Satisfaction and Adherence in Patients with Iron Overload Receiving Iron Chelation Therapy as Assessed by a Newly Developed Patient Instrument
ABSTRACT
Objectives: This study assesses satisfaction with iron chelation therapy (ICT) based on a reliable and valid instrument, and explores the relationship between satisfaction and adherence to ICT.
Methods: Patients in the USA and UK completed a new “Satisfaction with ICT” (SICT) instrument consisting of 28 items, three pertaining to adherence. Simple and multivariate regression analyses assessed the relationship between satisfaction with different aspects of ICT and adherence.
Results: First assessments of the SICT instrument indicate its validity and reliability. Recommended thresholds for internal consistency, convergent validity, discriminant validity, and floor and ceiling effects were met. A number of variables were identified in the simple linear regression analyses as significant predictors of “never thinking about stopping ICT,” a proxy for adherence. These significant variables were entered into the multivariate model to assess the combined factor effects, explaining 42% of the total variance of “never thinking about stopping ICT.” A significant and positive relationship was demonstrated between “never thinking about stopping ICT” and age (P = 0.04), Perceived Effectiveness of ICT (P = 0.003), low Burden of ICT (P = 0.002), and low Side Effects of ICT (P = 0.01).
Conclusions: The SICT is a reliable and valid instrument which will be useful in ICT clinical trials. Furthermore, the administration of ICT by slow subcutaneous infusion negatively impacts on satisfaction with ICT which was shown to be a determinant of adherence. This points to the need for new more convenient and less burdensome oral iron chelators to increase adherence, and ultimately to improve patient outcomes.
Introduction
Approximately 900,000 individuals worldwide are expected to be born with thalassemia over the next 20 years [1]. Furthermore, about 250,000 individuals worldwide are diagnosed with sickle cell disease (SCD) per year [2], and myelodysplastic syndrome (MDS) is primarily manifested in older individuals with 20 to 50 out of 100,000 individuals per year diagnosed over the age of 60 years [3].
Blood transfusions are an essential part of therapy in the treatment of many patients with thalassemia, SCD, and MDS. One consequence of regular blood transfusions is excess iron intake, which cannot be excreted naturally from the body, and accumulates (hemosiderins) in insoluble ferritin complexes deposited mainly in the liver, spleen, a number of endocrine organs, and the myocardium. This leads to tissue damage and fibrosis [4,5]. Further, without treatment for iron overload, patients may not survive, and cardiac complications are usually the main cause of death.
To avoid complications of iron overload, chronically transfused patients must receive life-long iron chelation therapy (ICT) [6]. Deferoxamine or desferal (DFO) is administered via subcutaneous infusion at home by the patient overnight or during the day over 8 to 12 hours, at least 5 days per week [7]. Although DFO-related ICT has an acceptable safety profile, the treatment regimen is not only time-consuming but burdensome to patients [7], and negatively impacts on their health-related quality of life (HRQoL) [8].
In one study, univariate analysis found that the degree of discomfort with DFO-related ICT and ferritin level significantly influenced patients' perceptions of their HRQoL, and the risk of a low HRQoL score increased with the degree of discomfort [9]. Another study in Malaysia explored HRQoL in children with thalassaemia compared to healthy controls using the 23-item Pediatric Quality of Life Inventory (PedsQL 4.0 Generic Core Scales), and revealed that patients with thalassemia receiving DFO via blood transfusions have a lower HRQoL compared to healthy controls regardless of age, sex, ethnicity, and household income [10]. Furthermore, in a US study cohort of patients receiving DFO, HRQoL was compromised in patients receiving DFO. The differences between the Short Form 36-Item Health Survey (SF-36) domain scores and age-matched norms were often of the magnitude of at least 3 to 5 units, which indicates that these results were clinically meaningful and significant [11]. In other studies, the HRQoL domains affected by DFO-related ICT include: depression, fatigue, dyspnoea, physical functioning, psychological distress, general health, and decrease in HRQoL during hospitalization [11–13].
Further, local injection site reactions that are generally not serious but bothersome to patients include: bumps, rashes and bruises, and infections [14,15]. Patients on DFO-related ICT may experience other side effects such as: neutropenia, hematological toxicity, shortness of breath, headaches, and dizziness [16].
Given the significant negative impact of DFO-related ICT on patients' HRQoL, and the potential side effects experienced, it is perhaps not surprising that many patients do not adhere to their DFO regimen as recommended by their doctors [17–20], and it is estimated that up to 50% of patients in the UK may not fully adhere to their DFO-related ICT regimen [21].
Less demanding to adhere to is the oral chelator deferasirox (Exjade Novartis Pharma Stein AG, Stein, Switzerland) [22,23]. In some countries (although not in the USA or Canada), deferiprone (Ferriprox Apotex, Inc., Weston, ON, Canada) is an approved alternative oral chelator with various restrictions on the label. In patients with thalassemia, it has been shown to be less effective at lowering hepatic iron than subcutaneous DFO [24,25].
There is an expanding body of evidence in patients with thalassemia and SCD that suggests increased satisfaction with ICT would reduce the likelihood of patients stopping their treatment [26,27]. Consequently, patient satisfaction with ICT is an important patient outcome and should be considered during patients' overall treatment management.
While some research indicates that satisfaction with treatment and care is associated with factors such as age, dosage, and side effects [28], to our knowledge, there are no studies which considered all the factors associated with satisfaction with ICT, nor any adequate instrument available to reliably quantify the concept [8]. This finding supports the assertion of Linder-Pelz (1982) that there is very little satisfaction research that tests or builds on theory, and which provides data to explain the association between satisfaction with treatment and patient behaviors such as adherence [29].
The objectives of this article are threefold. Specifically to: 1) describe the development scoring, and validity of the Satisfaction with ICT instrument (SICT) instrument; 2) report satisfaction results of a binational, multicenter, retrospective chart review, and semiprospective study in the USA and UK; and 3) investigate based on empirical findings the relationship between satisfaction and adherence of ICT.
Patients and Methods
Study Design and Patient Population
As part of a retrospective chart review and semiprospective investigation [11], patients with thalassaemia, SCD, or MDS from eight study sites (four per country: USA and UK) completed the SICT instrument at one study visit. Study protocols and questionnaires were reviewed by an ethics committee and approval was obtained by the investigator at each participating center.
Patient eligibility was verified by the principal investigator. The inclusion criteria were: 1) females or males ≥6 years of age; with 2) diagnosis of thalassaemia, SCD, or MDS; 3) minimum of 3 months of ongoing ICT at the time of study enrolment; and 4) provided written informed consent. Exclusion criteria were: 1) patients at the study site for less than 6 months before first study visit; 2) those less than 18 years of age without (available) caregiver; or 3) patient was deemed too unwell as a result of comorbid medical conditions.
Because there is no formal method to establish sample size to conduct exploratory factor analysis, the generally accepted rule of five to 10 participants for every item was employed [30]. With 26 items to be included in the factor analysis (two items were not included because their answer choice was not an ordinal scale), it was estimated that 130 patients was a sufficient sample size. Nevertheless, because of the low prevalence of such disorders [2,3], a lower number of patients was considered acceptable.
SICT Instrument
A 28-item SICT instrument was developed based on a literature review, patient interviews, clinician interviews, item generation, and face and content validity testing.
The first draft of the instrument was created based on the results of three expert clinician interviews, and four patient interviews (two thalassaemia, one SCD, and one MDS patient). A further nine patient interviews (four thalassaemia, one SCD, and four MDS patients) were conducted. The open-ended questions ensured all relevant concepts were covered and cognitive debriefing ensured patient understanding of the instrument. The 28-item SICT instrument included an assessment of satisfaction with prior experience with ICT, adherence to treatment, and preferences. The majority of items were measured on a 5-point Likert scale, where 1 represented “always” and 5 “never,” or 1 being “very satisfied” and 5 “very dissatisfied.” This scale allows for non–forced-choice answers so that respondents could answer neutrally [31]. Furthermore, Likert scales are more valid than forced-choice scales, reduce consenting response bias, and are therefore very reliable [32]. Multiitem scale scores were calculated as the mean of the items if at least half of the items within a scale were completed.
Based on the literature searches, three expert interviews, and 13 patient interviews, it was hypothesized that the newly developed instrument should have eight domains: satisfaction with ICT effectiveness, safety/side effects, convenience of ICT, costs, overall satisfaction, ICT impact on daily life, patient adherence, and ICT preferences.
Patient-Reported Outcome Measures
Participants also completed four well-established HRQoL measures to assess the concurrent validity of the daily life domain of the SICT instrument (how well concepts are measured). These were SF-36 [33], the Child Health Questionnaire (CHQ) [50-item parent form, for parents of children 5–17 years of age (CHQ-PF50) or the 87-item child form, for children of 10 years and older, (CHQ-CF87)][34], and the Medical Outcomes Study (MOS)-Sleep Scale (12-item questionnaire) [35].
Statistical Procedures
Descriptive and psychometric analyses were performed on the SICT. Although PRO data are frequently nonnormal, parametric statistical methods were used because they are robust to nonnormality. Specifically, Principal Component Analysis (PCA) was used as a type of exploratory factor analysis using orthogonal (varimax) rotation to make sense of the complex factors associated with satisfaction, and to observe relationships between items of the SICT instrument.
Multitrait analysis was conducted to establish the item convergent validity, item discriminant validity, and internal consistency (Cronbach's Alpha ≥0.7). Pearson correlations between the SICT instrument and well-established HRQoL measures were conducted to test for the concurrent validity.
Simple linear regressions were performed to examine relationships with the SICT instrument domains and adherence as defined by “never thinking about stopping ICT.” A Multiple Linear Regression with backward selection of the variables was also conducted (variables were retained at P ≤ 0.05) to assess the combined effects of the principal components of SICT in predicting adherence. All tests were two-tailed, with statistical significance level of P < 0.05.
All data were analyzed using Statistical Analysis System (SAS) version 9, or Multi-trait Analysis Program-Revised (MAP-R) version 1.
Results
Demographics and Clinical Characteristics
Demographic data are presented in Table 1 for the total cross-sectional sample (USA and UK). In total, 107 patients with thalassaemia, SCD, or MDS, currently undergoing ICT, participated in the binational study (USA: thalassaemia n = 41 and SCD n = 8; UK: thalassaemia n = 39, SCD n = 13, and MDS n = 6).
| Demographic and clinical characteristics | Cross-sectional(N = 107) | USA sample(N = 49) | UK sample(N = 58) | |
|---|---|---|---|---|
| Gender | ||||
| Male | n (%) | 44 (41.12) | 24 (48.98) | 20 (34.48) |
| Female | n (%) | 63 (58.88) | 25 (51.02) | 38 (65.52) |
| Employment | ||||
| Full-time | n (%) | 35 (32.71) | 19 (38.78) | 16 (27.59) |
| Part-time | n (%) | 16 (14.95) | 9 (18.37) | 7 (12.07) |
| Unemployed | n (%) | 50 (46.73) | 21 (42.86) | 29 (50.00) |
| Retired | n (%) | 6 (5.61) | 0 (0.00) | 6 (10.34) |
| Age (years) | Mean (SD) | 31.51 (14.65) | 28.54 (9.60) | 34.01 (17.54) |
| Patient disease | ||||
| Thalassaemia | n (%) | 80 (74.77) | 41 (83.67) | 39 (67.24) |
| SCD | n (%) | 21 (19.63) | 8 (16.33) | 13 (22.41) |
| MDS | n (%) | 6 (5.61) | 0 (0.00) | 6 (10.34) |
| Ferritin levels (mg/L) | ||||
| Mean level of ferritin in the most recent year | Mean (SD) | 2888 (2247) | 2750 (2505) | 3006 (2015) |
| Number of side effects in the previous year | ||||
| 0 | n (%) | 79 (73.83) | 37 (75.51) | 42 (72.41) |
| 1 | n (%) | 18 (16.82) | 8 (16.33) | 10 (17.24) |
| 2 and above | n (%) | 10 (9.35) | 4 (8.16) | 6 (10.34) |
| Number of doses/week patients are suppose to take | ||||
| 1 to 5 | n (%) | 59 (55.14) | 36 (73.47) | 23 (39.66) |
| 6 and above | n (%) | 48 (44.86) | 13 (26.53) | 35 (60.34) |
- SCD, sickle cell disease; MDS, myelodysplastic syndrome.
The majority of the sample were female (58.9%, n = 63), and the mean age was 31.5 years (range 10–85 years). Specifically, 86.9% (n = 93) were adults (over 18 years), 13.1% (n = 14) adolescents (10–18 years).
Overall, 70.9% of patients were currently receiving DFO-related ICT (n = 78), 17.2% on deferiprone (n = 19), and 11.8% on combined therapy (n = 13).
United States and UK demographic details were broadly comparable. Nevertheless, the majority of patients in the USA (98%, n = 48) were on DFO-related ICT with only one patient on combination of oral and DFO-related ICT, whereas in the UK, 46.6% (n = 27) patients were on DFO-related ICT, 31% (n = 18) were on oral ICT, and 22.4% (n = 13) were on combination ICT.
Psychometric Characteristics of the SICT Instrument
Item review and reduction. The PCA and multitrait analysis were performed to explore the structure of the 26-item questionnaire from complete patient responses (n = 92). Items 17 and 28 were excluded from the PCA because the answer choices were not an ordinal scale; thus, these were analyzed separately.
A four-dimensional structure was indicated by the PCA and confirmed by the multitrait analysis. Seven items were excluded from the four-dimensional structure and were analyzed separately:
- •
Items 26 and 27 related to previous experience and intention and failed to correlate to any of the identified dimensions.
- •
Items 5 and 24 were deleted because they did not meet minimal criteria for convergent validity and failed to load on a factor:
- •
Item 5: In general in the last 4 weeks, how often did you feel worried that you were not receiving an adequate dose of medication?
- •
Item 24: Overall, how did the side effects of chelation therapy meet your expectations?
- •
- •
Items 14 to 16 were adherence items:
- •
Item 14: In general in the last 4 weeks, how often did you have trouble remembering to take your chelation therapy?
- •
Item 15: In general in the last 4 weeks, how often did you think about stopping your chelation therapy?
- •
Item 16: In general in the last 4 weeks, how often did you follow the chelation therapy regimen exactly as recommended by your doctor?
- •
The final PCA was conducted on the 19 SICT items, yielding to four domain scores. The rotated factor pattern for the varimax PCA is presented in Table 2. The first four factors (domains) represented 63% of the variability of the items.
| Rotated factor pattern | |||||
|---|---|---|---|---|---|
| Perceived Effectiveness | Burden | Acceptance | Side Effects | ||
| Item 1 | Your current chelation therapy would help you live longer? | 0.852 | 0.015 | 0.090 | −0.063 |
| Item 3 | Your chelation therapy would stop the iron overload from getting worse? | 0.851 | 0.039 | −0.028 | 0.028 |
| Item 2 | Your chelation therapy would get rid of the iron overload? | 0.850 | 0.006 | −0.003 | 0.048 |
| Item 4 | Your chelation therapy was working? | 0.713 | 0.129 | 0.252 | −0.174 |
| Item 20 | Overall, how worthwhile was your chelation therapy? | 0.640 | −0.209 | 0.106 | −0.027 |
| Item 13 | That chelation therapy was worth taking/having? | 0.636 | −0.072 | 0.0001 | −0.059 |
| Item 9 | That chelation therapy made you dependent on others? | −0.028 | 0.766 | 0.049 | 0.114 |
| Item 8 | That your chelation therapy regimen stopped you from getting a good night sleep? | −0.017 | 0.741 | −0.124 | 0.337 |
| Item 19 | Overall, how bothered were you by the amount of time it took to take your chelation therapy? | −0.146 | 0.675 | −0.355 | 0.217 |
| Item 6 | That your chelation therapy regimen limited your evening or night time activities? | 0.021 | 0.666 | −0.264 | 0.338 |
| Item 7 | That your chelation therapy regimen limited your daytime activities? | 0.017 | 0.655 | −0.159 | 0.090 |
| Item 22 | How easy or difficult was it to take chelation therapy? | 0.032 | −0.419 | 0.754 | −0.209 |
| Item 18 | Overall, how convenient or inconvenient was it for you to take your chelation therapy? | −0.038 | −0.236 | 0.721 | −0.103 |
| Item 25 | Overall, how satisfied or dissatisfied were you with the form of your chelation therapy (oral pill/capsules or pump)? | −0.006 | −0.052 | 0.717 | −0.194 |
| Item 23 | Overall, how well did the benefits of chelation therapy meet your expectations? | 0.255 | −0.024 | 0.665 | 0.243 |
| Item 21 | Was chelation therapy as difficult as you expected it would be? | −0.200 | 0.098 | −0.661 | 0.353 |
| Item 11 | Upset about the side effects of your chelation therapy (e.g., pain)? | −0.064 | 0.284 | −0.034 | 0.841 |
| Item 12 | That chelation therapy negatively affected the appearance of your body or skin? | −0.046 | 0.270 | −0.154 | 0.754 |
| Item 10 | Pain because of your chelation therapy? | −0.091 | 0.322 | −0.315 | 0.701 |
| *Eigenvalues and cumulative variance: | ||
|---|---|---|
| Factor | Eigenvalue | Cumulative |
| Perceived Effectiveness | 6.249 | 0.240 |
| Burden | 3.603 | 0.379 |
| Acceptance | 2.214 | 0.464 |
| Side Effects | 1.418 | 0.519 |
- SICT, satisfaction with iron chelation therapy. Bold items correspond to the appropriate SICT domain.
The first satisfaction factor or domain was labeled “Perceived Effectiveness of ICT” and consisted of six items [1–4,13,20], pertaining to patient perceptions regarding the beneficial outcome of ICT, and rotated factor coefficients ranged from 0.64 to 0.85.
The second factor or domain, “Burden of ICT,” included five items [6–9,19] with rotated factor coefficients ranging from 0.66 to 0.77. The items related to this factor measure the negative impact incurred from ICT on activities of daily living, sleep, time to take ICT, and dependency.
Five items [18,21–23,25] loaded on the third factor labeled “Acceptance of ICT” with rotated factor coefficients ranging from 0.66 to 0.75. The items defining this factor reflect positive orientations toward ICT with regard to expectations and convenience of taking ICT.
The fourth factor or domain labeled “Side Effects of ICT” consisted of three items [10–12] with rotated factor coefficients ranging from 0.70 to 0.84. The items pertaining to this factor assess the potential unwanted side effects of ICT and their impact on the individual's appearance.
Item convergent and discriminant validity. The SICT met an acceptable threshold for item-discriminant validity and all 19 items met the minimum threshold for item-convergent validity (>0.4). Item-scale correlations ranged from 0.43 to 0.76, demonstrating homogeneity within each domain:
- •
Satisfaction with Perceived Effectiveness of ICT: 0.51 to 0.76;
- •
Acceptance of ICT: 0.43 to 0.74;
- •
Burden of ICT: 0.51 to 0.68;
- •
Side Effects of ICT: 0.63 to 0.71.
Internal consistency. Internal consistency for all subscales was good, with alpha coefficients meeting the minimum recommended threshold >0.7: Satisfaction with Perceived Effectiveness of ICT 0.86, Acceptance of ICT 0.80, Burden of ICT 0.82, and Side Effects of ICT 0.81.
Concurrent validity. In general, the Burden domain of the SICT instrument indicated low to moderate correlations with HRQoL measures: SF-36: r = 0.29 to 0.45, n = 93; CHQ-PF50: r = 0.00 to 0.33, n = 14; CHQ-CF87: r = 0.03 to 0.78, n = 14; and MOS-Sleep Scale r = −0.02 to 0.36, n = 93 (Table 3).
| Perceived effectiveness with ICT | Acceptance of ICT | Burden of ICT | Side effects of ICT | |
|---|---|---|---|---|
| SF-36 Physical Functioning | 0.193 | 0.143 | 0.355 * | 0.248 * |
| SF-36 Role physical | 0.090 | 0.2 | 0.294 * | 0.187 |
| SF-36 Pain index | 0.168 | 0.225 | 0.446 * | 0.336 * |
| SF-36 General Health perceptions | 0.225 * | 0.218 * | 0.285 * | 0.310 * |
| SF-36 Vitality | 0.064 | 0.174 | 0.370 * | 0.233 * |
| SF-36 Social Functioning | 0.237* | 0.191 | 0.419 ** | 0.346 * |
| SF-36 Role Emotional | 0.130 | 0.164 | 0.327 * | 0.214 * |
| SF-36 Mental Health Index | 0.174 | 0.157 | 0.314 * | 0.398 * |
| SF-36 Health Transition Index | 0.08 | −0.142 | −0.028 | −0.007 |
| SF-36 Physical Component Summary Score | 0.166 | 0.201 | 0.352 | 0.238 * |
| SF-36 Mental Component Summary | 0.155 | 0.167 | 0.350 * | 0.338 * |
| SF-6D Utility Score | 0.206 * | 0.194 | 0.364 * | 0.334 * |
| CHQ-PF50 Global Behaviour | 0.140 | 0.206 | −0.272 | 0.110 |
| CHQ-PF50 Family Cohesion | −0.040 | 0.235 | −0.300 | 0.118 |
| CHQ-PF50 Physical Functioning | −0.018 | 0.211 | 0.329 | 0.570 * |
| CHQ-PF50 Role Emotional/behaviour | −0.198 | 0.380 | 0.217 | 0.418 |
| CHQ-PF50 Role physical | −0.055 | 0.369 | 0.000 | 0.265 |
| CHQ-PF50 Bodily Pain | 0.197 | 0.424 | 0.334 | 0.335 |
| CHQ-PF50 Behaviour | 0.052 | 0.253 | −0.121 | 0.220 |
| CHQ-PF50 Mental Health | 0.013 | 0.094 | −0.40 | 0.224 |
| CHQ-PF50 Self Esteem | −0.022 | 0.416 | 0.143 | 0.561 |
| CHQ-PF50 General Health | −0.039 | 0.082 | 0.311 | −0.174 |
| CHQ-PF50 Parent Impact Emotional | 0.025 | 0.403 | 0.03 | 0.321 |
| CHQ-PF50 Parent Time Impact | 0.026 | 0.396 | 0.129 | 0.585 * |
| CHQ-PF50 Family Activities | 0.103 | 0.330 | 0.057 | 0.487 |
| CHQ-PF50 Physical Summary Score | 0.009 | 0.434 | 0.265 | 0.496 |
| CHQ-PF50 Psychosocial Summary Score | −0.020 | 0.346 | −0.056 | 0.402 |
| CHQ-CF87 General Health | 0.608 * | 0.515 | 0.029 | 0.345 |
| CHQ-CF87 Global Behaviour | 0.251 | 0.412 | 0.331 | 0.250 |
| CHQ-CF87 Family Cohesion | 0.357 | −0.017 | 0.061 | −0.197 |
| CHQ-CF87 Physical Functioning | 0.146 | 0.228 | 0.277 | 0.298 |
| CHQ-CF87 Role Functioning Emotional | 0.314 | 0.548 * | 0.290 | 0.369 |
| CHQ-CF87 Role Functioning Behaviour | 0.054 | 0.322 | −0.050 | 0.121 |
| CHQ-CF87 Role Functioning Physical | 0.259 | 0.512 | 0.401 | 0.419 |
| CHQ-CF87 Bodily Pain | 0.227 | 0.497 | 0.275 | 0.297 |
| CHQ-CF87 Behaviour | 0.355 | 0.192 | −0.171 | −0.037 |
| CHQ-CF87 Mental Health | 0.352 | 0.404 | 0.249 | 0.323 |
| CHQ-CF87 Self-Esteem Scale | 0.166 | 0.327 | 0.180 | 0.231 |
| CHQ-CF87 General Health Scale | 0.552 * | 0.768 * | 0.782 * | 0.611 * |
| CHQ-CF87 Family Activities | 0.481 | 0.431 | 0.640 * | 0.453 |
| MOS-Sleep Quantity of Sleep | −0.03 | 0.072 | 0.028 | −0.030 |
| MOS-Sleep Disturbance | −0.169 | −0.207 * | −0.314 | −0.275 |
| MOS-Sleep Snoring | 0.01 | −0.062 | −0.019 | −0.035 |
| MOS-Sleep Adequacy | 0.239 * | 0.128 | 0.272 * | 0.144 |
| MOS-Sleep Somnolence | −0.397 ** | −0.057 | −0.236 * | −0.156 |
| MOS-Sleep Problem Index (6 items) | −0.235 * | −0.117 | −0.345 * | −0.266 * |
| MOS-Sleep Problem Index (9 items) | −0.256 * | −0.169 | −0.357 * | −0.264 * |
| SICT Perceived Effectiveness with ICT | — | 0.20 | 0.09 | 0.14 |
| SICT Acceptance of ICT | 0.20 | — | 0.48 | 0.43 |
| SICT Burden of ICT | 0.09 | 0.48 | — | 0.62 |
| SICT Side Effects of ICT | 0.14 | 0.43 | 0.62 | — |
- * P < 0.05;
- ** P < 0.0001.
- SICT, satisfaction with iron chelation therapy; HRQoL, health-related quality of life; SF-36, Short Form 36-Item Health Survey; MOS, Medical Outcomes Study; CHQ, Child Health Questionnaire; —, not applicable. Bold numbers indicate significant correlations.
Correlations between each of the SICT domains compared to the other domains of the SICT are also presented in Table 3.
Description of Satisfaction Scores by treatment group. Because a score of 5 represents “very satisfied” for all items in a domain, a mean of 3.5 or more suggests that most patients were satisfied or very satisfied with most or all of the items in the domain. Figure 1 shows satisfaction results by type of treatment. Closer observation shows that patients treated with oral ICT were more satisfied with acceptance, burden, and side effects of ICT compared to patients treated by DFO-related ICT or a combination of DFO-related and oral ICT. Indeed, the type of treatment had a statistically significant effect on the satisfaction scores (P(SE) = 0.0010; P(AC) < 0.0001; P(BD) = 0.0004).
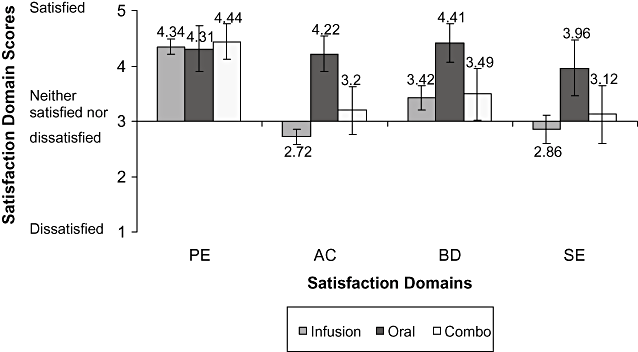
Mean satisfaction scores and standard deviations for overall cross-sectional data per type of treatment. AC, Acceptance of ICT; BD, Burden of ICT; PE, Perceived Effectiveness; SE, Side Effects of ICT.
Discriminant validity. Statistically significant differences in mean satisfaction scores were observed between: patients who experience side effects and those who did not (P < 0.001 for all scales) and patients whose number of doses per week were ≤5 and those with >5 doses (Acceptance of ICT: x̄ = 2.70 and x̄ = 3.45, P = 0.001; Burden of ICT: x̄ = 3.41 and x̄ = 3.82, P = 0.03; Side Effects of ICT: x̄ = 2.77 and x̄ = 3.46, P = 0.002) (Table 4).
| Characteristic | n | Perceived Effectiveness of ICT | Acceptance of ICT | Burden of ICT | Side Effects of ICT | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Gender | |||||||||
| Male | 44 | 4.37 | 0.58 | 3.12 | 0.75 | 3.70 | 0.94 | 3.34 | 1.13 |
| Female | 63 | 4.33 | 0.74 | 2.98 | 0.92 | 3.52 | 1.02 | 2.90 | 1.15 |
| Age group | |||||||||
| Adolescents | 14 | 4.20 | 0.58 | 3.04 | 0.72 | 3.20 | 1.11 | 2.64 | 1.24 |
| Adults | 93 | 4.37 | 0.69 | 3.04 | 0.87 | 3.65 | 0.96 | 3.15 | 1.14 |
| Employment | |||||||||
| Full-time | 35 | 4.58 | 0.46 | 3.07 | 0.81 | 3.91 | 0.75 | 3.53 | 0.92 |
| Unemployed | 50 | 4.28 | 0.70 | 3.04 | 0.89 | 3.32 | 1.03 | 2.77 | 1.20 |
| Country | |||||||||
| UK | 58 | 4.36 | 0.66 | 3.28 | 0.96 | 3.52 | 1.09 | 3.14 | 1.27 |
| USA | 49 | 4.34 | 0.71 | 2.75 | 0.60 | 3.68 | 0.85 | 3.01 | 1.01 |
| Any side effects in the previous 30 days | |||||||||
| No | 46 | 4.39 | 0.69 | 3.42 | 0.95 | 4.03 | 0.92 | 3.69 | 1.18 |
| Yes | 61 | 4.32 | 0.67 | 2.75 | 0.64 | 3.27 | 0.91 | 2.62 | 0.91 |
| Number of doses per week that patient is supposed to take | |||||||||
| ≤5 | 59 | 4.38 | 0.65 | 2.70 | 0.58 | 3.41 | 0.93 | 2.77 | 1.09 |
| >5 | 48 | 4.31 | 0.71 | 3.45 | 0.95 | 3.82 | 1.01 | 3.46 | 1.13 |
| Mean level of ferritin in most recent year | |||||||||
| Data missing | 1 | 4.67 | — | 2.60 | — | 1.60 | — | 1.33 | — |
| ≤1500 | 39 | 4.33 | 0.81 | 3.06 | 0.81 | 3.72 | 0.96 | 3.15 | 1.03 |
| 1500–3000 | 28 | 4.38 | 0.57 | 3.04 | 0.80 | 3.46 | 1.05 | 3.11 | 1.17 |
| >3000 | 39 | 4.33 | 0.63 | 3.03 | 0.95 | 3.62 | 0.94 | 3.04 | 1.28 |
- SICT, satisfaction with iron chelation therapy.
There were no statistically significant differences in mean SICT scores for any domains for the following variables: age, sex, and mean ferritin levels.
Predictors of adherence. Demographics and clinical characteristics, as well as SICT domains, were explored as potential predictors of adherence (defined by never thinking about stopping ICT) (Table 5). Simple regression analyses showed that of 18 variables tested, 10 significantly predicted adherence. Patient disease (thalassemia, SCD or MDS) explained the most variance associated with never thinking about stopping chelating therapy (r2 = 31.7%, P < 0.0001), followed by Burden of ICT (r2 = 29.7%, P < 0.0001), Side Effects of ICT (r2 = 26.0%, P < 0.0001), Acceptance of ICT (r2 = 15.5% P < 0.0001), age (r2 = 10.8%, P = 0.0005), full-time employment (r2 = 10.2%, P = 0.0008), Satisfaction with Perceived Effectiveness of ICT (r2 = 9.3%, P = 0.0014), unemployment (r2 = 5.5%, P = 0.0151), and number of doses missed in the last 7 days (r2 = 5.2%, P = 0.019).
| Description of the relationship with adherence* | Simple regression analysis | ||
|---|---|---|---|
| r 2 (%) | P-value | ||
| Patient disease | MDS: 4.33SCD: 2.38Thal: 4.19 | 31.7 | <0.0001 |
| Satisfaction with Burden of ICT | 0.55 | 29.7 | <0.0001 |
| Satisfaction with Side Effects of ICT | 0.51 | 26.0 | <0.0001 |
| Satisfaction with Acceptance of ICT | 0.39 | 15.5 | <0.0001 |
| Age | 0.33 | 10.8 | 0.0005 |
| Full-time | No: 3.56Yes: 4.43 | 10.2 | 0.0008 |
| Satisfaction with Perceived Effectiveness of ICT | 0.31 | 9.3 | 0.0014 |
| Unemployed | No: 4.12Yes: 3.52 | 5.5 | 0.0151 |
| Number of doses missed in the past 7 days | −0.23 | 5.2 | 0.0185 |
| Experience of side effects | No: 4.13Yes: 3.62 | 3.8 | 0.0433 |
- * Pearson correlation coefficients for continuous predictors; mean adherence score per subgroups for categorical predictors.
- ICT, iron chelation therapy; MDS, myelodysplastic syndrome; SCD, sickle cell disease; Thal, thalassemia.
Multivariate regression analysis was conducted based on independent variables that significantly predicted never thinking about stopping ICT. Four independent variables significantly predicted never thinking about stopping ICT (r2 = 42.3%): age (P = 0.04), Perceived Effectiveness of ICT (P = 0.003), low Burden of ICT (P = 0.002), and low Side Effects of ICT (P = 0.01).
Discussion
The study objectives were to: 1) describe the development, scoring, and validity of the SICT instrument; 2) report satisfaction results of a binational, multicenter, retrospective, and semiprospective study in the USA and UK; and 3) investigate the relationship between satisfaction and adherence of ICT.
The PCA revealed four domains based on 19 items (Satisfaction with Perceived Effectiveness of ICT, Acceptance of ICT, Burden of ICT, and Side Effects of ICT) accounting for 63% of the systematic covariance of the satisfaction items. Seven items were not included in the four-dimensional structure and were analyzed separately: two nonordered items assessing reasons for not taking chelation therapy as directed and preference for type of chelation therapy; three items related to adherence; and two items related to the previous experience of the patients. Nevertheless, the items “In general, in the past 4 weeks, how often did you feel worried you were not receiving an adequate dose of medication?” and “Overall, how did the side effects of ICT meet your expectations?” did not load on any of the factors and were deleted from the questionnaire. Further research is warranted to confirm with greater certainty the factor structure of the SICT instrument.
All four domains of the SICT instrument had satisfactory internal consistency reliability, exceeding Nunnally's threshold of 0.70 [36]. Although there were low to moderate correlations between the Burden domain of the SICT and HRQoL measures, future research could better assess the concurrent validity of the SICT by observing correlations with the generic Treatment Satisfaction Questionnaire for Medication [37].
The second objective was to report the satisfaction results of this binational, multicenter, retrospective, and semiprospective study in the USA and UK.
Overall, mean satisfaction scores revealed that the majority of scores were positive. Closer observation revealed that orally treated patients were more satisfied with acceptance, burden, and side effects of ICT compared to patients treated by DFO-related or a combination of DFO-related ICT and oral ICT.
This finding is supported by previous research which indicates that DFO-related ICT is often burdensome to patients and negatively impacts on patient's HRQoL [8,10,11,20]. These findings can be used as a benchmark for future studies although further research is necessary on this subject.
The third objective of this article was to describe, based on empirical findings, the relationship between satisfaction with ICT and adherence. Results indicated that satisfaction with ICT (specifically satisfaction with: perceived effectiveness, burden, and side effects) and age are significant predictors of “never thinking about stopping ICT” (Fig. 2). These findings were in line with previous research [26,27], explaining 42% of the total adherence variance. This finding is significant because to our knowledge, few studies have explored the association of satisfaction and adherence to ICT regimens. Nevertheless, the remaining 58% of unexplained variance suggests that apart from age and satisfaction with ICT, there may be other factors that have significant importance in explaining adherence to ICT. For example, an international survey exploring views of patients receiving DFO-related ICT revealed that the level of support received from specialist organizations may directly influence patient adherence to ICT [17]. In another study, a sharing of responsibilities for ICT between both parents and children with SCD was associated with a higher level of adherence, as was low family stress although home care regimens and convenience were not reported as useful predictors of adherence. It is possible, however, that treatment location is important for adult patients and this may have accounted for some of the differences in satisfaction with the pill versus infusion modalities [38].
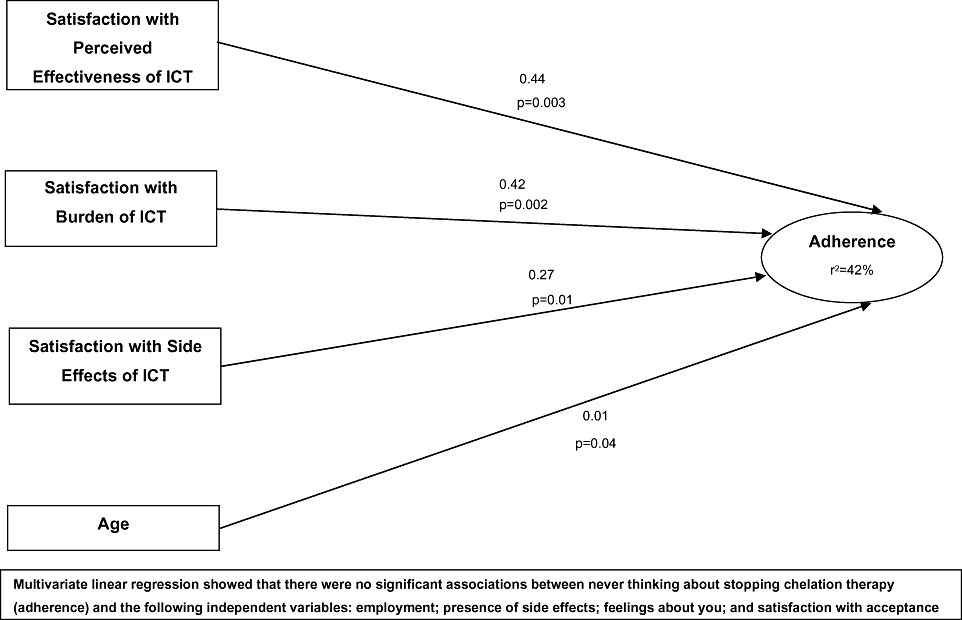
A supported conceptual framework of satisfaction with ICT in patients with iron overload. ICT, iron chelation therapy.
Alternatively, a possible explanation for the unexplained variance in this study is that the study design may have impacted on the ability to determine significant predictors of adherence—because cross-sectional designs are limited in determining cause and effect relationships, and there is potential for confounding variables [39].
This study extends previous research efforts to include aspects perceived important by both patients and health professionals, to develop an instrument to quantify satisfaction with ICT. The utility of this instrument might be directed to other clinical trials which assess satisfaction with ICT or a short form of the scale might be used in routine clinical practice to assess aspects of ICT relevant to patient satisfaction. This would allow clinicians to acknowledge, target, and improve aspects of ICT that patients are perhaps dissatisfied with (e.g., side effects), therefore managing patients' medication programs more effectively as well as improving and maintaining patient well-being. Nevertheless, the usage of a shorter form of the scale for clinical practice would require further psychometric validation. The SICT could be used to provide an indication of best practice and could provide a point of reference for future research and development in clinical practice.
Conclusions
In conclusion, the initial psychometric analyses of the SICT instrument suggest that it is a reliable and valid instrument. Further validation is required to assess its test–retest reliability and responsiveness. The satisfaction results from the study reported here indicated that DFO-related ICT negatively impacts satisfaction and that SICT is a determinant of adherence. This points to the need for a new, more convenient, and less burdensome oral iron chelators, which will increase adherence and ultimately to improve patient outcomes.
Source of financial support: Novartis has commissioned Mapi Values to advise on patient reported outcome strategies for their clinical trials.




