Systematic position of Pyloetis mimosae (Stainton) (Lepidoptera: Tineidae), with redescriptions of the adults and immature stages
Abstract
Morphological characters of adults and immature stages of Pyloetis mimosae (Stainton 1859) are redescribed mainly on the basis of material collected from seed pods of Leucaena leucocephala (Lem.) in the Ryukyus, Japan. Although the systematic position of this species in the family Tineidae has been problematic, we conclude that it should be placed in the subfamily Erechthiinae based on morphological characters. Pyloetis mimosae is newly recorded from Japan.
INTRODUCTION
Pyloetis mimosae (Stainton 1859) is widely distributed in the Indo-Australian region, extending to the Solomon Islands, and is known as a pest of legume seeds (Fletcher 1921, 1933; Robinson et al. 1994). The species was originally described as Laverna mimosae by Stainton (1859) from India, being placed in Elachistidae. Some synonyms of the species were described in Tineidae by Walsingham (1899), Van Deventer (1904), and Meyrick (1907). Fletcher (1921) briefly reviewed the species as Pyloetis mimosae Stainton, 1859, with Ereunetis seminivora Walsingham and Pyloetis ophionota Meyrick as synonyms, and placed it in Lyonetiidae. Later, Fletcher (1933) transferred it to Tineidae as Spatularia mimosae Stainton, adding Spatularia fuligineella van Deventer as its synonym. Because the old genus name Spatularia (van Deventer 1904) is a junior homonym of Spatularia Shaw, 1804 (Nye & Fletcher 1991), the generic name Pyloetis established by Meyrick (1907) is used for containing just one species: P. mimosae (Robinson et al. 1994; Robinson & Tuck 1996; Robinson 2001).
Thus, P. mimosae is currently placed in the family Tineidae, but its systematic position is still problematic. In the present study, the morphology of the male and female genitalia and the immature stages are redescribed in detail, and the systematic position at subfamily level is discussed.
MATERIALS AND METHODS
Adult specimens examined are from Japan (the Ryukyus), Thailand and Vietnam, and are deposited in the collection of the Entomological Laboratory, Osaka Prefecture University.
Larvae for morphological study were collected from seed pods of Leucaena leucocephala (Lem.) (Fig. 1C) in the Ryukyus (Okinawa Island, Ishigaki Island and Iriomote Island), Japan, during April 2001 and April 2002. Some larvae were reared in plastic cups for 1 month to obtain pupae and adults in the laboratory. Male and female genitalia were dissected after being macerated for 4–5 min in 10% KOH heated in a water bath, and illustrated using a binocular microscope. The microstructure of the egg, larva, pupa and the head (labial palps) of the adult were observed by scanning electron microscopy (HITACHI S-3400N: Hitachi High- Technologies Co., Osaka, Japan).
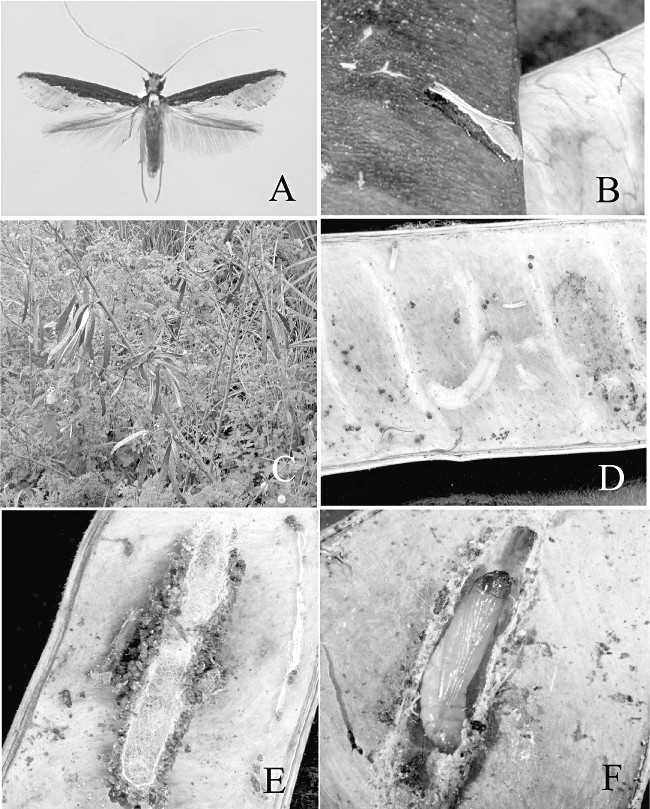
Adult, larva, cocoon, pupa and host plant of Pyloetis mimosae. (A) Adult ♀. (B) Adult, resting posture. (C) Host plant (Leucaena leucocephala). (D) Lavae within seed pod. (E) Cocoon. (F) Pupa in cocoon.
RESULTS
Description
Pyloetis mimosae Stainton, 1859 (1-7).
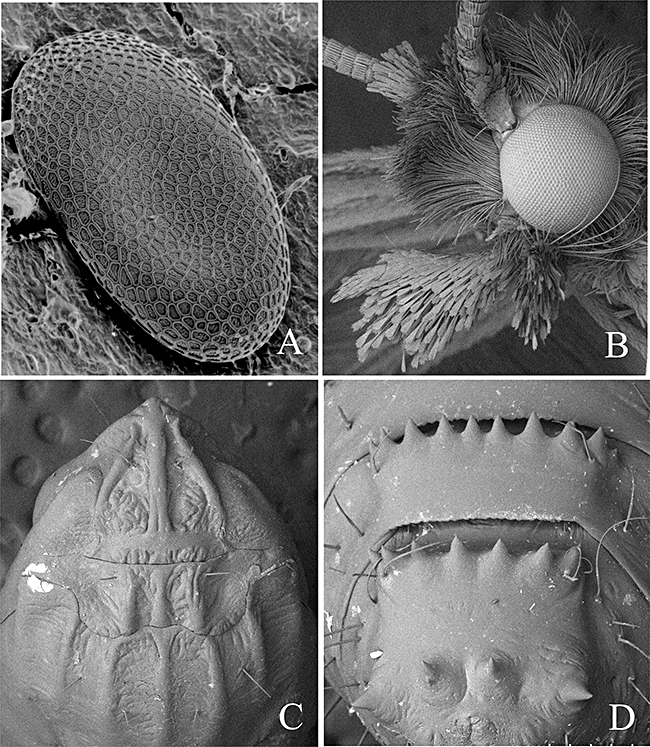
Scanning electron micrograph images of Pyloetis mimosae. (A) Egg, dorsal view. (B) Adult head, showing scales of labial palpus. (C) Head and thorax of pupa, dorsal view. (D) Terminal segments of pupa, dorsal view.
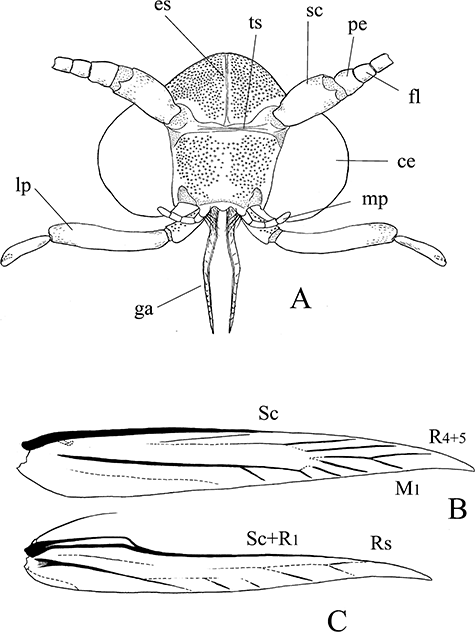
Adult head and wing venation of Pyloetis mimosae. (A) Head of adult, scales removed. (B) Wing venation, forewing. (C) Wing venation, hindwing. ce, compound eye; es, epicranial suture; fl, flagellum; ga, galea; lp, labial palp; mp, maxillary palp; pe, pedicel; sc, scape; ts, transfrontal sulcus.
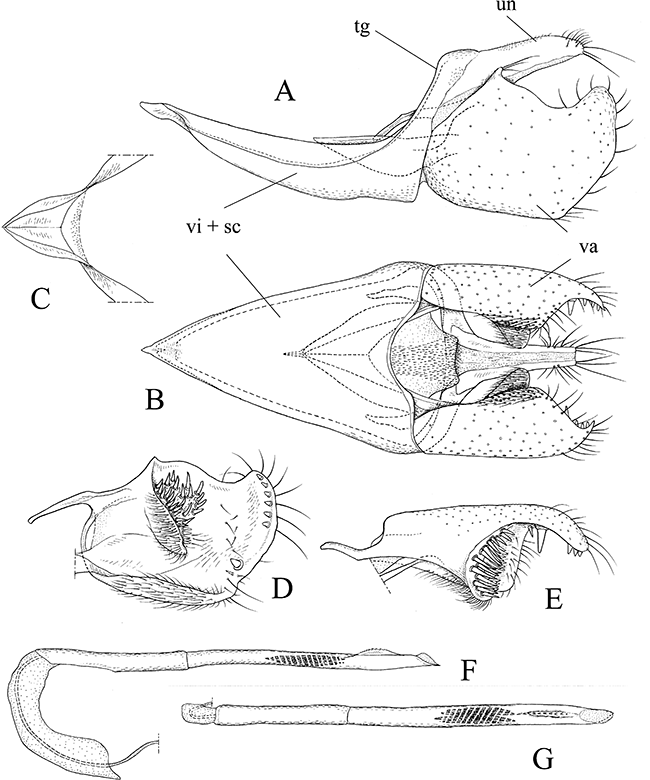
Male genitalia of Pyloetis mimosae. (A) Whole genitalia except aedeagus, lateral view. (B) Whole genitalia except aedeagus, ventral view. (C) Juxta, dorsal view. (D) Valva, inter view. (E) Ditto, dorsal view. (F) Aedeagus, lateral view. (G) Ditto, dorsal view. sc, saccus; tg, tegumen; un, uncus; va, valva; vi, vinculum.
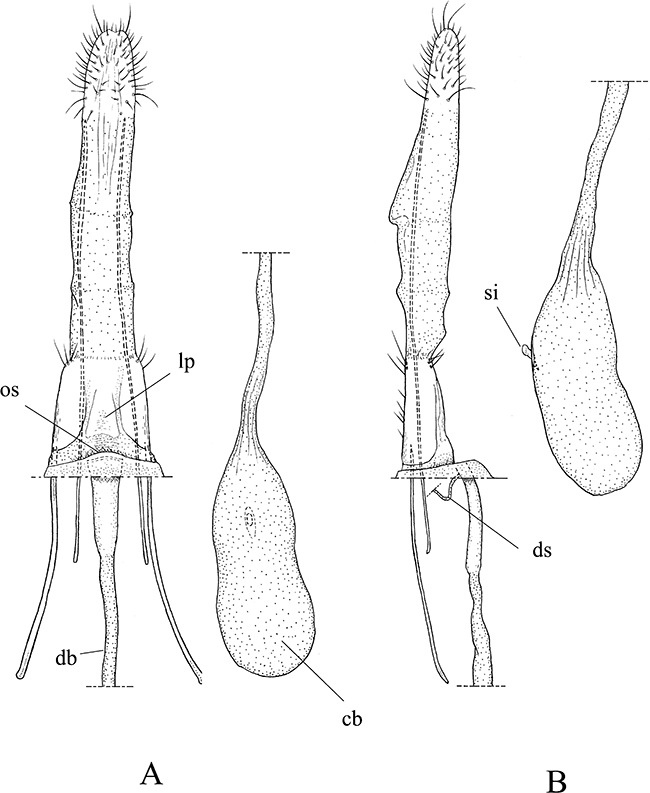
Female genitalia of Pyloetis mimosae. (A) Ovipositor and eighth segment, ventral view. (B) Ditto, lateral view. cb, corpus bursae; db, ductus bursae; ds, ductus seminalis; lp, lamella postvaginalis; os, ostium; si, signa.
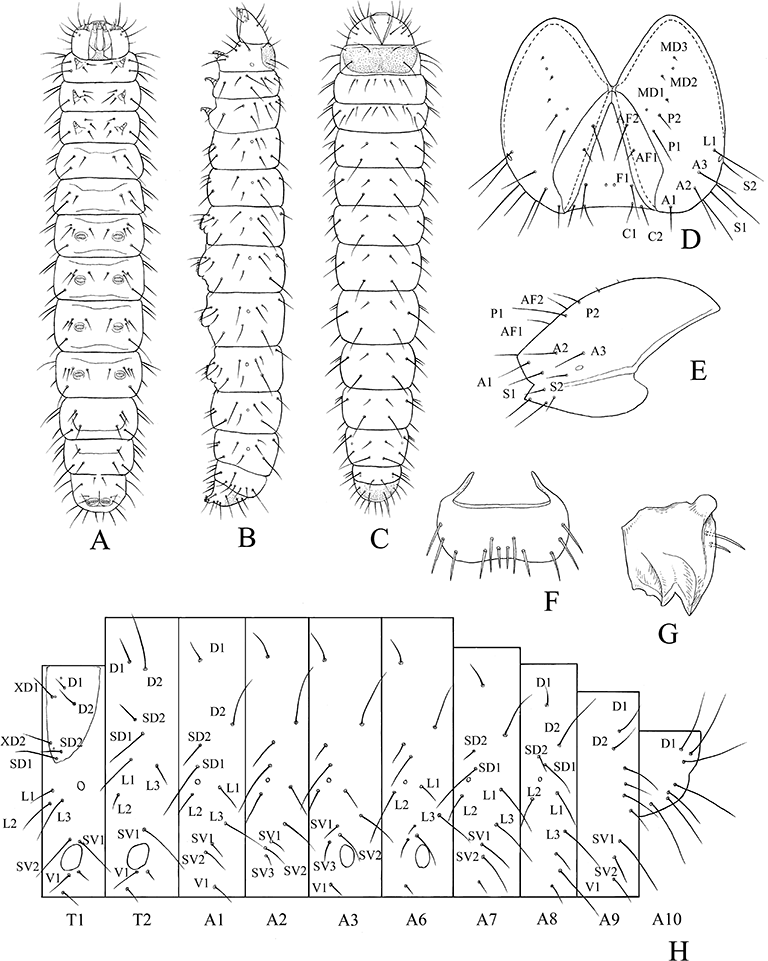
Mature larva of Pyloetis mimosae. (A) Ventral view. (B) Lateral view. (C) Dorsal view. (D) Head capsule, frontal view. (E) Ditto, lateral view. (F) Labrum, frontal view. (G) Mandible, inner view. (E) Setal map.
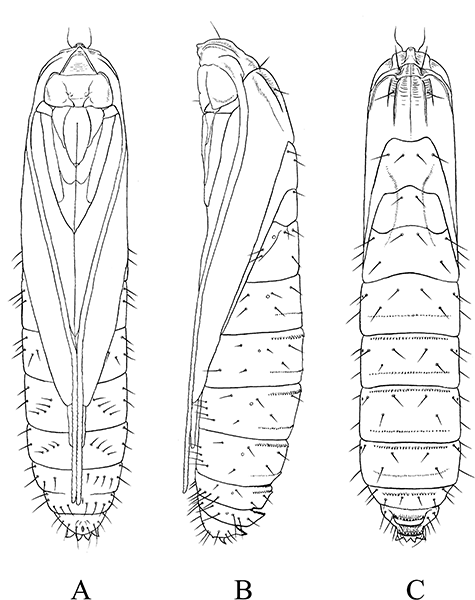
Pupa of Pyloetis mimosae. (A) Ventral view. (B) Lateral view. (C) Dorsal view.
Laverna mimosae Stainton, 1859: 126, from India (in Elachistidae).
Ereunetis seminivora Walsingham, 1899: 107, from India (in Tineidae).
Spatularia fuligineella Van Deventer, 1904: 1, from Indonesia (Java) with descriptions of life history (egg, larva and pupa) (in Tineidae).
Pyloetis ophionota Meyrick, 1907: 752, from Sri Lanka (in Tineidae).
Spatularia mimosae Fletcher, 1933: 75 (in Tineidae).
Pyloetis mimosae Fletcher, 1921: 178 (in Lyonetiidae); Robinson et al. 1994: 31; Robinson and Tuck, 1996: 15 (in Tineidae).
Adult (1)
Slender-winged. Wingspan 11–13 mm in male, 12–18 mm in female.
Head: Eye medium-sized, interocular index 1.3–1.4 in male, 1.2–1.3 in female. Epicranial suture weak. Occiput with two tufts of erect whitish-ochreous filiform scales. Vertex and frons covered with erect dense black filiform scales. Transfrontal suture present. Antenna 0.9–1.0 length of forewing, filiform, whitish-ochreous, except blackish portion at base of scape. Galea (Fig. 3A) moderate, 0.5× length of labial palpus, surface with segmental structure. Maxillary palpus 5-segmented, very short, as long as first segment of labial palpus. Labial palpus (2, 3) well developed, 1.4× height of head, 3-segmented, segmental ratio 1:3:1.4, densely covered with long spatulate blackish-brown scales.
Thorax: Pro- and mesothoraces clothed with whitish-ochreous scales. Tegula covered with blackish-brown scales.
Wing: Forewing narrow and elongate, apex pointed; ground color blackish-brown, with a narrow whitish-ochreous edging along dorsal margin from base to apex; distal half of dorsal margin with long yellowish-brown scales. Hindwing more elongate, lanceolate, apex pointed; semihyaline with long yellowish-brown fringes; two rows of spine-like scales arising from base of anterior margin.
Wing venation: Forewing (Fig. 3B): Sc strong, along anterior margin, ending at one-half of costa. R1 approximate to Sc at costa. R5 completely fused with R4, ending at costa near apex. R4+5 and M1 stalked. Hindwing (Fig. 3C): All veins except Sc+R1 weak. Sc+R1 arising from wing base and ending near apex, separated from Rs. Leg: Fore- and midlegs almost blackish-brown. Hindlegs blackish-brown mixed with whitish-ochreous scales; tibiae with long ochreous hairs dorsally.
Abdomen: Covered with pale ochreous scales. Terminal segment with dense ochreous scales covering genitalia.
Male genitalia (Fig. 4): Uncus narrow, posterior margin sclerotized with long stout bristles at apex. Gnathos absent. Tegumen and vinculum fused into ring. Vinculum + saccus triangular, long and caudally wide. Juxta V-shaped, forming a deep pouch. Valva large and broad; inner side with a cluster of strong bristles on cup-like projection; posterior margin with several short stout bristles; costa with a slender rod. Aedeagus slender, cylindrical, 2.5 times length of valva, with a small dorsal serrate keel near apex; vesica with cornuti composed of many spines.
Female genitalia (Fig. 5): Ovipositor, thick and stout with ostium membranous. Lamella postvaginalis weakly sclerotized. Ductus bursae membranous; caudal portion (antrum) relatively wide, weakly sclerotized. Ductus seminalis arising from caudal portion of ductus bursae near ostium. Corpus bursae elongately ovate, broad. Signum short dagger-shaped, with a papilliform projection.
Immature stages
Egg (Fig. 2A): Semi-oblate, flat, strongly depressed, surface finely reticulated.
Final instar larva (6)
Head (Fig. 6D,E): Head capsule light brown, width 0.8–1.0 mm, with only one stemma. Labrum (Fig. 6F) basic with six pairs of setae. Mandible (Fig. 6G) with two prominent outer teeth and internal ridges.
Body: Cylindrical. Body color translucent milky white. Length 8–12 mm.
Thorax: Thoracic shield (prothorax) amber. Pinaculum absent. Prothorax with l-group trisetose.
Abdomen: A1 to A8 with D2 displaced ventrad, rather approximate to SD-group. A1 and A7 to A9 with SV3 absent. Prolegs on A3 to A6 and A10, with crochets 29–32 in uniordinal transverse bands.
Pupa (2, 7): Length 75–95 mm. Pale brown to amber, head and nine segments dark brown. Head: Frons slightly projected anteriorly, with a pair of setae on near antennal bases. Antennae extending to near anterior margin of A8. Eyes prominent, labrum trapezoid. Thorax: Prothorax with three dorsal carinae; mesothorax with five dorsal carinae (Fig. 2C). Abdomen: Posterior margin of A3–A9 with a slight transverse ridge armed with a row of numerous stout spines. A8 and A9 with 7–10 well-developed spines. Cremaster four short, stout spines (Fig. 2D).
Specimens examined
Japan. Okinawa Island Kunigami: 1♀, Benoki, 9.vi.1996, T. Ueda; 1♀, Yona, 19.x.1998, T. Ohno; 1♂, Ohkuni-rindo, 14.v.1998, T. Ueda; 3♀, Hiji-Ohhashi, 14–19.v.1998, T. Ueda; 1♀, Hiji, 18.v.2002, Y. Sawada; 6♂3♀, Yaese-town, Okinawa Island, 30. iv.−8.v.2006, S. Tominaga.
Ishigaki Island: 1♂4♀, Mount Omotodake, 8.v.1998, T. Ueda; 3♀, Nagura, 13–15.xi.1982, T. Tanabe; 1♂1♀, Takeda-rindo, 15.v.2002, Y. Sawada.
Iriomote Island: 2♀, Uehara, 23.iii.2001, T. Saito; 1♀, Uehara, 3.x.2001, 15♂18♀, 11–17.x.2001, 1♀, 15.iii.2002, Y. Miyamoto; 1♂, Funaura, Iriomote Island, 13.v.2002, Y. Sawada; 2♂1♀, Uhsihku forest, Iriomote Island, 13.x.1998, T. Ohno; 1♂, Ohara, 7.x.1979, S. Hashimoto.
Thailand: 2♂, Fang, Chiang Mai, 13–16.ix.1987, S. Moriuti, T. Saito, Y. Arita and Y. Yoshiyasu; 1♀, same locality, 18.vii.1987, H. Kuroko, S. Moriuti, Y. Arita and Y. Yoshiyasu; 2♂, Tam Tarn Lod, Kanchanaburi, 21.viii.1981, same collectors; 1♂, Erawan, Kanchanaburi, 19.viii.1981, same collectors; 1♂, Phliu, Chanthaburi, 23&26.viii.1987, S. Moriuti, T. Saito, Y. Arita and Y. Yoshiyasu; 1♂2♀, Khao Soi Dao, Chanthaburi, 7–8.x.1985, H. Kuroko, S. Moriuti, T. Saito and Y. Arita; 1♂, same locality, 7.vi.1983, H. Kuroko, S. Moriuti, Y. Arita and Y. Yoshiyasu; 1♀, Khao Yai, Nakhon Nayok, 16.vi.1983, same collectors; 1♂, same locality, 18.vi.1983, same collectors;
1♀, Wang Ta Krai, Nakorn Nayok, 6.viii.1981, same collectors; 1♀, Loei, Phu Rua, 15–19.viii.1987, S. Moriuti, T. Saito, Y. Arita and Y. Yoshiyasu; 1♂, Chulabhorn Dam, Chaiyaphum, 14.viii.1987, same collectors.
Vietnam: 3♀, Cuc Phuong, Ninh Binh, 22–29.iv.1998, T. Hirowatari; 2♂1♀, same locality, 4.v.2004, B. W. Lee.
Distribution
India, Sri Lanka, Thailand, Malaysia, Vietnam, Hong kong, Taiwan, Japan (Ryukyus), Java, Sulawesi, Moluccas Island, Solomon Islands. New to Japan.
Biology
Eggs were laid on the surface of dried seedpods of L. leucocephala (Lem.) in the laboratory. The larvae tie dried pods with silk and remain inside, feeding on seeds and shells. Various stage larvae were observed in the same pod through the year (Fig. 1D).
Pupation takes place within a cocoon, its outer wall camouflaged with larval frass and the dorsal surface covered with a silk mesh (Fig. 1E,F). The pupa protrudes its head and thorax from the pod when the adult emerges. Adults were observed flying around the host plant in the daytime and often perched on the dried seedpods (Fig. 1B); they are sometimes attracted to light traps at night. Adults seem to fly throughout the year.
Hosts include the following Leguminosae: Mimosa arabica Lam. (=Acacia nilotica L.) (Stainton 1859), Albizia lebbeck L. (Robinson et al. 1994), Leucaena glauca Benth (Van Deventer 1904), Leucaena leucocephala (Lem.), Cassia occidentalis L. and Cassia fistula L. (Walsingham 1899), Cassia corymbosa Lam. (Fletcher 1921), Caesalpinia pulcherrima L. (Robinson et al. 1994), Sesbania grandiflora L. (originally in Agathi) & Sesbania aegyptiaca Poiret (=Sesbania sesban L.) (Van Deventer 1904).
Leucaena leucocephala (Lem.), the host plant of P. mimosae in the Ryukyus, was introduced from tropical Asia after 1910 (Yamamura 2002). The tineid may also be introduced in Japan, having been carried with the host plant from overseas.
DISCUSSION
Although it is doubtless that P. mimosae belongs to Tineidae (Davis 1992; Robinson & Nielsen 1993;Robinson et al. 1994; Robinson & Tuck 1996), its taxonomic position at subfamily level has been unclear. Davis (1992) placed it in the subfamily Tineinae, while Robinson et al. (1994) simply noted that its relationships are obscure. Judging from the morphological characters described above, however, we conclude that this species should be placed in the subfamily Erechthiinae (sensuDavis & Robinson 1998). In the male genitalia, the juxta forms a deep pouch and the saccus is large and broad. In the female genitalia, the ovipositor is pudgy without ventral rods and the signum is of a characteristic dagger-shaped form. In the larval characters, the abdominal D2 setae are displaced ventrad and roughly level with the SD group.
Erechthiinae comprise two genera, Erechthias Meyrick, 1880 and Comodica Meyrick, 1880 (Davis & Robinson 1998). Among them, Pyloetis mimosae seems to be rather closely related to the species of Erechthias in having the single dagger-shaped signum, the juxta forming a deep pouch, and the ovipositor lacking ventral rods. The cluster of strong bristles on a cup-like projection of the valva in P. mimosae may be homologous with the costal patch of spine-like setae or those on the elongate basicostal process in Erechthias. Pyloetis mimosae lacks a notched flagellum and a pair of hair pencils on the second abdominal segment, which are characteristics of Comodica (Robinson & Nielsen 1993). Thus, it is possible that Pyloetis is congeneric with Erechthias, but further study is required to determine its generic status within the Erechthiinae.
Larvae of Erechthias feed on plant detritus, dead moribund plant tissue or, in a few cases, on living leaf-tissue (Robinson 1983; Robinson & Nielsen 1993). In Japan, the most common species Erechthias atririvis Meyrick, is found on bark or dead branches of Fagaceae, Leguminosae, Anacardiaceae, and Caricaceae (Moriuti & Kadohara 1994), and canker on bark of Sophora japonica L. (Leguminosae) (Yoshimatsu 1992). In Hawaii, Zimmerman (1978) reported that they are scavengers in dead tree trunks, stems, leaves and seed pods of many plants. Feeding on dried legume seeds in P. mimosae larvae may indicate one of variable feeding habits of Erechthiinae.
ACKNOWLEDGMENTS
We wish to express our thanks to Dr G. S. Robinson (Natural History Museum, UK) for critical reading of the manuscript of this paper, and Prof. M. Ishii and Dr N. Hirai (Osaka Prefecture University, Japan) for their invaluable advice and hospitality during this study. Our thanks are also due to Dr M. Sakai (Osaka, Japan) for giving critical advice and literatures, and to Dr M. Kinjyo (Ryukyu University, Japan) for his kind help to our study in Iriomote Island. We are also indebted to the following entomologists for their kind help and for valuable specimens: Dr Y. Sawada, Dr N. Ahn, Dr B. Lee (Osaka Prefecture University, Japan), Dr F. Komai (Osaka University of Arts, Osaka), S. Tominaga (Okinawa, Japan), Dr T. Saito (Ikeda, Osaka) and Dr T. Ueda (Chiikikankyokeikaku Co. Ltd, Osaka). We thank the staff of the Yona Field, Subtropical Field Science Center, Faculty of Agriculture, and the Iriomote Station, Tropical Biosphere Research Center, University of the Ryukyus, for providing access and field facilities.




