Budgetary Impact of Varenicline in Smoking Cessation in the United Kingdom
ABSTRACT
Objectives: To estimate the budgetary impact of varenicline in the United Kingdom (UK) in the first 5 years after its introduction to the smoking-cessation aid market, from the National Health Service (NHS) pharmacy perspective.
Methods: The economic impact of varenicline to the national health budget is estimated in a population of current, former, and new smokers. The analyses are based on data from a variety of secondary sources including national health data, clinical trials, and meta-analyses of smoking-cessation aids. The number of patients seeking aid and the treatment patterns are estimated using 2004 national health surveys, costs for medications from national prescription drug pricing tariffs, and efficacy of the various smoking-cessation aids from clinical trial data. Sensitivity analyses were performed to evaluate the impact of varying the patient parameters and costs.
Results: Model estimates suggest that the budgetary impact of varenicline would be £3.6 million in the second year after its introduction, with a 95% confidence interval of £0.63 to £7.2 million, and a resultant increase of 0.05% to the total NHS pharmacy budget. The model predicts that the addition of varenicline to the market would result in an additional 162,000 successful smoking-cessation attempts and 103,000 fewer smokers over 5 years, when compared to the world without varenicline.
Conclusion: The introduction of varenicline is likely to result in greater numbers of individuals succeeding at smoking cessation, with an approximately £3.6 million (0.05%) increase in the NHS pharmacy budget.
Introduction
Cigarette smoking is the leading preventable cause of death and disease in the United Kingdom (UK), attributable for 114,000 deaths annually [1] and £1.5 billion in National Health Service (NHS) expenditures (e.g., hospital, primary care, and pharmaceutical fees) [2]. In 2004, there were 12 million adult smokers in the UK [3], and it is expected that one-half of the habitual smokers within this group will die as a result of their addiction to nicotine [4]. Although smoking increases the risk of developing a wide array of diseases, the most fatal of the smoking-related diseases are lung cancer, chronic obstructive lung disease (bronchitis and emphysema), and coronary heart disease [1].
Because of the economic burden that nicotine addiction places on the UK health-care system, the Department of Health allots an average of £46 million annually for the NHS Stop Smoking Services [5]. Of the 600,000 smokers who enlisted the aid of the Stop Smoking Services in 2005, 82% received some form of nicotine-replacement therapy (NRT) and 5% received bupropion [5]. NRT encompasses a wide variety of delivery systems—including gums, lozenges, transdermal patches, nasal sprays, and inhalers—but all share in common the objective of alleviating the symptoms of nicotine withdrawal [6]. Although the percentages of smokers who have attempted to quit smoking has increased over the past 5 years [7], many smokers who try to quit either fail to do so, or relapse soon after quitting. A 2006 report on smoking behavior in the UK showed that 80% of the individuals who were smoking in 2005 had tried to quit at least once at some point in the past [7]. For those smokers who had failed to quit smoking, 32% had relapsed within 2 weeks of their last cessation attempt.
Varenicline is a new oral medication with a novel mechanism of action designed specifically for smoking cessation. In contrast to NRT, in which small quantities of nicotine are provided to ease nicotine withdrawal symptoms, varenicline partially activates the α4β2 nicotinic acetylcholine receptors in the brain that are associated with smoking, while simultaneously blocking nicotine from binding with those same receptors. Consequently, varenicline eases the craving and withdrawal symptoms associated with smoking cessation, while also blocking the reward-based sensations that result from smoking a cigarette should a relapse occur [8,9].
Although varenicline has the potential to improve smoking-cessation initiatives in the UK, concerns may arise regarding its impact on pharmacy budgets. Accordingly, we used modeling techniques and data from a variety of secondary sources to project the likely budgetary impact of varenicline from the UK NHS pharmacy perspective.
Methods
Methodologic Overview
Estimation of budgetary impact of a new intervention involves comparison of estimated health-care costs before versus after its introduction—in effect, a contrast of the “world without” to the “world with” the new intervention. The net difference in estimated health-care costs between these two scenarios constitutes the budgetary impact of the intervention. There are three main components of a budgetary impact model: population, marketplace dynamics, and costs. The population component reflects the number of individuals who are eligible to receive a new therapy. The marketplace dynamics component predicts market uptake of the new therapy, along with consequent changes in market shares of competing therapies and overall growth in the market. The cost or economic component predicts changes in costs and sometimes outcomes of care associated with the introduction of the new therapy to the marketplace.
Model Structure
We developed a state-transition model reflecting relevant health states for current and former adult smokers in the UK. The model, illustrated in Figure 1, includes the following mutually exclusive health states: current smokers eligible to quit using prescription-based smoking-cessation therapy; current smokers ineligible for such therapy; recent quitters (defined as those who had quit in the prior model cycle); sustained quitters (defined as those who quit in a prior model cycle and did not relapse in the most recent cycle); former smokers (defined as those who have a history of smoking but had quit before entering the model) [10]; and individuals who have never smoked. The current smoker eligible to quit state includes new smokers; current smokers who are attempting or not attempting to quit; and recent quitters, sustained quitters, and former smokers who relapsed in the previous model cycle. The model is estimated as a Markov process over a period of 5 years, with a 6-month cycle length.
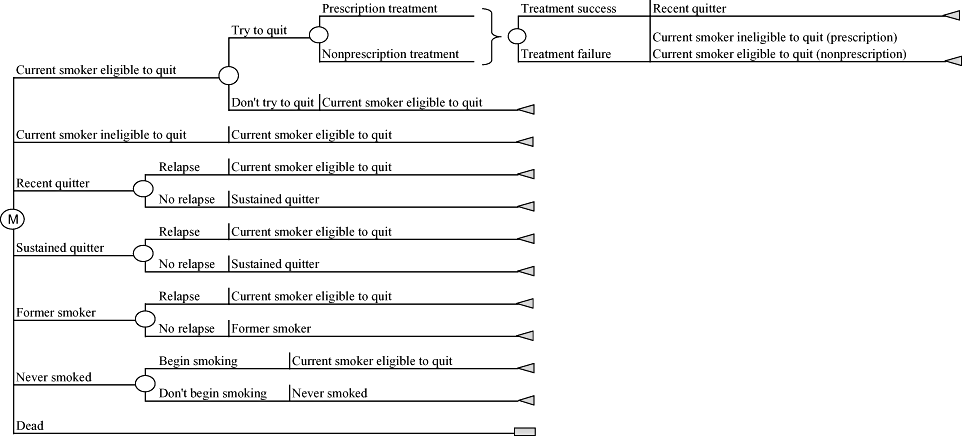
Decision tree diagram of Markov states. Individuals enter the model in either the “Current smoker eligible to quit,”“Former smoker,” or “Never smoked” state.
Individuals enter the model in one of three states: as a current smoker eligible to quit, a former smoker, or as someone who has never smoked. For convenience, we assume that all current smokers enter the model eligible to quit and that no one begins the model as a recent or sustained quitter (data limitations preclude assignment of the UK population to these states). In any given cycle of the model, current smokers eligible to quit may or may not decide to do so, and those who do attempt to quit may or may not receive prescription therapy. All those attempting to quit may or may not be successful—those who are successful transition to the recent quitter health state in the next model cycle, while those who are not successful become current smokers ineligible or eligible to quit depending on whether they had or had not received prescription therapy in the current model cycle. Because the cycle-length of the model is identical to the UK's 6-month waiting period between prescription-based quit attempts, all current smokers ineligible to quit in a given model cycle immediately become eligible in the next cycle.
All persons with a history of smoking—including recent quitters, sustained quitters, and former smokers—are at risk of relapse in each model cycle; those who do resume smoking become current smokers eligible to quit in the next model cycle. Recent quitters who do not relapse become sustained quitters in the next model cycle, consistent with our previous definitions of these health states. Finally, individuals who have never smoked may begin to smoke in any given model cycle and in so doing become current smokers eligible to quit in the next cycle.
Model Estimation
Model estimation involves predicting and tracking patients' transitions across the health states over the 5-year time horizon of the model, which was chosen to allow estimation of the budgetary impact of varenicline over time, as its penetration into the marketplace takes effect. The smoking-cessation therapies that were explicitly modeled include varenicline, bupropion, prescription NRT, and nonprescription treatments. The nonprescription treatment category includes both unaided cessation and over-the-counter NRT.
Two inflows of new smokers are added to the current smoker eligible to quit state in each year of the model, consisting of 17-year-old individuals [11] who turn 18 years old and are then eligible for varenicline therapy (i.e., according to prescribing instructions), and 18- to 24-year-old individuals who never smoked and then start smoking. We added two inflows of new smokers to the 18- to 24-year-old age group only, because according to a 2004 UK household survey, 94% of male and 83% of female current or former smokers began smoking when they were less than 25 years old [3]. The number of new smokers in the 18- to 24-year-old age group was determined by multiplying the annual probability of becoming a new smoker in that age group [3] by the number of individuals who never smoked. Those individuals turning 25 years old in the model were no longer eligible to become a new smoker.
Candidates for smoking-cessation treatment are determined by multiplying age- and sex-specific prevalence rates for smoking by the number of individuals in each age–sex group. The result is then multiplied by the percentage of smokers attempting to quit and the percentage electing to use pharmacologic therapy. Finally, the number of smokers attempting to quit with pharmacologic therapy is then multiplied by the market share for each of the smoking-cessation treatments, which yields the number of individuals receiving each product in each model cycle.
We assumed that secular trends in the growth of the smoking-cessation marketplace would continue throughout the period of analysis and that the use of varenicline would result in reduced market shares for existing products, including bupropion and prescription NRT. That is, varenicline would take the place of other prescription therapies and not cause an expansion of the smoking-cessation aid marketplace. We based this assumption on the “UK Smoking Related Behaviour and Attitudes Survey” from 2004, which showed that the annual percentage of smokers using prescription aids changed little after bupropion was introduced to the smoking-cessation aid marketplace in 2000 [11].
Model Inputs
The total population and percentages of current and former smokers in the model are contained in Table 1, and are stratified by age and sex. Table 2 shows the clinical efficacies for the smoking-cessation treatments that were used in the model. The efficacies were obtained from clinical trial data and published sources. Clinical efficacy is defined as carbon monoxide (CO)-confirmed continuous smoking abstinence through both 6 and 12 months. Because the prescription NRT formulations in the meta-analyses showed similar clinical efficacy to nonprescription NRT formulations, an overall odds ratio for successful smoking cessation was assumed [6,14], and we used pooled placebo rates to calculate overall NRT efficacy [8,9].
| Male | Female | |
|---|---|---|
| Total population [12] | ||
| 18–24 years old | 2,723,100 | 2,642,100 |
| 25–44 years old | 8,506,800 | 8,622,600 |
| 45–64 years old | 7,170,400 | 7,368,000 |
| ≥65 years old | 4,091,100 | 5,488,800 |
| Current smokers [13] | ||
| 18–24 years old | 25.00% | 29.00% |
| 25–44 years old | 31.11% | 27.46% |
| 45–64 years old | 22.16% | 22.62% |
| ≥65 years old | 8.74% | 10.94% |
| Former smokers [13] | ||
| 18–24 years old | 5.00% | 7.00% |
| 25–44 years old | 17.75% | 17.08% |
| 45–64 years old | 36.62% | 26.86% |
| ≥65 years old | 58.10% | 31.58% |
| Smoking-cessation treatment | Abstinence rate % (SD) |
|---|---|
| Varenicline [8,9] | |
| 6 months | 29.8 (6.12) |
| 12 months | 22.5 (4.88) |
| Bupropion [8,9] | |
| 6 months | 20.5 (3.33) |
| 12 months | 15.7 (3.01) |
| Prescription NRT [6,8,9,14] | |
| 6 months | 19.1 (1.05) |
| 12 months | 15.5 (0.79) |
| Nonprescription NRT [6,8,14] | |
| 6 months | 19.1 (1.05) |
| 12 months | 15.5 (0.79) |
| Unaided cessation [15,16] | |
| 6 months | 6.0 (0.60) |
| 12 months | 5.0 (0.50) |
- NRT, nicotine-replacement therapy; SD, standard deviation.
Three types of relapse rates were used within the model: treatment-specific relapse rates, relapse among sustained quitters who quit smoking during the model, and relapse among former smokers who quit smoking at some point before the start of the model. The clinical efficacies were used to calculate the treatment-specific relapse rates in the model. Each relapse rate was calculated as the difference between the 6- and 12-month efficacies, divided by the 6-month efficacy. The relapse rates for the sustained quitters and the former smokers were estimated separately, because relapse probability varies with the duration of smoking abstinence. We used a 6-month relapse rate of 3.3% for sustained quitters, and 0.6% for former smokers [10].
With respect to treatment costs, a “full course” of treatment is typically 9 to 12 weeks in length, and costs £165.66 for varenicline [17], £81.56 for bupropion [18], and £115.81 for NRT [18]. Nevertheless, the first prescription in the UK only covers 2 to 4 weeks of smoking-cessation treatment. Consequently, a “partial course” cost was calculated for those patients who failed at their attempt to quit smoking before they could start a second prescription. The cost for a partial course of treatment was calculated as £28.23 for varenicline, £40.78 for bupropion, and £39.54 for NRT. All costs used in the analysis are expressed in 2006 pounds sterling.
The top half of Table 3 contains the percentage of smokers who attempt to quit smoking annually. In addition, 25% of the smokers who will attempt to quit smoking in each year of the model will use a pharmacological treatment. The bottom half of Table 3 contains the market share projections for each of the smoking-cessation treatments, in each year of the model. We assumed that bupropion and prescription NRT are the only available smoking-cessation treatments on the market in the “world without” varenicline. Varenicline is added as a treatment in the “world with” scenario.
| 2007 | 2008 | 2009 | 2010 | 2011 | |
|---|---|---|---|---|---|
| Percentage of smokers attempting to quit annually [11] | |||||
| 18–24 years old | 21.8% | 22.6% | 23.4% | 24.2% | 25.1% |
| 25–44 years old | 35.2% | 36.6% | 37.9% | 39.2% | 40.6% |
| 45–64 years old | 24.9% | 25.8% | 26.8% | 27.7% | 28.6% |
| ≥65 years old | 13.5% | 14.0% | 14.5% | 15.0% | 15.5% |
| Market shares without varenicline* | |||||
| Bupropion | 4.36% | 4.54% | 4.71% | 4.89% | 5.06% |
| NRT | 95.64% | 95.46% | 95.26% | 95.11% | 94.94% |
| Market shares with varenicline* | |||||
| Varenicline | 11.09% | 16.01% | 20.94% | 25.86% | 30.79% |
| Bupropion | 4.00% | 3.60% | 3.21% | 2.81% | 2.42% |
| NRT | 84.91% | 80.38% | 75.85% | 71.33% | 66.80% |
- * Pfizer, Inc. market projections.
- NRT, nicotine-replacement therapy.
Analyses
Budgetary impact was calculated by taking the difference in aggregate drug costs in the “world without” and the “world with” varenicline. The per-treatment-success budgetary impact was calculated by dividing the aggregate drug costs by the number of treatment successes.
One-way sensitivity analyses were performed to assess the robustness of model results to plausible variation in parameters. Where possible, the ranges of selected model parameters (e.g., abstinence rates) were varied according to published sources [6,11,13], otherwise parameters were varied through a range of 75% to 125% of the base-case estimate.
To assess uncertainty, probabilistic sensitivity analyses were performed on the budgetary impact in the second year of the model to allow enough time for market penetration yet not relying heavily on model assumptions and extrapolations. The drug-specific abstinence rates, and relapse probabilities, were assumed to have a beta distribution that was based on the point estimates, and the standard deviations of the estimates, where available. A random number generator was used to draw a set of parameter values from these distributions; the parameter set was then inserted into the model and the budgetary impact recalculated in the usual manner. The process of drawing parameters and running the model was repeated 1000 times.
Results
Budgetary Impact Analysis
The overall budgetary impact is estimated to be £2.5 million in 2007, £3.7 million in 2008, £4.8 million in 2009, £5.9 million in 2010, and £7.0 million in 2011. The per-treatment-success budgetary impact is estimated to be £120.18 in 2007, £120.48 in 2008, £120.76 in 2009, £121.04 in 2010, and £121.31 in 2011. The budgetary impact data for overall and per treatment success are presented in Table 4.
| Budgetary impact | Year | ||||
|---|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2010 | 2011 | |
| Overall (£ millions) | 2.48 | 3.63 | 4.68 | 5.71 | 6.73 |
| Per treatment success (£) | 120.18 | 120.48 | 120.76 | 121.04 | 121.31 |
Annual prescription costs for smoking-cessation treatments in the “world without” varenicline were estimated to be £79.8 million in 2007, £83.9 million in 2008, £85.1 million in 2009, £86.3 million in 2010, and £87.3 million in 2011. Annual prescription costs in the “world with” varenicline were estimated to be £82.3 million in 2007, £87.6 million in 2008, £90.0 million in 2009, £92.2 million in 2010, and £94.3 million in 2011. The increased costs in the “world with” varenicline are due to the higher costs of varenicline relative to other smoking-cessation aids, the increasing numbers of current smokers attempting to quit [7,11], and the increasing numbers of patients who take varenicline instead of other prescription smoking-cessation aids.
Treatment with smoking-cessation aids in the “world with” varenicline yielded more than 2.7 million quitters (i.e., 12 months of sustained smoking abstinence), and approximately 3.7 million treatment successes (i.e., 6 months of sustained smoking abstinence) by the end of the 5-year model. Furthermore, the addition of varenicline to the smoking-cessation aid market resulted in an additional 162,000 successful smoking-cessation attempts and 103,000 fewer smokers in the “world with” versus the “world without” varenicline.
Sensitivity Analyses
A one-way sensitivity analysis was conducted on model parameters to assess robustness. The following parameters were included in the analysis: treatment success and failure costs for varenicline, NRT, and bupropion; 6- and 12-month abstinence rates for varenicline, NRT, and bupropion; the relapse probabilities for recent quitters and former smokers; percentage of individuals taking prescription treatment; and the percentage of individuals in each age group attempting to quit. The one-way sensitivity analysis showed that the budgetary impact estimate was most sensitive to variations in the costs of varenicline and NRT, and least sensitive to NRT efficacy (Fig. 2).
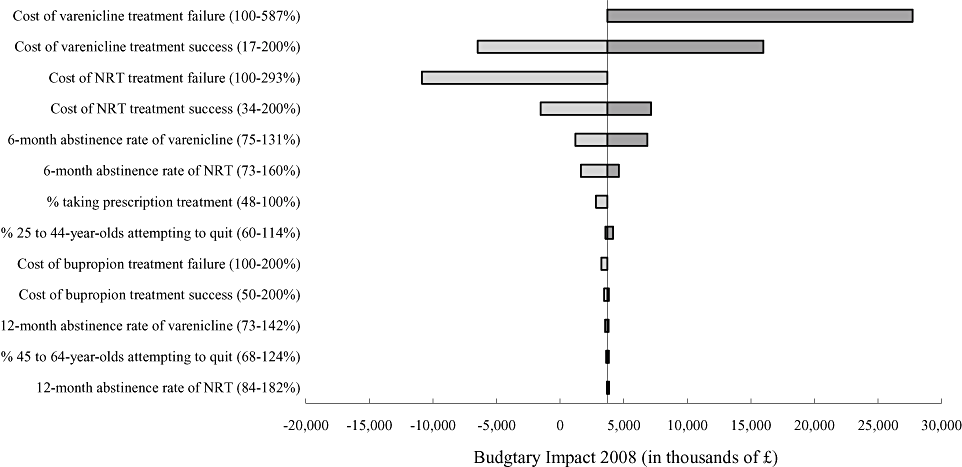
Tornado diagram depicting sensitivity of 2008 budgetary impact to model parameters. Range (%) is expressed as percentage of the base-case estimate. NRT, nicotine-replacement therapy.
To assess uncertainty, a probabilistic sensitivity analysis was performed on the budgetary impact in the second year of the model and suggests that the mean budgetary impact would be £3.6 million, with a 95% confidence interval of £0.634 to £7.2 million.
Discussion
The overall budgetary impact of varenicline was estimated to be £2.5 million in the first year after introduction, £4.8 million in 2009, and £7.0 million in 2011 for the UK. The increase in the overall budgetary impact of varenicline across the 5-year time horizon of the model is due to the higher cost of varenicline, when compared to other smoking-cessation treatments, the increasing number of smokers trying to quit, and the increasing numbers of individuals taking varenicline. Although the overall budgetary impact increased from 2007 to 2011, the per-treatment-success budgetary impact changed very little across the 5-year time horizon (e.g., £120.18 in 2007, £120.76 in 2009, and £121.31 in 2011).
In spite of the robustness of the model in predicting budgetary impact, there are some limitations. Our model assessed cost and impact on the NHS pharmacy budget. Costs incurred by other payers such as employers and patients are excluded, although they may impact the utilization of certain smoking-cessation therapies. Also, no cost offsets are included from reduced medical care for not smoking, which could decrease the budgetary impact of the NHS as a whole. Although not likely substantial in the short run, these cost offsets would certainly accumulate over time because of avoidance of costly and deadly smoking-related illnesses, such as lung cancer, chronic obstructive pulmonary disease, and cardiovascular disease. In fact, Rasmussen et al. [19] have estimated the direct lifetime health-care cost-savings to be about €10,000 on average, or about £6,700, for a moderate smoker who quits smoking at the age of 35 years. The savings were based on hospital, physician, and drug costs for the treatment of various smoking-related diseases (e.g., cancer, vascular, respiratory). Rasmussen's estimates were based on the 1999 data collected in Denmark, and rising health-care costs could result in even larger lifetime savings.
Finally, our estimates of the number of smokers who are attempting to quit and using pharmacological treatment are based on national surveys. Although the 2004 “National Health Survey for England”[13] and the 2004 “Smoking Related Behaviour and Attitudes Survey”[11] are well-documented and established, these may overestimate the actual numbers and percentages of smokers attempting to quit. This is because survey participants may sometimes exhibit what is referred to as “Evaluation Apprehension”[20], where participants respond in such a way as to put themselves in the best light (e.g., saying that they intend to quit smoking). A resulting decrease in the number of individuals attempting to quit would decrease the budgetary impact.
Our analysis sheds light on the probable impact of the introduction of varenicline in the UK. Our model suggests that introduction of varenicline will likely result in a greater number of smoking-cessation treatment successes, and thus fewer smokers, when compared to traditional smoking-cessation therapies such as NRT. A reduction in the number of smokers would result in a reduction in the medical costs that are linked to smoking behaviors. The introduction of varenicline in the UK would only increase the UK pharmacy budget by 0.05%, based on the 2005 to 2006 Family Health Service drugs bill expenditure of £7,235 million that was reported in the 2007 UK Department of Health Report [21].
As experience with varenicline in the UK accumulates, additional data on its financial impact on NHS budgets will become available. Until then, our projections of budgetary impact may be of use to NHS administrators, formulary committee members, clinicians, patients, and others interested in the economics of smoking cessation in the UK.
Source of financial support: Pfizer Inc., New York, NY, USA.




