Long-Term Cost-Effectiveness of Pioglitazone versus Placebo in Addition to Existing Diabetes Treatment: A US Analysis Based on PROactive
ABSTRACT
Objective: To estimate the long-term cost-effectiveness of adding pioglitazone versus placebo to standard treatment in high-risk patients with type 2 diabetes.
Methods: The validated CORE Diabetes Model was modified to project long-term clinical and cost outcomes associated with pioglitazone versus placebo, based on results from PROactive. The model retained basic structure and functionality, with interdependent Markov submodels, Monte Carlo simulation and user interface. Adjustments to submodels were made to accommodate the PROactive primary end points. The analysis was from the perspective of a third party US health-care payer perspective, projected over a lifetime horizon using a 3% annual discount.
Results: Over a lifetime horizon, addition of pioglitazone was associated with increased life expectancy (0.237 life-years) and quality-adjusted life expectancy (QALE) [0.166 quality-adjusted life-years (QALYs)] versus placebo. Estimated long-term complication rates showed that pioglitazone reduced the number of events versus placebo for most outcomes. Lifetime total direct costs were marginally higher with pioglitazone versus placebo ($272,694 vs. $265,390, difference $7,305). The incremental cost-effectiveness ratio for pioglitazone versus placebo was $44,105 per QALY gained. Probabilistic sensitivity analysis indicated a 55% likelihood that pioglitazone would be considered cost-effective in the United States, with a willingness to pay of $50,000 per QALY gained.
Conclusions: The addition of pioglitazone to existing therapy in high-risk patients with type 2 diabetes was projected to improve life expectancy, QALE and complication rates compared with placebo. Addition of pioglitazone was in the range generally considered acceptable.
Introduction
The global prevalence of type 2 diabetes is approximately 200 million and is expected to increase to nearly 300 million by 2025 [1]. The disease is associated with an enormous health-care burden, accounting for approximately 10% of global health-care expenditure [2]. Macrovascular complications, including coronary artery disease, myocardial infarction, and stroke, account for almost 80% of all diabetes-related mortality, contributing to an average reduction in life expectancy of approximately 10 years for diabetes patients versus the general population [3].
One of the main aims of treating type 2 diabetes is to prevent or delay the onset of micro- and macrovascular complications. It is well established that improving patients' glycemic control (in terms of glycosylated hemoglobin), as well as cardiovascular risk factors (blood pressure, serum lipid levels, etc.), can substantially reduce the risk of diabetes-related complications [4–8]. Pioglitazone is a member of the thiazolidinediones class of oral antidiabetic agents and has been demonstrated to have blood glucose-lowering properties, as well as beneficial effects on triglyceride and high-density lipoprotein levels [9–11]. The PROspective pioglitAzone Clinical Trial In macrovascular Events (PROactive) was the first large-scale cardiovascular outcomes study to investigate prospectively the effect of an oral hypoglycemic agent on macrovascular outcomes [12].
PROactive was a prospective, international, multicenter, double-blind, randomized, parallel group study that enrolled 5238 patients with type 2 diabetes and established macrovascular disease defined as one or more of the following: more than 6-month history of myocardial infarction (MI), coronary artery revascularization, stroke; 3-month history of acute coronary syndrome (ACS), other evidence of coronary artery disease or peripheral arterial obstructive disease. The study's design and results have been described in detail elsewhere [13,14]. Briefly, pioglitazone or placebo was administered on top of existing diabetes treatments, including exercise and diet and other glucose-lowering therapies with or without insulin, and titrated up to 45 mg daily as tolerated. Additionally, patients were treated as necessary with cardiovascular medications, including lipid-lowering therapies and antihypertensives. Throughout the study, diabetes and cardiovascular therapies were adjusted as necessary according to the International Diabetes Federation (Europe) guidelines [15]. At baseline, 10% and 20% of patients were receiving metformin (MET) and sulfonylurea (SU) monotherapy, respectively; 25% were receiving MET + SU combination therapy, and 34% were receiving insulin. Pioglitazone was administered to 2605 patients and placebo to 2633 patients. The study population had a mean age of 61.8 years and an average duration of diabetes of 6 years. Over 80% of patients within each cohort completed the study while on study medication (Fig. 1), and the mean exposure to study drug was 30.4 months.
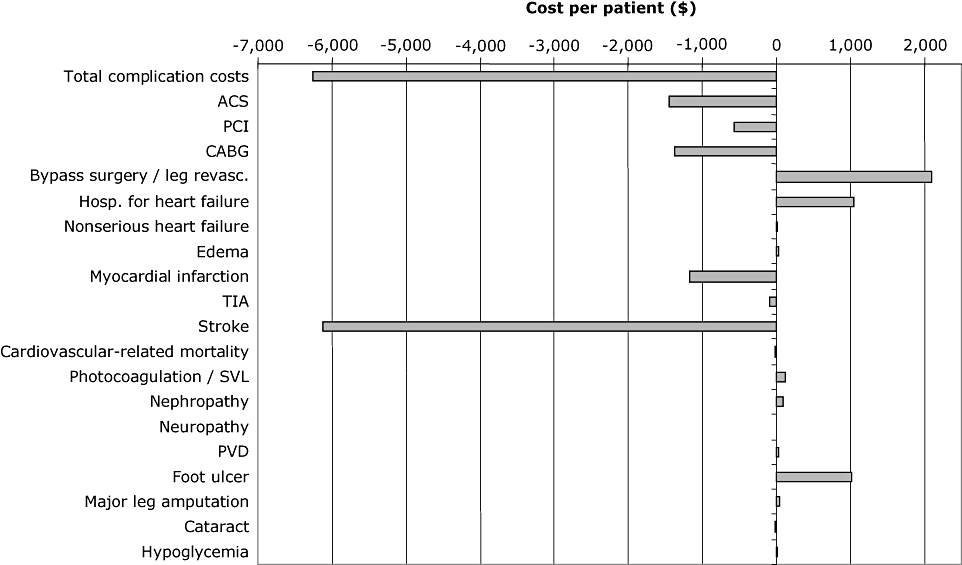
Summary of complication costs over patient lifetimes. ACS, acute coronary syndrome; CABG, coronary artery bypass grafting; PCI, percutaneous coronary intervention; PVD, peripheral vascular disease; SVL, severe vision loss; TIA, transient ischemic attack.
After 36 months of follow-up (average follow-up, 34.5 months), there was a nonsignificant 10% relative risk reduction with pioglitazone in the primary end point (P = 0.095), which was a composite of all-cause mortality, nonfatal myocardial infarction (including silent myocardial infarction), stroke, acute coronary syndrome, endovascular or surgical intervention in the coronary or leg arteries, and amputation above the ankle. Pioglitazone was, however, associated with a significant relative risk reduction of 16% (P = 0.027) in the main secondary end point of all-cause mortality, myocardial infarction (excluding silent myocardial infarction), and stroke. Additionally, pioglitazone treatment was associated with significant decreases in triglyceride levels (−11.4% vs. 1.8% with placebo; P < 0.0001) and increases in high-density lipoprotein cholesterol (HDL-C) levels (19.0% vs. 10.1% with placebo; P < 0.0001). Despite a significantly greater increase in low-density lipoprotein cholesterol (LDL-C) observed with pioglitazone (7.2% vs. 4.9% with placebo; P < 0.003), there was significant reduction in the LDL-C to HDL-C ratio with pioglitazone compared with placebo (−9.5 vs. −4.2: P < 0.0001). Pioglitazone also reduced the number of patients progressing to long-term insulin therapy by half compared with placebo (P < 0.0001). The observed increased risk of any reported heart failure (11% vs. 8% with placebo; P < 0.0001) with (6% vs. 4% with placebo; P < 0.007) and without hospitalization (5% vs. 3% with placebo; P < 0.003) were also included in our analyses.
We developed a working hypothesis that the clinical benefits observed with pioglitazone in PROactive could lead to economic benefits in terms of reduced complication costs and insulin treatment costs. These cost reductions could, at least in part, offset the additional costs of pioglitazone treatment. To test our hypothesis, we adapted the CORE Diabetes model, a validated, published, Internet-based model of type 2 diabetes, to the PROactive clinical end point data and projected diabetes-related complications and associated direct medical costs by treatment over the lifetime horizon in the US setting.
Methods
The CORE Diabetes model (CDM) was used to estimate the health and economic impact of pioglitazone treatment versus placebo over the lifetime horizon based on short-term clinical data (0–3 years) from PROactive [16,17]. The model was used to project life expectancy, quality-adjusted life expectancy [based on quality of life data from the Cost of Diabetes in Europe—Type II study (CODE-2)][18], cumulative incidence of diabetes-related complications and direct medical costs, and to calculate cost-effectiveness.
Model Description
A brief overview of the CORE Diabetes model is provided here, but the interested reader is referred elsewhere for additional detail [16,17]. The CDM is an interactive, Internet-based computer simulation model developed to estimate the long-term health outcomes and economic consequences of type 1 and type 2 diabetes interventions. The model is nonproduct-specific and designed to support diabetes policy decision-making. It performs real-time simulations, taking into account various insulin therapy regimens, oral antidiabetic agents, and screening and treatment strategies for microvascular complications. Diabetes progression is based on a series of interdependent Markov submodels that simulate the micro-and macrovascular, and acute metabolic complications of diabetes. Each submodel uses diabetes type-dependent health state transition probabilities derived from published sources and can interact with other complication submodels. Traditional Markov mathematical models do not possess virtual memory. One of the shortcomings of a standard Markov model is that there is no memory (i.e. the model cannot record when a patient experiences an event in the simulation). This is overcome using tracker variables in the sub-models of the CORE Diabetes Model. Tracker variables act as flags in the model, which are raised when a patient experiences events or complications during the simulation, and this in turn can be used to influence the calculation of quality-adjusted life expectancy, costs and to adjust the risk of subsequent events in the simulation (including across different sub-models). For example, a tracker variable is used record the onset of angina in a simulated patient. This is recorded in the database and the risk of subsequent events for that patient, for instance myocardial infarction or stroke, is adjusted appropriately. Patient cohorts are defined in terms of diabetes type, demographics, baseline risk factors, and preexisting complications. Economic and clinical data can be edited by the user, enabling new data to be incorporated as they become available. Diabetes management strategies geared toward improving the quality of care for diabetes patients can be compared in a variety of clinical settings. The reliability of simulated outcomes has been tested, with results validated against those reported by clinical trials and epidemiological studies [17].
Modifications to the CORE Diabetes Model for PROactive
A description of the changes made to the CDM to incorporate data from the PROactive study has been previously published [19]. To incorporate clinical end point data from PROactive into the CORE Diabetes model, a number of new health state submodels were developed to include the following events: acute coronary syndrome, coronary artery bypass grafting and percutaneous coronary intervention, bypass surgery/revascularization of the leg, above-ankle amputation, peripheral edema, photocoagulation/severe vision loss, hospitalization following heart failure, transient ischemic attack, cardiovascular-related mortality, and all-cause mortality. Two of the existing submodels were modified in line with the available clinical data from PROactive. These were the myocardial infarction (nonfatal myocardial infarction) and stroke (nonfatal stroke) submodels. The original version of the CDM had six submodels that were inactivated, with most replaced with PROactive-specific submodels. First, the angina submodel of the CDM was replaced by the ACS, coronary bypass graft (CABG) and percutaneous coronary intervention (PCI) submodels, allowing additional details on risk adjustment to be introduced. Second, the congestive heart failure (CHF) submodel of the CDM was replaced by the hospitalization following heart failure submodel, which made the risk adjustment more specific to PROactive and the thiazolidinedione (TZD) class of oral antidiabetic drugs. Third, nonspecific mortality in the CDM was replaced by the all-cause mortality and cardiovascular mortality submodels in PROactive, which were collected as part of the study. Fourth, the macular edema submodel of the CDM was broadened to include edema, as this was a very important finding of the PROactive study. Lastly, the ketoacidosis and lactic acidosis submodels of the CDM were inactivated as they have not been observed to be outcomes relevant to TZD treatment and were also not observed in PROactive. Beyond the submodel additions and adjustments, the overall architecture of the CORE Diabetes model was analogous to the original version, with all submodels running in parallel to simulate the progression of disease and the development of diabetes-related complications.
PROactive Event Rates
Event rates in the placebo arm were calculated directly from the annual hazard rates observed over months 0–36 of PROactive assuming constant risk. Constant risk, or the annual average for each event, was assumed for application of hazard ratios to calculate event rates in the pioglitazone arm for all end points with the exception of edema, where hazard ratios were sampled from separate distributions for the first year and subsequent years of the simulation. Hazard ratios were then applied to the event rates from the placebo arm in line with the relative risk observed for each event during PROactive to calculate event rates in the pioglitazone treatment arm. To capture the statistical uncertainty in the trial data, hazard ratios in the modeling simulations were randomly sampled from a log-normal distribution generated from mu and sigma values derived directly from the trial data, which correspond to geometric means and standard deviations. Constant risk was assumed for application of hazard ratios to calculate event rates in the pioglitazone arm for all end points with the exception of edema, where hazard ratios were sampled from separate distributions for the first year and subsequent years of the simulation.
During simulations, event rates in all years beyond the trial period (i.e. years 4+) were calculated by applying a relative risk adjustment for each additional life-year gained (i.e., as the patient gets older, his/her risk of experiencing an event increases). Each event was associated with a specific relative risk adjustment based on published data as previously described [19].
In all complication submodels, the occurrence of events resulted in the accrual of event costs, and, where applicable, subsequent state costs as well as application of the appropriate quality of life utility values.
Estimation of Mortality
All-cause and cardiovascular mortality rates were derived from the PROactive Study for years 1–3 of the simulation, with rates subsequently doubling every 10 years [20]. Event rates for all subsequent years were calculated by applying relative risk adjustments [21–24] for each additional life-year gained (i.e., as the patient gets older, his/her risk of experiencing an event increases). In all submodels, the occurrence of events resulted in the accrual of event costs, and, where applicable, subsequent state costs as well as an assignment of the appropriate disutility values.
Simulated Cohort
Two cohorts of patients were defined with baseline demographics, baseline complications, and important concomitant medications representative of the two treatment arms from PROactive (Table 1). The use of a combined cohort is common in health economic analysis so that baseline risk factors do not act as a confounder to the application of treatment effects during the simulation [25]. Long-term outcomes were calculated in the model using a simulated population of 1000 patients, with baseline characteristics consisting of 66.1% male and a mean age of 61.8 years. At baseline, patients had a mean duration of diabetes of 10 years, with a mean HbA1c level of 8.1%. For the purposes of this analysis, patients were assumed to remain on the same treatment regimen for the duration of the simulation (35 years or death).
| Characteristic | Value | Data source |
|---|---|---|
| Demographics | ||
| Proportion male (%) | 66.1 | PROactive |
| Mean age (years) | 61.8 | PROactive |
| Duration of diabetes (years) | 10 | PROactive |
| Ethnic group | ||
| Proportion white (%) | 98.6 | PROactive |
| Proportion black (%) | 1.4 | PROactive |
| Baseline risk factors | ||
| HbA1c (%-points) | 8.1 | PROactive |
| Systolic blood pressure (mmHg) | 143.4 | PROactive |
| Body mass index (kg/m2) | 30.9 | PROactive |
| HDL-C (mg/dl) | 46.44 | PROactive |
| LDL-C (mg/dl) | 116.10 | PROactive |
| Total cholesterol (mg/dl) | 201.52 | PROactive |
| Triglycerides (mg/dl) | 194.92 | PROactive |
| Proportion smokers (%) | 13.8 | PROactive |
| Baseline complications | ||
| Acute coronary syndrome(%) | 13.65 | PROactive |
| CABG/PCI (%) | 30.75 | PROactive |
| Peripheral vascular disease (%) | 24.3 | PROactive |
| Myocardial infarction (%) | 47.0 | PROactive |
| Stroke (%) | 19.0 | PROactive |
| Microalbuminuria (%) | 14.3 | PROactive |
| Neuropathy (%) | 25.6 | PROactive |
- CABG, coronary artery bypass graft surgery; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; PCI, percutaneous coronary intervention.
Intervention Effects
For the base-case simulation, the clinical effects associated with the pioglitazone and placebo treatment regimens were applied as observed from PROactive. Treatment effects on HbA1c were applied separately in simulation years 1, 2, and 3 based on the findings from PROactive, and, in subsequent years, followed the United Kingdom Prospective Diabetes Study (UKPDS) long-term pattern [4]. Changes in HbA1c and other parameters for pioglitazone and placebo regimen effects were applied as summarized in Table 2. The long-term progression of all of these clinical parameters subsequently followed the patterns previously described by Palmer et al. in their description of the CORE Diabetes Model [16]. HbA1c levels were assumed to increase by 0.15% points in both treatment arms from year 4 until the end of the simulation, in line with observations made in the UKPDS [4].
| Pioglitazone | Placebo | |
|---|---|---|
| Change in HbA1c in year 1 (%-points) | −0.9 | −0.3 |
| Change in HbA1c in year 2 (%-points) | +0.1 | +0.1 |
| Change in HbA1c in year 3 (%-points) | +0.3 | +0.2 |
| Change in subsequent years | +0.15 | +0.15 |
| Total cholesterol (mg/dl) | +34.6 | +21.96 |
| HDL-C (mg/dl) | +21.0 | +11.9 |
| LDL-C (mg/dl) | +13.6 | +8.7 |
| Triglycerides (mg/dl) | −5.7 | +6.8 |
| Systolic blood pressure (mmHg) | −3.8 | −2.4 |
| Body mass index (kg/m2) | +1.1 | −0.1 |
| Hypoglycemic event rate (per 100 patient years) | +9.29 | +6.68 |
- HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol.
Costs
Direct medical costs over the lifetime horizon were accounted from a third-party health-care payer perspective (Medicare) in the United States. All costs were expressed in 2005 US dollars ($). Costs retrieved from published sources that were not expressed in 2005 values were inflated using the US Bureau of Labor Statistics Consumer Price Index calculator [26]. Direct medical costs comprised treatment, patient management, and complications costs.
In all submodels, the occurrence of events resulted in the accrual of event costs, and, where applicable, subsequent state costs. Treatment costs were calculated on an annual basis as the sum of study medication costs, other antidiabetes medication costs, and cardiovascular medication costs, based on the daily resource use data from PROactive, for the first 3 years. The daily pharmacy costs for pioglitazone in the United States were $2.86, $4.59, and $4.98 for 15 mg, 30 mg, and 45 mg doses, respectively [27]. Cardiovascular medication costs are summarized in Table 3. For all subsequent years in the simulation, the annual treatment costs of year 3 were applied. Patient management costs included the costs of screening for retinopathy and microalbuminuria, and the costs of lower extremity ulcer treatments. The cost of complications accounted for events that occurred during the simulation, and, for certain events, included the state or follow-up costs, as projected by the model. The unit costs of complications are summarized in Table 4.
| Medication | Daily cost of medication ($) |
|---|---|
| Beta-blockers | 8.712 |
| ACE inhibitors | 0.921 |
| Calcium channel blockers | 1.294 |
| Nitrates | 0.412 |
| Angiotensin II antagonists | 0.024 |
| Alpha blockers | 0.024 |
| Thiazide diuretics | 0.024 |
| Loop diuretics | 0.931 |
| Potassium sparing diuretics | 1.773 |
| Cardiac glycosides | 0.123 |
| Antiarrhythmics | 0.291 |
| Acetylsalicylic acid | 0.160 |
| Ticlopinide/clopidogrel | 3.530 |
| Oral anticoagulants | 0.402 |
| Statins | 3.722 |
| Fibrates | 3.624 |
- Source: Grover et al. [27].
- ACE, angiotensin converting enzyme.
| Event cost | Follow-up cost | Reference(s) | |
|---|---|---|---|
| Death (all causes) | 0 | 0 | Assumed |
| CVD death | 920 | 0 | [37] |
| MI (excluding silent MI) | 20,060 | 1,937 | [38] |
| Silent MI | 0 | 0 | Assumed |
| Acute coronary syndrome | 25,288 | 1,937 | [38,39] |
| CABG only | 37,071 | 1,937 | [37,38] |
| PCI only | 15,904 | 1,937 | [37,38] |
| Coronary revascularization (CABG + PCI) | 52,975 | 1,937 | [37,38] |
| Stroke | 46,420 | 15,492 | [38] |
| Leg amputation (major, above ankle) | 35,043 | 1,260 | [38] |
| Bypass surgery/revascularization of leg | 23,577 | 2,104 | [40] |
| Transient ischemic attack | 4,472 | 164 | [38] |
| Retinal photocoagulation | 971 | 87 | [38] |
| Hospitalization for CHF | 6,874 | 0 | [37] |
| Peripheral edema | 0 | 0 | Assumed to be zero, as patients were already receiving diuretics in PROactive, to avoid double counting. |
| Peripheral vascular disease, onset | 4631 | 0 | [41] |
| Hemodialysis | 44,901 | 44,901 | [38] |
| Peritoneal dialysis | 44,901 | 44,901 | [38] |
| Kidney transplant | 44,901 | 44,901 | [38] |
| Cataract extraction | 2,612 | 0 | [38,41] |
| Neuropathy, onset | 401 | 0 | [38] |
| Uninfected ulcer | 1,741 | 0 | [42] |
| Infected ulcer | 3,147 | 0 | [42] |
| Gangrene | 6,139 | 0 | [42] |
| Major hypoglycemic event | 269 | 0 | [38] |
| Ketoacidosis | 13,146 | 0 | [38] |
- CABG, coronary artery bypass grafting; CHF, congestive heart failure; CVD, cardiovascular disease; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Quality of Life Calculation
For the base case analysis, health state utilities for the events reported in PROactive were derived, wherever possible, from the CODE-2 disutility data [18]. Where PROactive events were not taken into consideration in the CODE-2 formula, no substitute values were used. All other quality of life utilities used in the sensitivity analyses have been described previously by Palmer et al. [16]. Univariate sensitivity analyses were performed to investigate the impact of including alternative disutilities.
A function that enabled evaluation of the impact of insulin treatment on quality of life was added to the CORE Diabetes model for the purposes of this analysis. Recent studies have suggested that insulin treatment reduces quality of life, and this has been quantified by the CODE-2 [18] and the University of Michigan [28]. The model assigned quality of life disutilities associated with insulin use based on the proportion of patients in the simulated cohort receiving insulin. This proportion of insulin users could be set for each of the first 5 years of the simulation, with the year-5 value carried forward for subsequent years. In the base case analysis, the disutility for insulin use was set to −0.049 as specified in CODE-2.
Patient Management
The proportion of patients receiving angiotensin converting enzyme (ACE) inhibitors in the simulation cohort was set to 62.8% based on data from PROactive. Risk adjustment for the use of aspirins or statins was disabled in the CORE Diabetes model, as the influence of these agents was already taken into account, along with the impact of ACE inhibitors, in the cardiovascular event rates taken from PROactive. Other settings for patient management parameters (e.g., screening for renal and eye disease and foot ulcer prevention programs) were applied as previously described by Palmer et al. [16].
Time Horizon and Discounting
A lifetime horizon of 35 years was used in the analysis to capture all relevant long-term complications, their associated costs, and impact on life expectancy. Future costs and clinical benefits were discounted at 3.0% per annum in line with recent recommendations for analyses in the US setting [29].
Statistical Approach
The cost-effectiveness analysis was performed using a nonparametric bootstrapping approach in which the progression of diabetes was simulated in 1000 patients run through the model 1000 times to calculate the mean and standard deviation of costs, life expectancy, and quality-adjusted life expectancy using second order Monte Carlo simulation [30]. Mean results of each of the 1000 iterations were used to create a scatterplot that compared the differences in outcomes for the pioglitazone and placebo treatment regimens. These values were in turn used to generate an acceptability curve over a range of willingness to pay values in the US setting.
Sensitivity Analysis
Univariate sensitivity analyses were performed to investigate the robustness of the base case findings. To investigate the influence of time horizon on the findings, sensitivity analyses were performed with the time horizon set to 5, 10, and 20 years. The effect of the improvement in HbA1c associated with pioglitazone treatment was investigated with a sensitivity analysis in which the change from baseline in HbA1c reported in the placebo arm was applied to both treatment arms. A sensitivity analysis was performed where no risk adjustments for age were applied during the simulation (risk adjustment factors all set to equal one). To investigate the assumption that the effects associated with pioglitazone treatment were transient, a sensitivity analysis was performed whereby the clinical benefits associated with pioglitazone from PROactive were applied for only 5 years. Thereafter, both treatment arms followed the clinical progression based on event rates from the placebo arm of PROactive. The discount rate on costs and clinical benefits were varied in a further sensitivity analysis between 0% and 6%. To establish the influence of including quality of life disutilities not included in the CODE-2 formula (base case), sensitivity analysis was performed where an “all disutilities” scenario was considered in which the disutilities were included, in addition to those previously described from the CODE-2 formula. Incremental values for discounted quality-adjusted life expectancy and direct medical costs, as well as incremental cost-effectiveness ratios were reported from the sensitivity analyses.
Results
Based on data from the PROactive study, the CORE Model projected that pioglitazone increased life expectancy and quality-adjusted life expectancy compared with placebo. In the base case, mean discounted life expectancy increased by 0.237 years with pioglitazone versus placebo (Table 5). Evaluation of quality-adjusted life expectancy showed an improvement of 0.166 quality-adjusted life-years associated with pioglitazone versus placebo (Table 5).
| Outcome | Pioglitazone | Placebo | Difference (PIO–PLA) |
|---|---|---|---|
| Discounted life expectancy (life-years) | 12.001 (0.185) | 11.764 (0.181) | +0.237 |
| QALYs | 8.918 (0.133) | 8.752 (0.131) | +0.166 |
| Undiscounted life expectancy (years) | 16.392 (0.302) | 15.986 (0.293) | +0.406 |
- Values shown are means (SD).
- PIO, pioglitazon; PLA, placebo; QALY, quality-adjusted life expectancy.
Estimation of long-term complication rates demonstrated that over the lifetime horizon, pioglitazone was associated with a reduced number of events per 100 patients versus placebo for most cardiovascular outcomes (Table 6). Exceptions included hospitalization for heart failure and peripheral edema. Pioglitazone was associated with a reduced risk of stroke, MI, ACS, and CABG compared with placebo over patient lifetimes; however, the cumulative number of leg revascularization and amputation procedures was higher in the pioglitazone arm.
| Complication | Cumulative events per 100 patients | ||
|---|---|---|---|
| Pioglitazone | Placebo | Difference (PIO–PLA) | |
| Acute coronary syndrome | 24.9 | 31.0 | −6.1 |
| (1.9) | (1.9) | ||
| Percutaneous coronary intervention | 56.7 | 60.5 | −3.8 |
| (2.9) | (2.9) | ||
| Coronary artery bypass graft surgery | 27.2 | 31.6 | −4.4 |
| (2.0) | (1.8) | ||
| Bypass surgery/revascularization of the leg | 37.4 | 29.2 | 8.2 |
| (2.3) | (1.7) | ||
| Hospitalization for CHF | 73.9 | 50.9 | 23.0 |
| (3.3) | (2.4) | ||
| Perlpheral edema | 158.4 | 101.3 | 57.1 |
| (4.9) | (3.3) | ||
| Myocardial infarction | 43.3 | 51.4 | −7.9 |
| (2.4) | (2.7) | ||
| Transient ischemic attack | 22.5 | 25.0 | −2.5 |
| (1.9) | (1.7) | ||
| Stroke | 58.3 | 69.1 | −10.8 |
| (3.2) | (3.2) | ||
| Photocoagulation | 61.8 | 60.1 | 1.7 |
| (2.7) | (2.7) | ||
| Severe vision loss | 7.4 | 7.0 | 0.4 |
| (0.9) | (0.8) | ||
| Microalbuminuria | 28.1 | 28.0 | 0.1 |
| (1.5) | (1.4) | ||
| Gross proteinuria | 17.6 | 17.3 | 0.3 |
| (1.2) | (1.2) | ||
| End-stage renal disease | 6.9 | 6.7 | 0.2 |
| (0.8) | (0.8) | ||
| Neuropathy | 42.2 | 41.7 | 0.5 |
| (1.6) | (1.6) | ||
| Peripheral vascular disease | 23.2 | 21.8 | 1.4 |
| (1.4) | (1.3) | ||
| First foot ulcer | 37.0 | 36.0 | 1.0 |
| (1.5) | (1.5) | ||
| Above-ankle amputation | 8.9 | 8.8 | 0.1 |
| (1.1) | (0.9) | ||
| Cataract | 11.1 | 11.8 | −0.7 |
| (1.0) | (1.0) | ||
- Values shown are means with standard deviation in parentheses.
Over patient lifetimes, the pioglitazone treatment regimen from PROactive was associated with higher direct medical costs compared with the placebo regimen (Table 7). Although treatment and management costs with pioglitazone were approximately $13,569 higher than for placebo over patient lifetimes, this additional cost was substantially offset by the reduced cost of diabetes-related complications. Therefore, over patient lifetimes, total direct medical costs were approximately $7305 more on pioglitazone than on the placebo regimen. A breakdown of complication costs demonstrated that the main cost savings with pioglitazone treatment were due to a reduced incidence of stroke (saving $6117 per patient) and coronary revascularization (coronary artery bypass grafting and percutaneous coronary intervention), saving $1939 per patient (Fig. 1). In contrast, the costs associated with bypass surgery/revascularization of the leg were more expensive in the pioglitazone arm than in the placebo arm (+$2092 per patient), as was hospitalization for heart failure (+$1045 per patient).
| Cost (per patient) | Pioglitazone (PIO) | Placebo (PLA) | Difference (PIO–PLA) |
|---|---|---|---|
| Total direct costs ($) | 272,694 (6,795) | 265,390 (6,617) | 7,305 |
| Treatment and management costs ($) | 79,743 | 66,174 | 13,569 |
| Complication costs ($) | 192,953 | 199,216 | −6,263 |
| ICER based on life expectancy | $30,792 per life-year gained | ||
| ICER based on quality-adjusted life expectancy | $44,105 per QALY gained | ||
- Values shown are means (SD). Incremental values are given as the pioglitazone value minus the placebo value.
- ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life expectancy.
The incremental cost-effectiveness ratios for pioglitazone versus placebo were $30,792 per life-year gained, and, taking quality of life into account, $44,105 per quality-adjusted life-year gained (Table 7). The cost-effectiveness plane indicated that the majority of points were in the upper right quadrant of the plane, implying increased effectiveness and increased costs associated with pioglitazone versus placebo. The cost-effectiveness acceptability curve (Fig. 2), demonstrated that at a willingness to pay of $50,000 per quality-adjusted life-year there was a 55% likelihood that pioglitazone would be cost effective [31,32].
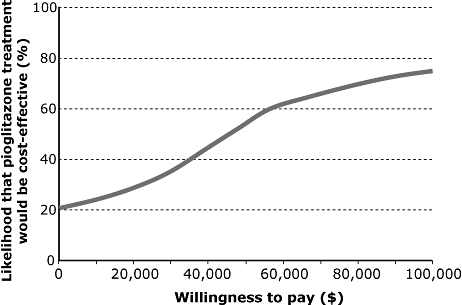
Cost-effectiveness acceptability curve: pioglitazone versus placebo.
Sensitivity Analyses
Sensitivity analysis demonstrated that the results were most sensitive to variation in the time horizon and assumptions on the duration of the benefits of pioglitazone treatment seen in the trial. Shorter time horizons meant that the development of many long-term complications were not captured in the analysis. For example, reduced rates of nephropathy and retinopathy complications, associated with improved HbA1c levels in patients on pioglitazone, were not observed at 5- and 10-year time horizons. When no discounting was applied to the long-term outcomes, mean life expectancy with pioglitazone was 0.406 years longer than with placebo. Limiting the duration of pioglitazone effect to 5 years resulted in patients experiencing complications at the same frequency in both arms of the model over subsequent years of the simulation, but still accumulating pioglitazone drug acquisition costs in the active treatment arm, which led to a relatively high incremental cost-effectiveness ratio of $710,455 per quality-adjusted life gained.
Inclusion of disutilities in the analysis for all macrovascular events led to a smaller improvement in quality-adjusted life expectancy with pioglitazone compared with the base case analysis. This increased the incremental cost-effectiveness ratio to approximately $58,921 per quality-adjusted life-year gained. Sensitivity analysis where both treatment regimens had an identical effect on HbA1c, indicated that most of clinical benefit associated with pioglitazone, in terms of quality-adjusted life expectancy, was driven by an improved cardiovascular risk profile rather than reduced HbA1c (incremental benefit 0.178 QALYs versus placebo, base case value 0.180 QALYs). Incremental costs were also comparable to the base case analysis, leading to an incremental cost-effectiveness ratio of approximately $42,258 per QALY gained for pioglitazone versus placebo.
Discussion
PROactive was the first clinical trial to demonstrate the potential for pioglitazone to reduce the incidence of cardiovascular events in patients with type 2 diabetes and evidence of macrovascular disease when added to existing therapy [13,14]. The findings of this long-term analysis of the cost-effectiveness of pioglitazone from PROactive in the United States indicate that the addition of pioglitazone would be associated with an incremental cost-effectiveness ratio of approximately $44,105 per quality-adjusted life-year gained, over the lifetime horizon, compared with placebo. In the base-case analysis, treatment with pioglitazone was associated with improvements in life expectancy of 0.237 years and quality-adjusted life expectancy of 0.166 QALYs, and slightly higher direct medical costs ($7305) over the lifetime horizon. Acceptability curve analysis indicated that there would be a 55% probability that pioglitazone would be cost-effective at a willingness to pay threshold of $50,000 per quality-adjusted life-year gained, compared with placebo.
The CORE Diabetes Model was used to estimate the long-term cost-effectiveness of pioglitazone versus placebo from PROactive by projecting short-term clinical and resource use data over the lifetime horizon. New submodels were designed to incorporate primary end point data from PROactive and represented disease- and procedure-related health states. To facilitate the modeling process beyond the duration of the PROactive study, some assumptions were necessary regarding future event risks, intervention effects, and annual costs. After year 4 in the model, data from sources other than PROactive were used to calculate age-related relative risk adjustments for the new submodels [21,23,24]. Furthermore, it was assumed that in the first 3 years, intervention effects of pioglitazone and placebo would be based on observations from PROactive, thereafter following the long-term pattern observed in the UKPDS [4,13,14]. In addition, the costs associated with medication were based on resource use data from PROactive for the first 3 years, and then it was assumed that year 3 treatment costs would apply for all subsequent years.
Sensitivity analyses were performed to evaluate the impact of variation in key parameters in the base case outcomes. This included a shorter time horizon, assuming the change in baseline HbA1c was equal for pioglitazone and placebo, removing age-related risk adjustments, shortening the duration of treatment effect of pioglitazone, varying the discount rate on clinical and cost outcomes, and applying alternative health state disutilities. The results were most sensitive to variation in the time horizon and the duration of pioglitazone effect. Over a 5-year time horizon, fewer long-term complications were captured so the long-term clinical benefits of pioglitazone were not observed. A shorter duration of pioglitazone treatment effect meant fewer long-term complications being avoided with pioglitazone versus placebo. The incremental cost-effectiveness ratio for pioglitazone versus placebo increased to $831,601 and $710,455 per quality-adjusted life-year gained for shorter time horizon and a shorter treatment effect, respectively.
This analysis estimated quality-adjusted life expectancy conservatively in the base case, using only data from CODE-2, which did not capture changes in quality of life associated with several macrovascular end points [18]. It is noteworthy that this methodology may have underestimated the improvements in quality-adjusted life expectancy, as the formula did not capture some of the benefits of pioglitazone treatment, including reduced rates of myocardial infarction, acute coronary syndrome, percutaneous coronary intervention, and coronary artery bypass graft surgery. The corollary is that certain disadvantages associated with pioglitazone treatment were, similarly, not captured, such as peripheral edema, hospitalization for heart failure, and revascularization of the leg. Sensitivity analysis, including disutilities to include these end points, produced an incremental cost-effectiveness ratio that was higher than base case ($58,921 per quality-adjusted life-year gained).
The primary end point of PROactive was a composite of both disease- and procedure-related events, while its principal secondary end point included disease states only [13,14]. Pioglitazone was associated with a nonsignificant reduction for the primary end point and a significant reduction for the principal secondary end point. The conclusions of PROactive have focused on the principal secondary end point, and the rationale for this approach has been the subject of some discussion [33–36]. The aim of this present health economic analysis was not to contribute to this discussion, but to take all the available outcomes data from PROactive and project the long-term health and economic outcomes using a published and validated diabetes model that derives transition rates and risk adjustments from epidemiological studies, including the UKPDS, Wisconsin Epidemiological Study of Diabetic Retinopathy (WESDR), and Framingham.
The base case mean price per day of therapy (DOT) for beta-blockers was $8.71 and was based on a weighted average across the beta-blocker class of therapy. We performed one final sensitivity analysis by lowering the price of beta-blocker therapy to $4.50/DOT to determine what kind of incremental cost-effectiveness ratio (ICER) change would result from this reduction in beta-blocker therapy price. The ICER changed from $44,105 per QALY gained in the base case to $44,708 per QALY gained, with the lower price for beta-blockers. Basically, there were more patients in the placebo arm taking beta-blockers. As a result, lowering the beta-blocker price benefits the placebo arm slightly over the pioglitazone arm. The result was a marginal increase in incremental costs and a slightly higher ICER, but not any dramatic differences.
The PROactive study did not demonstrate a statistically significant increased risk of bone fractures with pioglitazone use compared with placebo, and was therefore not included in this analysis.
Conclusions
The addition of pioglitazone to existing therapy in patients with type 2 diabetes and pre-existing macrovascular disease, based on data from the PROactive study, was projected to be cost-effective over the lifetime horizon compared with placebo, with an incremental cost-effectiveness ratio of $44,105 per quality-adjusted life-year gained. At a willingness to pay of $50,000 per quality-adjusted life-year gained in the United States, there was a 55% likelihood that pioglitazone would be considered cost-effective.
This study was supported by an unrestricted grant from Takeda Global Research and Development, Inc., Deerfield, IL, USA.
Source of financial support: This study was supported by an unrestricted grant from Takeda Global Research and Development, Inc, Deerfield, IL, USA.




