomes considered in the model were life years saved (LYS) and quality-adjusted life years (QALY). The base case was modeled using the later, as recommended by the Panel on Cost Effectiveness [29].
We searched MEDLINE for clinical trials and meta-analyses of ICD therapy in heart failure patients in order to obtain ICD effectiveness estimates. There are two meta-analyses published compiling data from ten primary prevention trials available. Al-Khatib et al. found a hazard ratio for total mortality of 0.71 (95% confidence interval [CI] 0.58–0.88), and an I2 of 70% [30]. This large heterogeneity was the reason given by Sanders et al. to analyze each trial separately in their cost-effectiveness report [21]. Nanthakumar et al. pooled the results from the 10 studies and estimated an RR of 0.75 (95% CI 0.63–0.91), also with a high heterogeneity (I2 of 69%) [16]. They attributed this finding to three trials with design very different from the other seven: the DINAMIT trial [14], which included patients with recent myocardial infarction (maximum 40 days); the MUSTT trial [8], which was not a randomized comparison of ICD use, and the coronary artery bypass graft patch [7] trial, in which the device was implanted during a scheduled coronary artery bypass surgery. A second analysis excluding these three trials (which comprised approximately 30% of the total sample) yielded a similar RR of 0.74 (95% CI 0.67–0.83) and a very low heterogeneity (I2 of 5.2%). For the current analyses, we decided to use the latter risk reduction in our base case.
Complications
For complications of ICD therapy, the evidence search comprised clinical trials, cohort studies and international registries. In order to best reflect these data under the use of present-day technology and expertise, we included only studies published since 1996, when the majority of lead systems were implanted transvenously.
A meta-analysis conducted by Ezekowitz et al. [31] provided rates for system infection (total number of patients = 12,436), peri-implantation mortality (N = 39,858, defined as mortality in the first 30 days following the procedure) and implantation failure (N = 11,129).
We performed random effects meta-analyses in order to calculate probabilities for lead dislodgement and death associated with ICD infection, using the incidence rates from individual studies. We included studies with at least 90% of ICDs with pectoral implants; there were four reporting lead dislodgement [32–35] and four reporting device infection-related mortality [9,32,33,36]. Meta-analyses were performed in Stata (version 9, Stata Corporation, College Station, TX).
Probabilities for lead dislodgement were applied only during the cycle immediately after ICD implantation, as this kind of complication rarely occurs after this period. If the patient underwent a new implant during the Markov process, after an infection, he would be at risk of lead dislodgement for another cycle. Probabilities for system infection were applied throughout the 20-year time horizon.
Data input for lead complications requiring its replacement (such as breaks and insulation defects) were collected from a work by Kleemann et al., which followed a cohort of patients up to 10 years [37]. We chose to use data solely from this article instead of a compilation of all evidence available since this study showed a progressive increase in the rate of this complication over time, what was already suggested by Luria et al. in an analysis with fewer patients [38]. As the number of patients accompanied for more than seven years was very small, we used the yearly rate until the 7th year presented in the study, and applied the rate of the seventh cycle to all remaining cycles in the model. Because our model simulates patients with single chamber ICDs, we did a transformation of the annual rates presented by the study, using the hazard ratio presented to single versus double chamber ICDs regarding this complication (0.69) and the number of single chamber ICDs implanted (46.6%). Our calculations generated a formula by which the annual rate of lead change for single chamber device was equal to the study value for a double chamber divided by 1.24.
Utilities
Utility data regarding CHF patients vary considerably in the literature, and we could not identify a quality of life study using appropriate methodology performed in Brazilian patients. Although other studies have described utility values as low as 0.52 for NYHA class III [39], we chose the utility value of 0.88 generated in the Beaver Dam Study [40], which was also adopted by Al-Khatib [24] and Sanders [21,23,41] in their analysis.
Inappropriate shocks associated with ICD can affect quality of life [42,43]. In the Cardiac Arrhythmia Patient Outcomes Research Team study, mean utility for low, moderate, and severe rates of device-related side effects were 0.76, 0.75, and 0.64, respectively [44]. Although this adverse effect on quality of life, two large studies, one evaluating patients from the SCD-HeFT and the other from the DEFINITE trial, did not observe any difference in quality of life in the comparison of ICD and conventional therapy patients [45,46]. These findings lead us to not incorporate any changes in quality of life in the ICD paths in our base case, although we considered these scenarios in our sensitivity analysis, where we ranged ICD utility from 0.88 (the same as in the conventional therapy) to 0.64, the lowest value described in the literature.
Costs
Annual costs of conventional therapy were derived from a cohort study of ambulatory patients from Southeastern Brazil [47]. We used their resource utilization data to calculate costs from the Public Healthcare System (PHS) perspective. PHS costs included expenditures with diagnostic tests, laboratory exams, hospitalizations, medical visits, and all medications paid by the government.
Medication costs were based on Brazilian retail sales price. Prices of medical visits, hospitalizations, ICD placement and complications, and laboratory and imaging tests were derived from codebooks (year 2007) used by the PHS for reimbursement in Brazil. These values have a fixed diagnoses (or procedure) related group price, that is, the PHS reimburse the same amount for a hospitalization for worsening heart failure, regardless of diagnostic work-up and therapies prescribed; this applies to ICD related costs. Costs are expressed in Brazilian Real (R$) and international dollars, applying the purchasing power parity conversion rate (PPP US$). In this procedure, the purchase power of money in different countries is taken into account, which is more appropriate than a simple exchange rate. According to the last report of the World Bank regarding conversion rates, 1 PPP US$ = 1.357 R$ [48].
Sensitivity Analyses
We performed one-way sensitivity analysis on most parameters in the model (Table 1). Rates of ICD effectiveness, perioperative death, system infection and its associated mortality, implantation failure, and lead dislodgement were varied between the boundaries of the meta-analyses confidence intervals [16,31]. Lead change rates were varied among their original values. Costs were varied between ± 50% of their original values and discount for both costs and utilities between 0% and 7%. Battery replacement frequency was oscillated between 3 and 7 years. Utilities variation was applied as described earlier.
| Input variable | Base-case | Variation for sensitivity analysis | Source |
|---|---|---|---|
| ICD variables | |||
| Procedural death (%) | 1.3 | 1.2–1.4 | [31] |
| Frequency of generator replacement (y) | 5 | 3–7 | |
| Risk reduction relative to conventional treatment (%) | 26 | 17–33 | [16] |
| Annual probability of system infection (%) | 0.6 | 0.5–0.8 | [31] |
| Annual probability of lead change (%) | [37] | ||
| 1st year | 2.36 | 2.36–2.93 | |
| 2nd year | 1.62 | 1.62–2.01 | |
| 3rd year | 2.09 | 2.09–2.59 | |
| 4th year | 2.19 | 2.19–2.71 | |
| 5th year | 3.16 | 3.16–3.92 | |
| 6th year | 5.44 | 5.44–6.75 | |
| 7th through 20th year | 6.72 | 6.72–8.33 | |
| Lead dislodgement (%) | 3.48* | 1.92–5.23 | [32–35] |
| Mortality associated with infection (%) | 21 | 0–50 | [9,32,33,36] |
| Implantation failure (%) | 1.1 | 0.9–1.3 | [31] |
| Minor procedure related complications† | 0 | 0–4 | [32] |
| Costs (PPP US$) | |||
| Initial ICD implantation, total costs | 22,447 | 11,223–33,669 | |
| Generator replacement | 21,671 | 10,836–32,507 | |
| Admission for lead replacement | 5,596 | 2,798–8,394 | |
| Admission for lead repositioning | 290 | 145–435 | |
| Admission for system infection (additional)‡ | 1,123 | 0–2,245 | |
| Annual cost of heart failure treatment | 2,329 | 1,164–3,493 | |
| Extra annual cost for ICD follow-up | 16 | 8–24 | |
| Utilities | |||
| Utility of a patient with heart failure | 0.88 | 0.71–0.88 | [21,23,24,41,57] |
| Utility of a patient with heart failure and ICD | 0.88 | 0.64–0.88 | [41] |
| Discount rate (%) | 0–7 |
- * Mean value calculated with incidence rates meta-analysis, random effect model from DerSimonian and Laird.
- † Minor peri-implant complications include pneumothorax, lower limbs deep vein thrombosis and brachial thrombosis.
- ‡ Additional cost for system infection, considering that a patient with this complication would be admitted for treatment of infection and implantable cardioverter-defibrillator (ICD) change, and would generate a cost of at least the same as the one for ICD implant.
We also performed an analysis modifying the survival curve in order to simulate the model in a population similar to the MADIT-I trial, which comprehended patients at higher risk of ventricular arrhythmias, changing the α and β in the exponential equation to 0.000367 and 7.762, respectively. Accordingly, we used the RR associated with ICD therapy achieved in that study (RR 0.41; 95%CI 0.24–0.69) [16].
The robustness of the model was tested in a Monte Carlo simulation, with generation of 1000 trials and variation of values in the range described earlier. The distributions used in the simulation were beta for probabilities, normal for the logarithm of RR, and triangular for costs. We also performed threshold analysis of the cost of the ICD in different clinical scenarios. Table 1 lists the base case assumptions and the range used in sensitivity analysis.
Results
For the base-case analysis, in the model starting at 60 years of age for patients with CHF class II or III, the predicted mean survival was 5.95 years with conventional treatment, 6.99 with ICD therapy, diminishing to 5.23 and 6.15 when adjusted for quality, respectively. The survival curve is shown in Figure 2. The undiscounted effectiveness gained for ICD compared with conventional therapy was 1.42 life-years and 1.24 QALYs. Total costs of therapy were PPP US$ 24,619 for conventional and PPP US$ 70,841 for ICD therapy. Table 2 shows the health benefits—expressed in LYS and in QALYs—the costs of each strategy, and the incremental cost effectiveness ratio (ICER). ICD therapy was both more expensive and effective when compared with conventional therapy, yielding an incremental cost-effectiveness ratio of PPP US$ 50,345 (R$ 68,318) per QALY and PPP US$ 44,304 (R$ 60,121) per LYS in the base case.
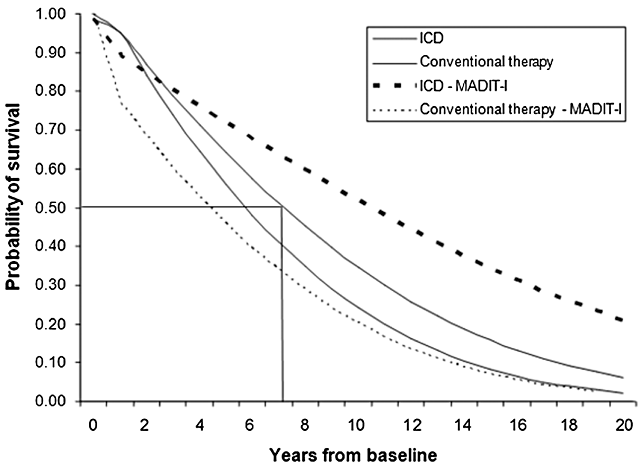
Undiscounted survival curve projections for conventional treatment and implantable cardioverter-defibrillator (ICD) therapy in the base case analysis and in the simulation resembling MADIT-I population.
| Total cost (US$ PPP) | Effectiveness | Incremental cost-effectiveness | |||
|---|---|---|---|---|---|
| Mean life years | Mean QALYs | US$ PPP/LYS | US$ PPP/QALY | ||
| Conventional treatment | 24,619 | 5.95* | 5.23* | — | — |
| ICD therapy | 70,841 | 6.99* | 6.15* | 44,304 | 50,345 |
- * All values are discounted.
- ICD, implantable cardioverter-defibrillator; LYS, life years saved; QALY, quality-adjusted life year; US$ PPP, purchasing power parity conversion rate.
In order to simulate the natural history of a heart failure population with increased risk of sudden death, resembling patients evaluated in the MADIT-I trial, we changed the survival curve and effectiveness parameters as described in the Methods section. In this analysis, we obtained a predicted mean survival of 4.37 QALYs in the conventional treatment arm and 7.63 QALYs in the ICD therapy. Survival curves of this simulation are displayed in Figure 2. In this scenario, the ICER decreased to PPP US$ 17,494 (R$ 23,739) per QALY and PPP US$ 15,394 (R$ 20,890) per LYS.
One-way sensitivity analyses are displayed in Table 3. Results were most sensitive to ICD and generator costs, generator replacement frequency, ICD effectiveness, and utility of a patient with ICD. Discount rate and utility of a patient with heart failure had moderate effect on the incremental cost-effectiveness ratio, and the remaining parameters had minimal effect on the overall result. If the utility of a patient with an ICD was set to 10% lower than patients in the conventional treatment, the ICER would become PPP US$ 149,665, and the ICD strategy would eventually be dominated with utilities for ICD patients 16% lower than conventional therapy patients. In the MADIT-I model, altering the RR of death associated with ICD from 0.24 to 0.69 yielded cost-effectiveness ratios of PPP US$ 13,496 (R$ 18,314) and PPP US$ 34,635 (R$ 47,000) per QALY, respectively.
| Variables | Lower ICER (US$ PPP/QALY) | Higher ICER (US$ PPP/QALY) |
|---|---|---|
| Mortality reduction with ICD | 38,300 | 83,678 |
| Probability of system infection | 49,751 | 51,561 |
| Probability of death for system infection | 48,245 | 53,570 |
| Probability of procedural death | 50,048 | 50,646 |
| Probability of implantation failure | 50,286 | 50,404 |
| Probability of minor peri-implant complications | 50,337 | 50,353 |
| Probability of lead dislodgement | 50,338 | 50,352 |
| Probability of lead replacement | 50,345 | 50,851 |
| Utility of a patient with heart failure | 50,345 | 62,399 |
| Utility of a patient with heart failure and ICD | 50,345 | Dominated |
| Discount rate | 41,994 | 63,691 |
| Battery replacement frequency | 29,016 | 72,087 |
| Cost of ICD implant and battery replacement† | 26,831 | 73,857 |
| Cost of heart failure optimal treatment per year | 49,021 | 51,668 |
| Cost of electrode replacement | 49,290 | 51,400 |
| Cost of system infection | 50,285 | 50,405 |
| Cost of ICD maintenance | 50,303 | 50,387 |
| Cost of lead dislodgement | 50,338 | 50,352 |
| Cost of minor peri-implant complications | 50,341 | 50,349 |
- * Parameter ranges as described in Table 1.
- † Cost of battery replacement was considered as 96.54% of ICD implant cost, and variation in the later fell on both parameters.
- ICD, implantable cardioverter-defibrillator; ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life year; US$ PPP, purchasing power parity conversion rate.
Figure 3 shows two-way sensitivity analyses. If the effectiveness of ICD, represented by RR reduction, associated with the ICD increases to 30% and the generator cost is reduced by 25%, ICER would also have a considerable decrease, achieving PPP US$ 32,779 (R$ 44,481) per QALY (Fig. 3a). If the generator replacement frequency increases to 6-year intervals and the ICD cost falls by 25%, ICER would drop to PPP US$ 35,141 (R$ 47,686) per QALY (Fig. 3b).
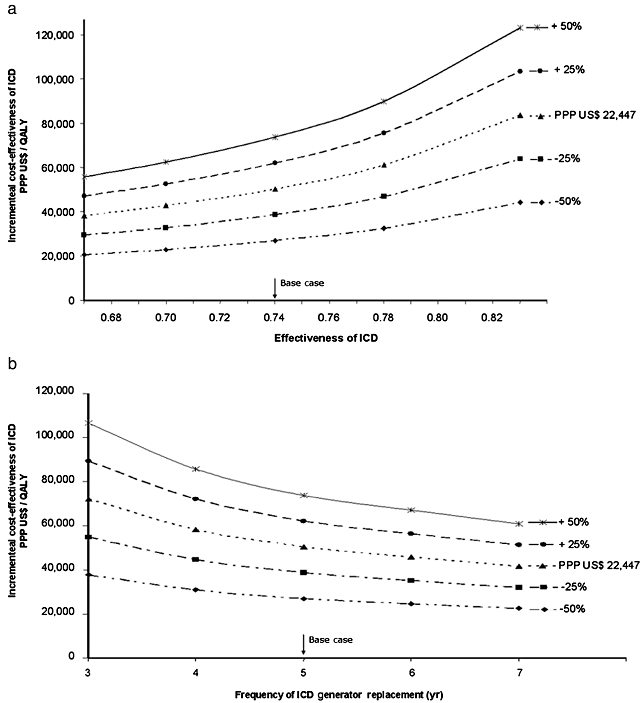
Two-way sensitivity analysis. (a) Implantable cardioverter-defibrillator (ICD) costs versus ICD effectiveness. (b) ICD costs versus generator replacement interval. Lines represent different ICD cost value inputs, ranging 25% and 50% more or less than base-case value (purchasing power parity conversion rate US$ 22,447).
In the Monte Carlo simulation, we evaluated the number of simulations that fell below the threshold of PPP US$ 27,495 (R$ 37,311), which represents three times the Brazilian gross domestic product (GDP) per capita in 2006 [49], according to the World Health Organization (WHO) recommendation [50]. Figure 4 shows the 1000 trials produced in the base case analysis; only 2% of them fell below this threshold. Conversely, in the Monte Carlo simulation with MADIT-I parameters, 84% of trials fell below PPP US$ 27,495 per QALY.
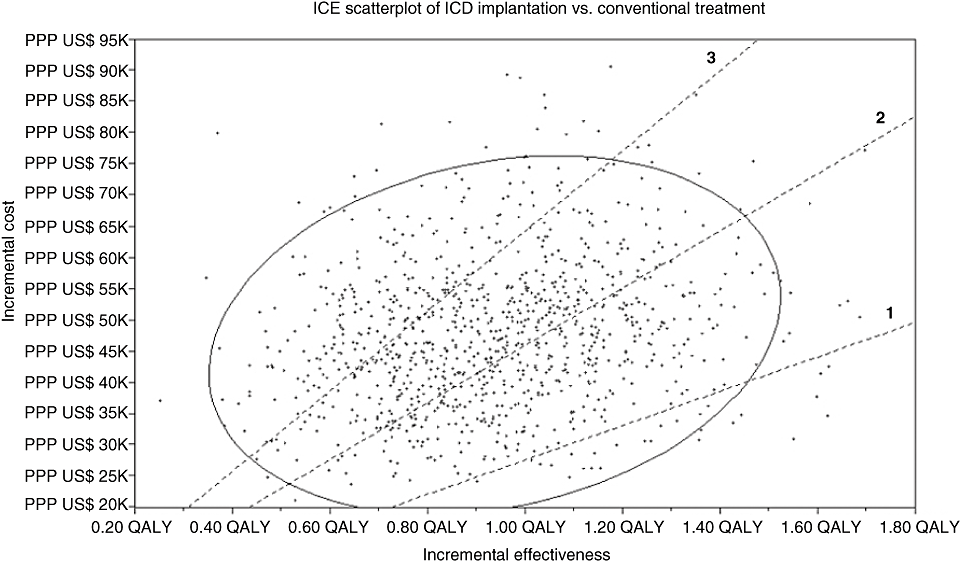
Monte Carlo 1000 trials scatter plot. The number of points below the threshold lines, from bottom to top, are as follows: 2.6% (line 1: purchasing power parity conversion rate [PPP US$] 27,495/QALY [three times Brazil's gross domestic product [GDP] per capita]), 37.5% (line 2: PPP US$ 45,825/QALY [five times Brazil's GDP per capita]), and 77.1% (line 3: PPP US$ 64,155/QALY [seven times Brazil's GDP per capita]).
Discussion
In this study, we developed a decision model to evaluate the cost-effectiveness of primary ICD in CHF patients in Brazil, and the results show that the incremental cost-effectiveness ratio of ICD implant versus optimal medical therapy was elevated, reaching PPP US$ 50,345 per QALY and PPP US$ 44,304 per LYS. Our analysis suggested that the model was robust, being more sensitive to ICD related costs and battery replacement frequency. In a scenario of increased risk of arrhythmias, cost-effectiveness was favorable for ICD therapy.
The results obtained were in the range of similar evaluations conducted in the United States. In the works by Sanders [21], Al-Khatib [24], and Zwanziger [25], whose model's assumptions were based on the MADIT-II trial, cost-effectiveness ratios were US$ 39,000, 50,500 and 78,000 per LYS, respectively. In the study by Mark, with data from SCD-HeFT, ICER was US$ 41,530 per QALY [18]. In another work by Sanders, where analyses were made separately for six primary prevention trials, ICER ranged from US$ 34,000 to US$ 70,200 per QALY [21].
Even though the absolute value of the ICER was similar to American studies and was close to values accepted as cost-effective in some developed countries, the parameters applied to define a technology as cost-effective vary worldwide. The ICER found in our study was elevated when compared to the WHO suggested benchmark, which is three times the GDP per capita of a country, namely PPP US$ 27,495 (R$ 37,311) for Brazil in 2006. In sensitivity analyses, the ICER would fall below this ratio only if ICD implant and generator cost would be reduced by 50%, and would get close to this value if the generator replacement frequency increased to 7 years intervals. All other parameters did not decrease substantially the ICER in the most favorable boundaries.
In the analysis where we projected a worst survival curve and used effectiveness data from the MADIT-I trial, where the population had a higher risk of ventricular arrhythmias, the ICER was much more favorable, and remained below the selected threshold in 84% of trials in the Monte Carlo simulation. Some details of this trial, however, suggests that it does not represent current standard of HF care—for example, use of beta-blockers was very low in the conventional treatment, what could have negatively biased the survival curve exaggerating the perceived efficacy of the ICD [51]. It is particularly disturbing that the conventional and ICD groups were slightly unbalanced in the end of the trial concerning beta-blockers use. However, although we acknowledge that the treatment received by the patients in that trial was suboptimal in both groups, the ICER in our MADIT-I scenario was far below the WHO's suggested threshold. This makes us believe that patients with similar clinical characteristics as that included in the MADIT-I trial, even if treated optimally, would still present an economically acceptable ICER for ICD implant.
Our study was based on data from almost 400 patients from a Brazilian heart failure cohort, and survival estimates were derived from local data. In previous studies, we have demonstrated that overall heart failure patients from Brazil had similar characteristics to the populations studied in the ICD clinical trials and other international cohorts [28,52]. It is important to emphasize that in this cohort only a small percentage of patients had Chagas's disease (<10%), which may not be representative of other regions of the country. There is a great debate in the literature whether ICD therapy holds similar benefit among patients with Chagas'disease [53], so that this results might not hold on these patients.
Besides using data from Brazilian population, the model was built using meta-analysis data for both effectiveness and complications, progressive rates of complications over time, especially the ones requiring lead change. The extended time horizon of our analytic strategy is also an important feature, since it has the ability to capture almost all costs and benefits yielded by each therapy.
The effectiveness parameter we used in the current evaluation came from a meta-analysis that included the COMPANION study, which evaluated heart failure patients with severe functional disability (class III and IV) and had a more complex device implanted, with resynchronization capacity. However, removing this study from the meta-analysis, the ICD would have a mortality benefit of 28% (95% CI 18–36%), yielding an ICER of PPP US$ 46,211/QALY, similar to the one we found using the original result. One also could argue whether the meta-analysis of complications that we used was an adequate strategy [31], as some of the patients included were not exactly the ones in the population we wished to simulate. However, this study is the best evidence about complications published to date, and, as seen in sensitivity analysis, those parameters had minimal influence on the results. In a Brazilian study reporting 26-month follow-up of a cohort of 155 patients who had an ICD implanted [54], ICD clinical effectiveness, including complications, was similar to the ones described in our study.
In the last decade, health economics evaluation has increased in importance to policymakers and clinicians in assessing health-care services and technologies in developing countries. Although there is no formal evaluation on the difference between high- and low-cost settings, it is rational to assume that lower labor costs and human capital may impact favorably on cost-effectiveness ratios of new interventions. This may be true to interventions involving low cost technology and high overall labor cost, such as demonstrated for screening programs for diabetes [55]. Nonetheless, in scenarios whether new technologies are associated with significant high costs and do not reduce or avert human cost, they tend to show elevated cost-effectiveness ratios. The case observed for drug-eluting stents [56] and in this article for ICD therapy for heart failure patients.
It is important to recognize some caveats associated with ICD economic analyses. For instance, we considered the benefit of ICD constant over time, which can overestimate its benefits as other cause of deaths have a tendency to grow with increasing age, decreasing the proportion of deaths due to arrhythmia. To overcome this problem, it would be necessary a clinical trial with extended follow-up, and available data from clinical trials so far have a follow-up of 5 years or less. In addition, there were no national data on utilities for heart failure, which might be different from one country to another. Thus, our result in QALYs might not be a perfect estimate for the Brazilian population. Finally, our analysis is limited to the PHS perspective, not including some costs that would be counted in the societal perspective.
This study represents a valuable tool for policymakers, although cost-effectiveness analyses should not be the only basis for decision making in health-care resource allocation. Strategies aiming at cost reduction of ICD should be pursued, in order to generate more favorable cost-effectiveness ratios for a larger number of patients. Considering present-day thresholds of acceptable cost-effectiveness ratios, ICD implantation should be strongly considered for patients at very high risk for ventricular arrhythmias in this Brazilian scenario, but usage in other eligible populations should be evaluated carefully.
Source of financial support:This project received financial support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).




